Wikipedia:Reference desk/Archives/Science/2020 July 4
| Science desk | ||
|---|---|---|
| < July 3 | << Jun | July | Aug >> | July 5 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is a transcluded archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
July 4[edit]
Tea bitterness[edit]
When you leave tea leaves in tea, tea quickly gets more bitter. I presume that's because of tannins from tea getting released into the water. However, if you remove the leaves and leave the tea alone for several hours, it also will have become quite a bit bitterer even though the source of the tannins has been taken out. How does this happen? — Preceding unsigned comment added by 93.136.4.100 (talk) 00:53, 4 July 2020 (UTC)
- Tannase. Abductive (reasoning) 07:47, 4 July 2020 (UTC)
- Care to elaborate? 93.136.4.100 (talk) 22:11, 4 July 2020 (UTC)
A hosepipe floating in water[edit]
Hi. For the purposes of a fictional world I want a situation where a typical hosepipe (like you'd use to water your garden with) drops into a large flowing body of water (river?) and is carried along. It struck me that this possibly isn't realistic because the pipe is hollow and it might immediately fill with water and sink. How can I make this work? If the pipe was jammed up with (say) dead leaves at both ends, would that allow it to float, since there would be inaccessible air in the middle? Or how else could I swing it? Thanks. Equinox ◑ 04:23, 4 July 2020 (UTC)
- It already works. Try throwing a garden hose in a swimming pool or bathtub. It will be very difficult trying to get the air out. You pretty much have to make every part of it "downhill" in the same direction. --Guy Macon (talk) 06:31, 4 July 2020 (UTC)
- Aren't any hosepipes made from a rubber, plastic, or other material that floats on water? If this isn't the case in the real world, could you not think of some justification for such a hosepipe in your fictional setting? {The poster formerly known as 87.81.230.195} 2.122.56.20 (talk) 15:31, 4 July 2020 (UTC)
Pumping water upwards[edit]
Hi. Me and my fictional world again. Suppose we have got an old-skool hand pump for water (as described at hand pump, maybe the suction kind, or anything that will take up water from a nearby source underground). And suppose further that we attach a hosepipe to the pump, so that pumped water goes through the pipe. And that pipe is carried up to a vertical height, and fixed there. It's two floors/storeys of a house. Will this "work" in terms of pumping water up there by hand, using the hand-pump lever? Or are there reasons (maybe related to pressure, or the general weight of the water) that will prevent this pumping from happening? I would like the "pumper" to be able to get several litres up to that height. Thanks. Equinox ◑ 04:26, 4 July 2020 (UTC)
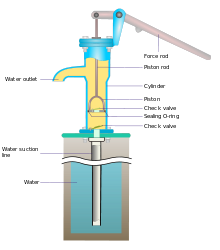
A hand pump - Typical hand pumps pump very well. The only problem is that they are slow. No matter how good the pump, you can only lift water about 10 meters if the pump is at the top. Any higher that that and you end up creating a vacuum. :In real life, the hose collapses long before that. Pumping from the bottom, no problem. at 10 meters you will only be adding 1 atmosphere to the hose. The normal range of water pressure in a house is between 2.5 and 5 atmospheres. --Guy Macon (talk) 06:44, 4 July 2020 (UTC)
- First, "the hose collapses" only if it is on the intake side of the pump. If the intake is a rigid pipe, how close to the 10-meter limit you can come depends on how good the pump is. Second, since you are designing a fictional world, you can adjust the surface gravity and the atmospheric pressure to be what you like. The limit of 10 meters for pumping from above is correct for Earth. --76.71.5.208 (talk) 09:02, 4 July 2020 (UTC)
- The person asking the original question specified a "hosepipe", which is UK for the US "garden hose". --Guy Macon (talk) 14:21, 4 July 2020 (UTC)
- It's still on the outlet not the inlet, so collapsing isn't the problem. DuncanHill (talk) 14:26, 4 July 2020 (UTC)
- I was careful to specify "if the pump is at the top". Please read the comments you are replying to before you start feverishly typing to tell experienced hydraulics engineers that they are wrong. --Guy Macon (talk) 16:47, 4 July 2020 (UTC)
- The OP clearly had the hosepipe going up from the pump. Try reading the question before feverishly trying to show off. DuncanHill (talk) 19:17, 4 July 2020 (UTC)
- I was careful to specify "if the pump is at the top". Please read the comments you are replying to before you start feverishly typing to tell experienced hydraulics engineers that they are wrong. --Guy Macon (talk) 16:47, 4 July 2020 (UTC)
- It's still on the outlet not the inlet, so collapsing isn't the problem. DuncanHill (talk) 14:26, 4 July 2020 (UTC)
- The person asking the original question specified a "hosepipe", which is UK for the US "garden hose". --Guy Macon (talk) 14:21, 4 July 2020 (UTC)
- First, "the hose collapses" only if it is on the intake side of the pump. If the intake is a rigid pipe, how close to the 10-meter limit you can come depends on how good the pump is. Second, since you are designing a fictional world, you can adjust the surface gravity and the atmospheric pressure to be what you like. The limit of 10 meters for pumping from above is correct for Earth. --76.71.5.208 (talk) 09:02, 4 July 2020 (UTC)
- You need some additional force. If the pump normally pumps the water 3 metres up and you want to pump it an additional 6 metres, you need 3 times the force to operate the handle of the pump. That shouldn't be a problem usually. Then there is the question of the seal. In the pump in the picture, the water outlet is below the level where the piston rod enters the body of the pump. This means that a proper seal between the piston rod and the cap at the top of the pump is not required. When you add a hose to the water outlet to bring the water further up, it may leak out of the top of the pump. PiusImpavidus (talk) 08:55, 4 July 2020 (UTC)
- This sounds immediately wrong to my ears - to raise water higher, you need more energy - and you don't necessarily need more force to get more energy. You can also use a longer lever; or you can use the same lever and same force applied for a longer amount of time; and so on. You could apply more force, but if your hand-pump works in the "conventional" way, by drawing a piston, more force won't do very much of anything helpful: what you'd actually want to do is to to pump more cycles using the same force. Review our basic physics definitions at force, potential energy, ... and so on.
- If this were just a pedantic nitpick, I and all other physicists could let it slide... but it's not pedantic - it's a core concept of physics that relates applied force to the work done. There are a dozen variations on the basic formulae and you can find them in a good introductory book like Giancoli's Physics.
- Nimur (talk) 16:12, 4 July 2020 (UTC)
- This is one of those areas where practical engineering takes the results from physics and runs with it. A typical hand pump is basically a hydraulic cylinder and some valves, and we know a lot about designing hydraulic cylinders. Make the diameter the size of a soda straw (remember to subtract the area used up by the rod) and a baby can lift a very tall column of water with one hand. Make it the size of a 55 gallon drum and you will have to be The Incredible Hulk to operate it. And that's assuming a fairly short lever for the handle. With many pumps you move the handle a lot to get the cylinder to move a little, making it even easier to pump (but sacrificing how much you can pump in a given amount of time) --Guy Macon (talk) 16:41, 4 July 2020 (UTC)
- You don't have to tell me about physics. I'm a physicist too. Maybe you were thinking about a different kind of pump than the one I put in the picture. A bucket on a rope maybe? (BTW, to nitpick, if you apply the same force for a longer amount of time, you get more impulse, not necessarily more energy).
- If you attach a hose to the output side of an existing pump to move the water higher, you need more pressure. Using a particular piston, this means more force on the piston. With a particular lever to move that piston, that means more force to move that lever. Sure, there are alternative ways to do this. You could move the water up one floor at a time, temporarily storing it in a bucket and moving the pump up each time. That means more pumping cycles at the same force (it would be easier to carry the filled bucked up the stairs). Or you could replace the pump with a different one, or add additional levers or pulleys to operate it. But the question was about taking a pump and adding a hose to it to pump the water higher than what would normally be the case, using the lever of the pump. That means more pressure and for a positive displacement pump (like all handpumps I've ever seen) that means more force to operate it.
- Or if you want to talk about energy, to move the water higher, you need more energy, so that's more force times distance. With our pre-existing pump, the distance we move the handle is directly proportional to the volume of water we move. To move it higher, we have to increase the force. PiusImpavidus (talk) 11:24, 5 July 2020 (UTC)
Pascal's principle relates the hydrostatic pressure to fluid density and height of the column of water. In english units the rule-of-thumb is 1 psi for every two feet of lift. For your piston pump you should be able to easily calculate the force on the handle from the surface area of the piston, the ambient pressure at the outlet, and difference in height between the outlet and top of the piston. On the intake side there are theoretical and practical limits to the height of the pump above the water source. If you are also concerned with the rate water can be pumped, then messy hydrodynamics and friction loss come into play. fiveby(zero) 13:17, 4 July 2020 (UTC)
- These are quite popular where the water table isn't too deep: [ https://www.youtube.com/watch?&v=rLRm0D6RmEU ]. I have seen them fitted with a hose on the output pumping to an elevated tank so the user gets gravity feed instead of having to pump every time they need water. --Guy Macon (talk) 14:36, 4 July 2020 (UTC)
- Guy is correct about the vacuum. A hose is designed for water to be pressured into it, not sucked out. But I think you will find it will not suck up through the hose at all. First you'd have to fill the hose with water, just to have a chance to suck anything through it at all, and even then it might start to flatten immediately when you try. Think about it this way, you will not be pushing water into the bottom of the pipe to fill it with water, you'll be sucking air from the top of it, and the air will suck out faster than the water will suck in. It will take less force to flatten the pipe slightly, than to suck water without any real pressure on the walls of the pipe at all. Each suck of the pump will flatten it slightly more, with exponential effect, until you reach a tipping point and it just flattens without drawing water at all. You might get somewhere with PEX pipe, which is flexible, but much more rigid than a hosepipe, but similar in price range and versatility, but
ideallyyou are looking at slightly more expensive HDPE pipe or PVC. The rigid plastics are not going to be unreachably more expensive at 10 meters, just somewhat more. Of course, some hosepipes will be relatively rigid and reinforced, and may work for a while, but over 10 meters with such force through a small hole to fill a big pump... you are definitely going to flatten the hosepipe. Also, PEX is pretty tough, but if you are talking about a pump which sucks through a much wider hole than the pipe, you might need a wider pipe or a truly rigid one over time. ~ R.T.G 20:11, 4 July 2020 (UTC)- Edit: Ideally you are looking at a length of pressure hose, more expensive than garden hose and plumbing pipes but not astronomical. I can't remember the exact pricing but I believe you'd get ten meters under 100 euros (might need to shop around a little) ~ R.T.G 20:16, 4 July 2020 (UTC)
- Of course, looking at the question you posted above as well, you may think that filling the hose with water then putting your hand over one end will solve the problem with flattening. It won't. It will just slow it down a little. Each time the pump sucks water out of the hose, it does not suck against the hole in the water at the bottom. It sucks against every part of the inside of the hose equally. Every molecule which comes through the hole at the bottom does so by tugging on the inside walls of the hose. It will flatten. Only a rigid pipe can suffice for you, sorry. ~ R.T.G 21:17, 4 July 2020 (UTC)
- Edit: Ideally you are looking at a length of pressure hose, more expensive than garden hose and plumbing pipes but not astronomical. I can't remember the exact pricing but I believe you'd get ten meters under 100 euros (might need to shop around a little) ~ R.T.G 20:16, 4 July 2020 (UTC)
- Guy is correct about the vacuum. A hose is designed for water to be pressured into it, not sucked out. But I think you will find it will not suck up through the hose at all. First you'd have to fill the hose with water, just to have a chance to suck anything through it at all, and even then it might start to flatten immediately when you try. Think about it this way, you will not be pushing water into the bottom of the pipe to fill it with water, you'll be sucking air from the top of it, and the air will suck out faster than the water will suck in. It will take less force to flatten the pipe slightly, than to suck water without any real pressure on the walls of the pipe at all. Each suck of the pump will flatten it slightly more, with exponential effect, until you reach a tipping point and it just flattens without drawing water at all. You might get somewhere with PEX pipe, which is flexible, but much more rigid than a hosepipe, but similar in price range and versatility, but
Global warming potential[edit]
Hello, please help me understand something about climate science.
Not all greenhouse gases are the same. The difference is measured as global warming potential. Carbon dioxide has a GWP of 1 as the basic unit of measurement. The gases also dissipate at different rates, so timeframe is relevant. In short, CO2 has a GWP of 1 over 100 years. Methane has a GWP of roughly (average estimates) 30 over 100 years. Nitrous oxide has a GWP of roughly 300 over 100 years. This means that these gases cause 30x and 300x the greenhouse effect (over a 100 year period).
And so, according to sources Wikipedia is using, in 2018, 72% of greenhouse emissions was CO2, 19% was methane, and 6% was nitrous oxide. When I multiply these figures by their GWP I get CO" - 72%, methane - 570%, and nitrous - 1800%. To compare these as a multiple of CO2 in particular, rather than a portion of the total, we can divide by 72 and multiply by 100. This gives methane at 790x and nitrous at 2500x the amount of CO2 global warming output in 2018...
Yet, when reporting the effect of methane and nitrous, sources generally say the effects of these gasses are far lower than CO2, like under 15% combined. I haven't found a source saying "Here is why methane and nitrous have a massively huge GWP but do not cause more global warming than CO2." Nor have I found a source which says, "Nirtous oxide is causing thousands of times more global warming than co2, and methane 800 times" How can that make sense?
For perspective, apparently most methane and nitrous are released through animal farming industries. The Humane Society of the USA has an interest in reducing animal agriculture, so they are going to be biased as much as they can towards negativity for methane and nitrous, or at least, they are not going to be biased in the other direction. Yet, according to the HSUS[1], animal industry accounts for less than one fifth. The wildest claim I can find is from the Daily Mail, which claims that replacing all animal industries would account for a 50% reduction (not enough to be worth it, of course, according to the ever popular Daily Mail).
So why is my direct application of GWP absolutely nothing like the figures the others turn out? What else is there in the calculation? ~ R.T.G 18:37, 4 July 2020 (UTC)
- There's a problem with your calculation. What it should give is 7.9x for methane and 25x for N2O. However I'm finding much smaller values for N2O concentration: [2]. Methane is similarly less than 1/200 as abundant as CO2 [3]. Where did you get the 72-19-6% numbers? 93.136.4.100 (talk) 18:53, 4 July 2020 (UTC)
- @93.136.4.100:Our global warming article references that to the IPCC as far as I recall... it is reliably sourced, there is no doubt, various figures are similar. As for the multiplication figure, what you have is after 500 years for methane, but for nitrous, after 500 years the GWP is still 150x. So I take it the 15% figures represent the total of the industrial era since the 1850s, in leave of the introduction and sustained increase of methane and nitrous production since the 1950s? ~ R.T.G 19:27, 4 July 2020 (UTC)
- It is referenced to a couple of books, 19% and 6%, but I have seen across various sources at similar or matching rates. ~ R.T.G 19:31, 4 July 2020 (UTC)
- I think the main issue here is the differing concentrations. GWP is the power per molecule. The concentrations and yearly emissions methane and N20 are significantly lower than CO2, see f.i.: https://www.epa.gov/ghgemissions/overview-greenhouse-gases. So even if they are stronger per molecule, they have an overall smaller effect. Femke Nijsse (talk) 19:52, 4 July 2020 (UTC)
- Also note, from the EPA source; Methane's lifetime in the atmosphere is much shorter than carbon dioxide [but it] is more efficient at trapping radiation. Think methane deteriorates leaving carbon dioxide, so in the longer run its pace of absorbing radiation steps down but keeps going at the carbon dioxide rate. . . dave souza, talk 20:28, 4 July 2020 (UTC)
- Sadly, if you account for GWP, the emissions of methane and nitrous are much greater, more than 34x the effect according to the figures in 2018, unless there is some further metric... Yes, Dave, see global warming potential. +If you look at the global warming article... the second image is a graph of temperature observations, which rises sharply in the 1950s, alarmingly more significant than the trends relevant to industrial advancement... but correlative to when methane and nitrous emissions became significant... ~ R.T.G 21:10, 4 July 2020 (UTC)
- I've found the source of the 72-19-6-3% distribution claim [4]. Unfortunately the table heading says "share gas in GHG" which is useless by itself as it doesn't say what has been divided by what. Looking at methane, N2O and flouride emission graphs on pp.22-24 we get figures of rougly 9.8, 2.7 and 1.7 Gt CO2 equivalent. The total in Gt CO2 equivalent in the graph on p.15 adds up to roughly 51 Gt. Taking percentages we get 19%, 5% and 3%, and the remainder is 73% for CO2. I think it's then reasonable to conclude that the emission numbers you gave have already been adjusted for GWP. 93.136.4.100 (talk) 22:11, 4 July 2020 (UTC)
- Aha! The IPCC full reports do indeed note when they are using co2 equivalents[5], while groups involved with education and awareness (Wikipedia for instance), generally do not. It's so much easier when you know straight out, what you are looking for. And so much more difficult, when you receive a skewed end unnecessarily. All the same, denying that continuing 1950s spike is like denying global warming itself. And that's kind of funny.. ~ R.T.G 00:08, 5 July 2020 (UTC)
- I've found the source of the 72-19-6-3% distribution claim [4]. Unfortunately the table heading says "share gas in GHG" which is useless by itself as it doesn't say what has been divided by what. Looking at methane, N2O and flouride emission graphs on pp.22-24 we get figures of rougly 9.8, 2.7 and 1.7 Gt CO2 equivalent. The total in Gt CO2 equivalent in the graph on p.15 adds up to roughly 51 Gt. Taking percentages we get 19%, 5% and 3%, and the remainder is 73% for CO2. I think it's then reasonable to conclude that the emission numbers you gave have already been adjusted for GWP. 93.136.4.100 (talk) 22:11, 4 July 2020 (UTC)
- Sadly, if you account for GWP, the emissions of methane and nitrous are much greater, more than 34x the effect according to the figures in 2018, unless there is some further metric... Yes, Dave, see global warming potential. +If you look at the global warming article... the second image is a graph of temperature observations, which rises sharply in the 1950s, alarmingly more significant than the trends relevant to industrial advancement... but correlative to when methane and nitrous emissions became significant... ~ R.T.G 21:10, 4 July 2020 (UTC)
- It is referenced to a couple of books, 19% and 6%, but I have seen across various sources at similar or matching rates. ~ R.T.G 19:31, 4 July 2020 (UTC)
- @93.136.4.100:Our global warming article references that to the IPCC as far as I recall... it is reliably sourced, there is no doubt, various figures are similar. As for the multiplication figure, what you have is after 500 years for methane, but for nitrous, after 500 years the GWP is still 150x. So I take it the 15% figures represent the total of the industrial era since the 1850s, in leave of the introduction and sustained increase of methane and nitrous production since the 1950s? ~ R.T.G 19:27, 4 July 2020 (UTC)
Is there still an open question, or can this be closed? NewsAndEventsGuy (talk) 21:50, 4 July 2020 (UTC)
Oxygen saturation in blood[edit]
If each haemoglobin may bind 4 oxygen molecules at the most, how could it be that oxygen saturation in the blood can be measured in values of 78% 92% etc. while considering on the fact that the haemoglobin can bind 4 molecules of O2, it should be measured in one of these four values only: 25% (1 molecule), 50% (2 molecules), 75% (3 molecules) and 100% 4 molecules. So where the other numbers may come from? (I thought maybe it's a combination of two parameters: 1. how many haemoglobins bind to oxygen. 2. how many molecules of O2 in each haemoglobin (not all haemoglobin have the same number of bound O2 molecules. Some may have more and some less, and the saturation is the mean and combination of all of these. Am I right?). --ThePupil (talk) 18:53, 4 July 2020 (UTC)
- How would you indicate blood where 99% of the haemoglobin has 4 oxygen molecules and 1% has 0? --Guy Macon (talk) 19:13, 4 July 2020 (UTC)
- Maybe by average as I said (in this case it's 99% saturation). Isn't it? --ThePupil (talk) 19:27, 4 July 2020 (UTC)
- Although Guy Macon answer is probably good enough, did you read Oxygen saturation (medicine)? It seems to describe the situation well enough. Nil Einne (talk) 05:28, 5 July 2020 (UTC)
- Did you not notice I mentioned this article in my question?:) --ThePupil (talk) 11:43, 5 July 2020 (UTC)
- I don't see where you mentioned the article. You mentioned the oxygen saturation article which is a different article. Also, a lot of people mention link to articles but either don't seem to have read them or don't seem to have understood them. It seems possible one of these apply here since as I said, the article seems to deal with the situation raised in your initial question. Indeed your clarification seems to be a further example of why it's uncertain. The medicine article is linked in the general article, but you linked to the general article even though it barely discusses the specifics of your question. Nil Einne (talk) 13:02, 5 July 2020 (UTC)
- Well, I see what happened. Before I asked my question, I read the same article your linked to, but what happened, probably when I linked it, I linked to something different with a similar name according to the suggestion of Wikipedia algorithms (What's funny, I've just opened what I linked and it was strange to me since I didn't read it at all, but only the one you linked to, and since I didn't find an answer I came to ask here my question). --ThePupil (talk) 13:59, 5 July 2020 (UTC)
- I don't see where you mentioned the article. You mentioned the oxygen saturation article which is a different article. Also, a lot of people mention link to articles but either don't seem to have read them or don't seem to have understood them. It seems possible one of these apply here since as I said, the article seems to deal with the situation raised in your initial question. Indeed your clarification seems to be a further example of why it's uncertain. The medicine article is linked in the general article, but you linked to the general article even though it barely discusses the specifics of your question. Nil Einne (talk) 13:02, 5 July 2020 (UTC)
- Did you not notice I mentioned this article in my question?:) --ThePupil (talk) 11:43, 5 July 2020 (UTC)
- Although Guy Macon answer is probably good enough, did you read Oxygen saturation (medicine)? It seems to describe the situation well enough. Nil Einne (talk) 05:28, 5 July 2020 (UTC)
- Maybe by average as I said (in this case it's 99% saturation). Isn't it? --ThePupil (talk) 19:27, 4 July 2020 (UTC)
Can hemoglobin be empty of oxygen?[edit]
Usually, each haemoglobin can be up to four O2 molecules. Can haemoglobin be empty of oxygen? or it must carry at least one molecule of oxygen? --ThePupil (talk) 18:55, 4 July 2020 (UTC)
- Yes, it can be empty. Dragons flight (talk) 00:30, 5 July 2020 (UTC)
- Or indeed, it can carry other things, though it's not a good thing when that happens (carbon monoxide, cyanide etc). Fgf10 (talk) 10:55, 5 July 2020 (UTC)

