Fatty acid: Difference between revisions
| Line 51: | Line 51: | ||
|} |
|} |
||
====Essential fatty acids==== |
====Essential fatty acids===='''ELLIOT SAM SHABANGU UNIV OF LIMPOPO TURF''' |
||
{{main|Essential fatty acid}} |
{{main|Essential fatty acid}} |
||
Revision as of 12:39, 1 May 2009
| Types of fats in food |
|---|
| Components |
| Manufactured fats |
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid often with a long unbranched aliphatic tail (chain), which is either saturated or unsaturated. Carboxylic acids as short as butyric acid (4 carbon atoms) are considered to be fatty acids, whereas fatty acids derived from natural fats and oils may be assumed to have at least eight carbon atoms, caprylic acid (octanoic acid), for example. Most of the natural fatty acids have an even number of carbon atoms, because their biosynthesis involves acetyl-CoA, a coenzyme carrying a two-carbon-atom group (see fatty acid synthesis).
Fatty acids are produced by the hydrolysis of the ester linkages in a fat or biological oil (both of which are triglycerides), with the removal of glycerol. See oleochemicals.
Fatty acids are aliphatic monocarboxylic acids, derived from, or contained in esterified form in an animal or vegetable fat, oil or wax. Natural fatty acids commonly have a chain of four to 28 carbons (usually unbranched and even numbered), which may be saturated or unsaturated. By extension, the term is sometimes used to embrace all acyclic aliphatic carboxylic acids.[1] This would include acetic acid, which is not usually considered a fatty acid because it is so short that the triglyceride triacetin made from it is substantially miscible with water and is thus not a lipid.
Types
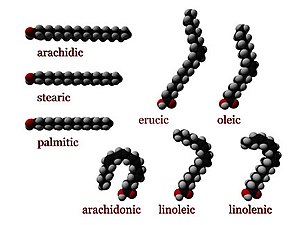
Fatty acids can be saturated and unsaturated, depending on double bonds. They differ in length as well.
===Unsaturated fatty acids===By ELLIOT SAM SHABANGU UNIV OF LIMPOPO TURF

Unsaturated fatty acids are of similar form, except that one or more alkenyl functional groups exist along the chain, with each alkene substituting a single-bonded " -CH2-CH2-" part of the chain with a double-bonded "-CH=CH-" portion (that is, a carbon double-bonded to another carbon).
The two next carbon atoms in the chain that are bound to either side of the double bond can occur in a cis or trans configuration.
- cis
- A cis configuration means that adjacent hydrogen atoms are on the same side of the double bond. The rigidity of the double bond freezes its conformation and, in the case of the cis isomer, causes the chain to bend and restricts the conformational freedom of the fatty acid. The more double bonds the chain has in the cis configuration, the less flexibility it has. When a chain has many cis bonds, it becomes quite curved in its most accessible conformations. For example, oleic acid, with one double bond, has a "kink" in it, whereas linoleic acid, with two double bonds, has a more pronounced bend. Alpha-linolenic acid, with three double bonds, favors a hooked shape. The effect of this is that, in restricted environments, such as when fatty acids are part of a phospholipid in a lipid bilayer, or triglycerides in lipid droplets, cis bonds limit the ability of fatty acids to be closely packed, and therefore could affect the melting temperature of the membrane or of the fat.
- trans
- A trans configuration, by contrast, means that the next two hydrogen atoms are bound to opposite sides of the double bond. As a result, they do not cause the chain to bend much, and their shape is similar to straight saturated fatty acids.
In most naturally-occurring unsaturated fatty acids, each double bond has three n carbon atoms after it, for some n, and all are cis bonds. Most fatty acids in the trans configuration (trans fats) are not found in nature and are the result of human processing (e.g., hydrogenation).
The differences in geometry between the various types of unsaturated fatty acids, as well as between saturated and unsaturated fatty acids, play an important role in biological processes, and in the construction of biological structures (such as cell membranes).
Examples of unsaturated fatty acids:
| Common name | Chemical structure | Δx | C:D | n−x |
|---|---|---|---|---|
| Myristoleic acid | CH3(CH2)3CH=CH(CH2)7COOH | cis-Δ9 | 14:1 | n−5 |
| Palmitoleic acid | CH3(CH2)5CH=CH(CH2)7COOH | cis-Δ9 | 16:1 | n−7 |
| Oleic acid | CH3(CH2)7CH=CH(CH2)7COOH | cis-Δ9 | 18:1 | n−9 |
| Linoleic acid | CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH | cis,cis-Δ9,Δ12 | 18:2 | n−6 |
| α-Linolenic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH | cis,cis,cis-Δ9,Δ12,Δ15 | 18:3 | n−3 |
| Arachidonic acid | CH3(CH2)4CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOHNIST | cis,cis,cis,cis-Δ5Δ8,Δ11,Δ14 | 20:4 | n−6 |
| Eicosapentaenoic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOH | cis,cis,cis,cis,cis-Δ5,Δ8,Δ11,Δ14,Δ17 | 20:5 | n−3 |
| Erucic acid | CH3(CH2)7CH=CH(CH2)11COOH | cis-Δ13 | 22:1 | n−9 |
| Docosahexaenoic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)2COOH | cis,cis,cis,cis,cis,cis-Δ4,Δ7,Δ10,Δ13,Δ16,Δ19 | 22:6 | n−3 |
====Essential fatty acids====ELLIOT SAM SHABANGU UNIV OF LIMPOPO TURF
The human body can produce all but two of the fatty acids it needs. These two, linoleic acid (LA) and alpha-linolenic acid (ALA), are widely distributed in plant oils. In addition, fish oils contain the longer-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Other marine oils, such as from seal, also contain significant amounts of docosapentaenoic acid (DPA), which is also an omega-3 fatty acid. Although the body to some extent can convert ALA into these longer-chain omega-3 fatty acids, the omega-3 fatty acids found in marine oils help fulfill the requirement of essential fatty acids (and have been shown to have wholesome properties of their own).
Since they cannot be made in the body from other substrates and must be supplied in food, they are called essential fatty acids. Mammals lack the ability to introduce double bonds in fatty acids beyond carbons 9 and 10. Hence linoleic acid and alpha-linolenic acid are essential fatty acids for humans.
In the body, essential fatty acids are primarily used to produce hormone-like substances that regulate a wide range of functions, including blood pressure, blood clotting, blood lipid levels, the immune response, and the inflammation response to injury infection.
Essential fatty acids are polyunsaturated fatty acids and are the parent compounds of the omega-6 and omega-3 fatty acid series, respectively. They are essential in the human diet because there is no synthetic mechanism for them. Humans can easily make saturated fatty acids or monounsaturated fatty acids with a double bond at the omega-9 position, but do not have the enzymes necessary to introduce a double bond at the omega-3 position or omega-6 position.
The essential fatty acids are important in several human body systems, including the immune system and in blood pressure regulation, since they are used to make compounds such as prostaglandins. The brain has increased amounts of linolenic and alpha-linoleic acid derivatives. Changes in the levels and balance of these fatty acids due to a typical Western diet rich in omega-6 and poor in omega-3 fatty acids is alleged [2] to be associated with depression and behavioral change, including violence. The actual connection, if any, is still under investigation. Further, changing to a diet richer in omega-3 fatty acids, or consumption of supplements to compensate for a dietary imbalance, has been associated with reduced violent behavior[3] and increased attention span, but the mechanisms for the effect are still unclear. So far, at least three human studies have shown results that support this: two school studies[citation needed][4] as well as a double blind study in a prison.[3][5][6]
Fatty acids play an important role in the life and death of cardiac cells because they are essential fuels for mechanical and electrical activities of the heart. [7] [8] [9] [10]
Trans fatty acids
A trans fatty acid (commonly shortened to trans fat) is an unsaturated fatty acid molecule that contains a trans double bond between carbon atoms, which makes the molecule less 'kinked' in comparison to fatty acids with cis double bonds. These bonds are characteristically produced during industrial hydrogenation of plant oils. Since they are also produced in bacterial metabolism, ruminant fats (e.g. in milk) also contain about 4% trans fatty acids[11]. Research suggests that amounts of trans fats correlate with circulatory diseases such as atherosclerosis and coronary heart disease more than the same amount of cis fats, for reasons that are not fully understood. It is known, however, that trans fats, just like saturated fats, raise the LDL ("bad") cholesterol and lowers the HDL ("good") cholestrol. They have also been shown to have other harmful effects such as increasing triglycerides and Lp(a) lipoproteins. They are also thought to cause more inflammation, which is thought to occur through damage to the cells lining of blood vessels.
Long and short
In addition to saturation, fatty acids are short, medium, or long.
- Short chain fatty acids (SCFA) are fatty acids with aliphatic tails of less than six carbons.
- Medium chain fatty acids (MCFA) are fatty acids with aliphatic tails of 6–12 [12] carbons, which can form medium chain triglycerides.
- Long chain fatty acids (LCFA) are fatty acids with aliphatic tails longer than 12 carbons[13].
- Very Long chain fatty acids (VLCFA) are fatty acids with aliphatic tails longer than 22 carbons
When discussing essential fatty acids (EFA), a slightly different terminology applies. Short-chain EFA are 18 carbons long; long-chain EFA have 20 or more carbons.[14]
Nomenclature
There are several different systems of nomenclature in use for fatty acids. The following table describes the most common systems.
| System | Example | Explanation |
|---|---|---|
| Trivial nomenclature | Palmitoleic acid | Trivial names (or common names) are non-systematic historical names which are the most frequent naming system used in literature. Most common fatty acids have trivial names in addition to their systematic names (see below). These names do not follow any pattern, but are concise and generally unambiguous. |
| Systematic nomenclature | (9Z)-octadec-9-enoic acid | Systematic names (or IUPAC names) derive from the standard IUPAC Rules for the Nomenclature of Organic Chemistry, published in 1979,[15] along with a recommendation published specifically for lipids in 1977.[16] Counting begins from the carboxylic acid end. Double bonds are labelled with cis-/trans- notation or E-/Z- notation, where appropriate. This notation is generally more verbose than common nomenclature, but has the advantage of being more technically clear and descriptive. |
| Δx nomenclature | cis,cis-Δ9,Δ12 | In Δx (or delta-x) nomenclature, each double bond is indicated by Δx, where the double bond is located on the xth carbon–carbon bond, counting from the carboxylic acid end. Each double bond is preceded by a cis- or trans- prefix, indicating the conformation of the molecule around the bond. For example, linoleic acid is designated . |
| n−x nomenclature | n−3 | n−x (n minus x; also ω−x or omega-x) nomenclature does not provide names for individual compounds, but is a shorthand way to categorize fatty acids by their physiological properties. A double bond is located on the xth carbon–carbon bond, counting from the terminal methyl carbon (designated as n or ω) toward the carbonyl carbon. For example, α-Linolenic acid is classified as a n−3 or omega-3 fatty acid, and so it shares properties with other compounds of this type. The ω−x or omega-x notation is common in popular literature, but IUPAC has deprecated it in favor of n−x notation in technical documents.[15] The most commonly researched fatty acid types are n−3 and n−6, which have unique biological properties. |
| Lipid numbers | 18:3 18:3, n−6 18:3, cis,cis,cis-Δ9,Δ12,Δ15 |
Lipid numbers take the form C:D, where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid. This notation can be ambiguous, as some different fatty acids can have the same numbers. Consequently, when ambiguity exists this notation is usually paired with either a Δx or n−x term.[15] |
Free fatty acids
Fatty acids can be bound or attached to other molecules, such as in triglycerides or phospholipids. When they are not attached to other molecules, they are known as "free" fatty acids.
The uncombined fatty acids or free fatty acids may come from the breakdown of a triglyceride into its components (fatty acids and glycerol). However as fats are insoluble in water they must be bound to appropriate regions in the plasma protein albumin for transport around the body. The levels of "free fatty acid" in the blood are limited by the number of albumin binding sites available.
Free fatty acids are an important source of fuel for many tissues since they can yield relatively large quantities of ATP. Many cell types can use either glucose or fatty acids for this purpose. In particular, heart and skeletal muscle prefer fatty acids. The brain cannot use fatty acids as a source of fuel; it relies on glucose, or on ketone bodies. Ketone bodies are produced in the liver by fatty acid metabolism during starvation, or during periods of low carbohydrate intake.
Fatty acids in dietary fats
The following table gives the fatty acid, vitamin E and cholesterol composition of some common dietary fats.[17] [18]
| Saturated | Monounsaturated | Polyunsaturated | Cholesterol | Vitamin E | |
|---|---|---|---|---|---|
| g/100g | g/100g | g/100g | mg/100g | mg/100g | |
| Animal fats | |||||
| Lard | 40.8 | 43.8 | 9.6 | 93 | 0.00 |
| Butter | 54.0 | 19.8 | 2.6 | 230 | 2.00 |
| Vegetable fats | |||||
| Coconut oil | 85.2 | 6.6 | 1.7 | 0 | .66 |
| Palm oil | 45.3 | 41.6 | 8.3 | 0 | 33.12 |
| Cottonseed oil | 25.5 | 21.3 | 48.1 | 0 | 42.77 |
| Wheat germ oil | 18.8 | 15.9 | 60.7 | 0 | 136.65 |
| Soya oil | 14.5 | 23.2 | 56.5 | 0 | 16.29 |
| Olive oil | 14.0 | 69.7 | 11.2 | 0 | 5.10 |
| Corn oil | 12.7 | 24.7 | 57.8 | 0 | 17.24 |
| Sunflower oil | 11.9 | 20.2 | 63.0 | 0 | 49.0 |
| Safflower oil | 10.2 | 12.6 | 72.1 | 0 | 40.68 |
| Hemp oil | 10 | 15 | 75 | 0 | |
| Rapeseed/Canola oil | 5.3 | 64.3 | 24.8 | 0 | 22.21 |
Acidity
Short chain carboxylic acids such as formic acid and acetic acid are miscible with water and dissociate to form reasonably strong acids (pKa 3.77 and 4.76, respectively). Longer-chain fatty acids do not show a great change in pKa. Nonanoic acid, for example, has a pKa of 4.96. However, as the chain length increases the solubility of the fatty acids in water decreases very rapidly, so that the longer-chain fatty acids have very little effect on the pH of a solution. The significance of their pKa values therefore has relevance only to the types of reactions in which they can take part.
Even those fatty acids that are insoluble in water will dissolve in warm ethanol, and can be titrated with sodium hydroxide solution using phenolphthalein as an indicator to a pale-pink endpoint. This analysis is used to determine the free fatty acid content of fats; i.e., the proportion of the triglycerides that have been hydrolyzed.
Reaction of fatty acids
Fatty acids react just like any other carboxylic acid, which means they can undergo esterification and acid-base reactions. Reduction of fatty acids yields fatty alcohols. Unsaturated fatty acids can also undergo addition reactions, most commonly hydrogenation, which is used to convert vegetable oils into margarine. With partial hydrogenation, unsaturated fatty acids can be isomerized from cis to trans configuration. In the Varrentrapp reaction certain unsaturated fatty acids are cleaved in molten alkali, a reaction at one time of relevance to structure elucidation.
Auto-oxidation and rancidity
Fatty acids at room temperature undergo a chemical change known as auto-oxidation. The fatty acid breaks down into hydrocarbons, ketones, aldehydes, and smaller amounts of epoxides and alcohols. Heavy metals present at low levels in fats and oils promote auto-oxidation. Fats and oils often are treated with chelating agents such as citric acid.
Circulation
Digestion and intake
Short- and medium chain fatty acids are absorbed directly into the blood via intestine capillaries and travel through the portal vein just as other absorbed nutrients do. However, long chain fatty acids are too large to be directly released into the tiny intestine capillaries. Instead they are absorbed into the fatty walls of the intestine villi and reassembled again into triglycerides. The triglycerides are coated with cholesterol and protein (protein coat) into a compound called a chylomicron.
Within the villi, the chylomicron enters a lymphatic capillary called a lacteal, which merges into larger lymphatic vessels. It is transported via the lymphatic system and the thoracic duct up to a location near the heart (where the arteries and veins are larger). The thoracic duct empties the chylomicrons into the bloodstream via the left subclavian vein. At this point the chylomicrons can transport the triglycerides to where they are needed.
Distribution
Blood fatty acids are in different forms in different stages in the blood circulation. They are taken in through the intestine in chylomicrons, but also exist in very low density lipoproteins (VLDL) and low density lipoproteins (LDL) after processing in the liver. In addition, when released from adipocytes, fatty acids exist in the blood as free fatty acids.
References
- ^ The Gold Book. IUPAC Compendium of Chemical Terminology (2nd ed.). International Union of Pure and Applied Chemistry. 1997. ISBN 052151150X. Retrieved 2007-10-31.
- ^ "Study Links Brain Fatty Acid Levels To Depression". ScienceDaily. Bethesda, MD: American Society For Biochemistry And Molecular Biology. 2005-05-25. Retrieved 2008-01-18.
- ^ a b C. Bernard Gesch, CQSW Sean M. Hammond, PhD Sarah E. Hampson, PhD Anita Eves, PhD Martin J. Crowder, PhD (2002). "Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behavior of young adult prisoners". The British Journal of Psychiatry. 181: 22–28. doi:10.1192/bjp.181.1.22. PMID 12091259. Retrieved 2006-06-27.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alexandra J. Richardson and Paul Montgomery (2005). "The Oxford-Durham study: a randomized controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder". Pediatrics. 115 (5): 1360–1366. doi:10.1542/peds.2004-2164. PMID 15867048.
- ^ Lawrence, Felicity (2004). Kate Barker (ed.). Not on the Label. Penguin. p. 213. ISBN 0-14-101566-7. OCLC 224019274 55482837 55588726 57432047.
{{cite book}}: Check|oclc=value (help) - ^ "Using Fatty Acids for Enhancing Classroom Achievement". Retrieved January 2004.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|dateformat=ignored (help) - ^ E Honoré, J Barhanin, B Attali, F Lesage, and M Lazdunski (1994 March 1). "External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids". Proc Natl Acad Sci USA. 91(5) (5): 1937–1941. PMID 8127910. Retrieved 2007-01-18.
{{cite journal}}: Check date values in:|year=(help); Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - see page 1 of this link - ^ Reiffel JA, McDonald A (2006). "Antiarrhythmic effects of omega-3 fatty acids". Am. J. Cardiol. 98 (4A): 50i–60i. doi:10.1016/j.amjcard.2005.12.027. PMID 16919517.
- ^ Landmark K, Alm CS (2006). "Alpha-linolenic acid, cardiovascular disease and sudden death". Tidsskr. Nor. Laegeforen. (in Norwegian). 126 (21): 2792–4. PMID 17086218.
- ^ Herbaut C (2006). "Omega-3 and health". Rev Med Brux (in French). 27 (4): S355–60. PMID 17091903.
- ^ F.D. Gunstone, J.L. Harwood & A.J. Dijkstra: The Lipid Handbook, 3rd ed., New York (Chapman & Hall) 2007, ISBN 978-0-849-39688-5
- ^ [http://emedicine.medscape.com/article/946755-overview
- ^ Christopher Beermann1, J Jelinek1, T Reinecker2, A Hauenschild2, G Boehm1, and H-U Klör2, "Short term effects of dietary medium-chain fatty acids and n-3 long-chain polyunsaturated fatty acids on the fat metabolism of healthy volunteers"
- ^ "Health Facts About Good Fats : The Basics : Omega-3s, Mono & Polyunsaturated Fatty Acids". Fats of Life Newsletter. Retrieved 2007-12-12.
- ^ a b c Rigaudy, J. (1979). Nomenclature of Organic Chemistry. Pergamon. ISBN 0080223699. OCLC 5008199.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "The Nomenclature of Lipids. Recommendations, 1976". European Journal of Biochemistry. 79 (1): 11–21. 1977. doi:10.1111/j.1432-1033.1977.tb11778.x.
{{cite journal}}: More than one of|work=and|journal=specified (help) - ^ Food Standards Agency (1991). "Fats and Oils". McCance & Widdowson's the Composition of Foods. Royal Society of Chemistry.
- ^ Ted Altar. "More Than You Wanted To Know About Fats/Oils". Sundance Natural Foods Online. Retrieved 2006-08-31.
See also
- List of saturated fatty acids
- Essential fatty acid
- Saturated fat
- Unsaturated fat
- Fatty acid synthesis
- Fatty acid metabolism
- Fatty acid synthase
- Vegetable oils
