Osteoporosis
| Osteoporosis | |
|---|---|
| Specialty | Endocrinology |
Osteoporosis ("porous bones", from Greek: οστούν/ostoun meaning "bone" and πόρος/poros meaning "pore") is a disease of bones that leads to an increased risk of fracture.[1] In osteoporosis the bone mineral density (BMD) is reduced, bone microarchitecture deteriorates, and the amount and variety of proteins in bone is altered. Osteoporosis is defined by the World Health Organization (WHO) as a bone mineral density that is 2.5 standard deviations or more below the mean peak bone mass (average of young, healthy adults) as measured by DXA; the term "established osteoporosis" includes the presence of a fragility fracture.[2] The disease may be classified as primary type 1, primary type 2, or secondary.[1] The form of osteoporosis most common in women after menopause is referred to as primary type 1 or postmenopausal osteoporosis. Primary type 2 osteoporosis or senile osteoporosis occurs after age 75 and is seen in both females and males at a ratio of 2:1. Finally, secondary osteoporosis may arise at any age and affect men and women equally. This form of osteoporosis results from chronic predisposing medical problems or disease, or prolonged use of medications such as glucocorticoids, when the disease is called steroid- or glucocorticoid-induced osteoporosis (SIOP or GIOP).
Osteoporosis risks can be reduced with lifestyle changes and sometimes medication; in people with osteoporosis, treatment may involve both. Lifestyle change includes diet and exercise, and preventing falls. Medication includes calcium, vitamin D, bisphosphonates and several others. Fall-prevention advice includes exercise to tone deambulatory muscles, proprioception-improvement exercises; equilibrium therapies may be included. Exercise with its anabolic effect, may at the same time stop or reverse osteoporosis. Osteoporosis is a component of the frailty syndrome.
Signs and symptoms
Osteoporosis itself has no symptoms; its main consequence is the increased risk of bone fractures. Osteoporotic fractures are those that occur in situations where healthy people would not normally break a bone; they are therefore regarded as fragility fractures. Typical fragility fractures occur in the vertebral column, rib, hip and wrist.
Fractures
Fractures are the most dangerous aspect of osteoporosis. Debilitating acute and chronic pain in the elderly is often attributed to fractures from osteoporosis and can lead to further disability and early mortality.[3] The fractures from osteoporosis may also be asymptomatic. The symptoms of a vertebral collapse ("compression fracture") are sudden back pain, often with radiculopathic pain (shooting pain due to nerve root compression) and rarely with spinal cord compression or cauda equina syndrome. Multiple vertebral fractures lead to a stooped posture, loss of height, and chronic pain with resultant reduction in mobility.[4]
Fractures of the long bones acutely impair mobility and may require surgery. Hip fracture, in particular, usually requires prompt surgery, as there are serious risks associated with a hip fracture, such as deep vein thrombosis and a pulmonary embolism, and increased mortality.
Fracture Risk Calculators assess the risk of fracture based upon several criteria, including BMD, age, smoking, alcohol usage, weight, and gender. Recognised calculators include FRAX[5] and Dubbo.
Falls risk
The increased risk of falling associated with aging leads to fractures of the wrist, spine, and hip. The risk of falling, in turn, is increased by impaired eyesight due to any cause (e.g. glaucoma, macular degeneration), balance disorder, movement disorders (e.g. Parkinson's disease), dementia, and sarcopenia (age-related loss of skeletal muscle). Collapse (transient loss of postural tone with or without loss of consciousness) leads to a significant risk of falls; causes of syncope are manifold but may include cardiac arrhythmias (irregular heart beat), vasovagal syncope, orthostatic hypotension (abnormal drop in blood pressure on standing up), and seizures. Removal of obstacles and loose carpets in the living environment may substantially reduce falls. Those with previous falls, as well as those with a gait or balance disorder, are most at risk.[6]
Risk factors
Risk factors for osteoporotic fracture can be split between non-modifiable and (potentially) modifiable. In addition, there are specific diseases and disorders in which osteoporosis is a recognized complication. Medication use is theoretically modifiable, although in many cases the use of medication that increases osteoporosis risk is unavoidable. Caffeine is not a risk factor for osteoporosis.[7]
Nonmodifiable
The most important risk factors for osteoporosis are advanced age (in both men and women) and female sex; estrogen deficiency following menopause or oophorectomy is correlated with a rapid reduction in bone mineral density, while in men a decrease in testosterone levels has a comparable (but less pronounced) effect. While osteoporosis occurs in people from all ethnic groups, European or Asian ancestry predisposes for osteoporosis.[8] Those with a family history of fracture or osteoporosis are at an increased risk; the heritability of the fracture as well as low bone mineral density are relatively high, ranging from 25 to 80 percent. There are at least 30 genes associated with the development of osteoporosis.[9] Those who have already had a fracture are at least twice as likely to have another fracture compared to someone of the same age and sex.[10] A small stature is also a non-modifiable risk factor associated with the development of osteoporosis.[1]
Potentially modifiable
- Excess alcohol—small amounts of alcohol are probably beneficial. Bone density increases with increasing alcohol intake. However chronic heavy drinking (alcohol intake greater than 3 units/day) probably increases fracture risk despite any beneficial effects on bone density.[11][12]
- Vitamin D deficiency[13]—low circulating Vitamin D is common among the elderly worldwide.[14] Mild vitamin D insufficiency is associated with increased Parathyroid Hormone (PTH) production.[14] PTH increases bone resorption, leading to bone loss. A positive association exists between serum 1,25-dihydroxycholecalciferol levels and bone mineral density, while PTH is negatively associated with bone mineral density.[14]
- Tobacco smoking—Many studies have associated smoking with decreased bone health, but the mechanisms are unclear. It has been proposed tobacco smoking inhibits the activity of osteoblasts, and is an independent risk factor for osteoporosis.[11][15] Another is that smoking results in increased breakdown of exogenous estrogen, lower body weight and earlier menopause, all of which contribute to lower bone mineral density.[14]
- Malnutrition—nutrition has an important and complex role in maintenance of good bone. Identified risk factors include low dietary calcium and/or phosphorus, magnesium, zinc, boron, iron, fluoride, copper, vitamins A, K, E and C (and D where skin exposure to sunlight provides an inadequate supply). Excess sodium is a risk factor. High blood acidity may be diet-related, and is a known antagonist of bone.[16] Some have identified low protein intake as associated with lower peak bone mass during adolescence and lower bone mineral density in elderly populations.[14] Conversely, some have identified low protein intake as a positive factor, protein is among the causes of dietary acidity. Imbalance of omega 6 to omega 3 polyunsaturated fats is yet another identified risk factor.[1]
- High protein diet—Research has found an association between diets high in animal protein and increased urinary calcium.[17][18][19] However, the relevance of this observation to bone density is unclear, since higher protein diets tend to increase absorption of calcium from the diet and are associated with higher bone density.[20] Indeed, it has recently been argued that low protein diets cause poor bone health.[21]
- Underweight/inactive—bone remodeling occurs in response to physical stress, and weight bearing exercise can increase peak bone mass achieved in adolescence.[14] In adults, physical activity helps maintain bone mass, and can increase it by 1 or 2%. [citation needed] Conversely, physical inactivity can lead to significant bone loss.[14] (Incidence of osteoporosis is lower in overweight people.)[22]
- Endurance training— In female endurance athletes, large volumes of training can lead to decreased bone density and an increased risk of osteoporosis.[23] This effect might be caused by intense training suppressing menstruation, producing amenorrhea, and it is part of the female athlete triad.[24] However, for male athletes the situation is less clear and although some studies have reported that low bone density in elite male endurance athletes,[25] others have instead seen increased leg bone density.[26][27]
- Heavy metals—a strong association between cadmium, lead and bone disease has been established. Low level exposure to cadmium is associated with an increased loss of bone mineral density readily in both genders, leading to pain and increased risk of fractures, especially in the elderly and in females. Higher cadmium exposure results in osteomalacia (softening of the bone).[28]
- Soft drinks—some studies indicate that soft drinks (many of which contain phosphoric acid) may increase risk of osteoporosis;[29] Others suggest soft drinks may displace calcium-containing drinks from the diet rather than directly causing osteoporosis.[30]
- High dietary protein intake increases calcium excretion in urine and has been linked to increased risk of fractures in research studies.[31] Other investigations have shown that protein is required for calcium absorption, but that excessive protein consumption inhibits this process. No interventional trials have been performed on dietary protein in the prevention and treatment of osteoporosis.[32]
Diseases and disorders
Many diseases and disorders have been associated with osteoporosis.[33] For some, the underlying mechanism influencing the bone metabolism is straightforward, whereas for others the causes are multiple or unknown.
- In general, immobilization causes bone loss (following the 'use it or lose it' rule). For example, localized osteoporosis can occur after prolonged immobilization of a fractured limb in a cast. This is also more common in active patients with a high bone turn-over (for example, athletes). Other examples include bone loss during space flight or in people who are bedridden or who use wheelchairs for various reasons.
- Hypogonadal states can cause secondary osteoporosis. These include Turner syndrome, Klinefelter syndrome, Kallmann syndrome, anorexia nervosa, andropause,[34] hypothalamic amenorrhea or hyperprolactinemia.[34] In females, the effect of hypogonadism is mediated by estrogen deficiency. It can appear as early menopause (<45 years) or from prolonged premenopausal amenorrhea (>1 year). A bilateral oophorectomy (surgical removal of the ovaries) or a premature ovarian failure cause deficient estrogen production. In males, testosterone deficiency is the cause (for example, andropause or after surgical removal of the testes).
- Endocrine disorders that can induce bone loss include Cushing's syndrome,[14] hyperparathyroidism,[14] thyrotoxicosis,[14] hypothyroidism, diabetes mellitus type 1 and 2,[35] acromegaly, and adrenal insufficiency. In pregnancy and lactation, there can be a reversible bone loss.[33]
- Malnutrition, parenteral nutrition[14] and malabsorption can lead to osteoporosis. Nutritional and gastrointestinal disorders that can predispose to osteoporosis include coeliac disease,[14] Crohn's disease, lactose intolerance, surgery[34] (after gastrectomy, intestinal bypass surgery or bowel resection) and severe liver disease (especially primary biliary cirrhosis).[34] Patients with bulimia can also develop osteoporosis. Those with an otherwise adequate calcium intake can develop osteoporosis due to the inability to absorb calcium and/or vitamin D. Other micro-nutrients such as vitamin K or vitamin B12 deficiency may also contribute.
- Patients with rheumatologic disorders like rheumatoid arthritis,[34] ankylosing spondylitis,[34] systemic lupus erythematosus and polyarticular juvenile idiopathic arthritis are at increased risk of osteoporosis, either as part of their disease or because of other risk factors (notably corticosteroid therapy). Systemic diseases such as amyloidosis and sarcoidosis can also lead to osteoporosis.
- Renal insufficiency can lead to osteodystrophy.
- Hematologic disorders linked to osteoporosis are multiple myeloma[34] and other monoclonal gammopathies,[35] lymphoma and leukemia, mastocytosis,[34] hemophilia, sickle-cell disease and thalassemia.
- Several inherited disorders have been linked to osteoporosis. These include osteogenesis imperfecta,[34] Marfan syndrome,[34] hemochromatosis,[14] hypophosphatasia, glycogen storage diseases, homocystinuria,[34] Ehlers–Danlos syndrome,[34] porphyria, Menkes' syndrome, epidermolysis bullosa and Gaucher's disease.
- People with scoliosis of unknown cause also have a higher risk of osteoporosis. Bone loss can be a feature of complex regional pain syndrome. It is also more frequent in people with Parkinson's disease and chronic obstructive pulmonary disease.
Medication
Certain medications have been associated with an increase in osteoporosis risk; only steroids and anticonvulsants are classically associated, but evidence is emerging with regard to other drugs.
- Steroid-induced osteoporosis (SIOP) arises due to use of glucocorticoids - analogous to Cushing's syndrome and involving mainly the axial skeleton. The synthetic glucocorticoid prescription drug prednisone is a main candidate after prolonged intake. Some professional guidelines recommend prophylaxis in patients who take the equivalent of more than 30 mg hydrocortisone (7.5 mg of prednisolone), especially when this is in excess of three months.[36] Alternate day use may not prevent this complication.[37]
- Barbiturates, phenytoin and some other enzyme-inducing antiepileptics - these probably accelerate the metabolism of vitamin D.[38]
- L-Thyroxine over-replacement may contribute to osteoporosis, in a similar fashion as thyrotoxicosis does.[33] This can be relevant in subclinical hypothyroidism.
- Several drugs induce hypogonadism, for example aromatase inhibitors used in breast cancer, methotrexate and other anti-metabolite drugs, depot progesterone and gonadotropin-releasing hormone agonists.
- Anticoagulants - long-term use of heparin is associated with a decrease in bone density,[39] and warfarin (and related coumarins) have been linked with an increased risk in osteoporotic fracture in long-term use.[40]
- Proton pump inhibitors - these drugs inhibit the production of stomach acid; it is thought that this interferes with calcium absorption.[41] Chronic phosphate binding may also occur with aluminium-containing antacids.[33]
- Thiazolidinediones (used for diabetes) - rosiglitazone and possibly pioglitazone, inhibitors of PPARγ, have been linked with an increased risk of osteoporosis and fracture.[42]
- Chronic lithium therapy has been associated with osteoporosis.[33]
Pathogenesis
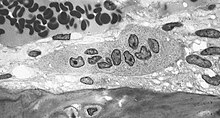
The underlying mechanism in all cases of osteoporosis is an imbalance between bone resorption and bone formation. In normal bone, there is constant matrix remodeling of bone; up to 10% of all bone mass may be undergoing remodeling at any point in time. The process takes place in bone multicellular units (BMUs) as first described by Frost in 1963.[43] Bone is resorbed by osteoclast cells (which derive from the bone marrow), after which new bone is deposited by osteoblast cells.[9]
The three main mechanisms by which osteoporosis develops are an inadequate peak bone mass (the skeleton develops insufficient mass and strength during growth), excessive bone resorption, and inadequate formation of new bone during remodeling. An interplay of these three mechanisms underlies the development of fragile bone tissue.[9] Hormonal factors strongly determine the rate of bone resorption; lack of estrogen (e.g. as a result of menopause) increases bone resorption as well as decreasing the deposition of new bone that normally takes place in weight-bearing bones. The amount of estrogen needed to suppress this process is lower than that normally needed to stimulate the uterus and breast gland. The α-form of the estrogen receptor appears to be the most important in regulating bone turnover.[9] In addition to estrogen, calcium metabolism plays a significant role in bone turnover, and deficiency of calcium and vitamin D leads to impaired bone deposition; in addition, the parathyroid glands react to low calcium levels by secreting parathyroid hormone (parathormone, PTH), which increases bone resorption to ensure sufficient calcium in the blood. The role of calcitonin, a hormone generated by the thyroid that increases bone deposition, is less clear and probably not as significant as that of PTH.[9]

The activation of osteoclasts is regulated by various molecular signals, of which RANKL (receptor activator for nuclear factor κB ligand) is one of best studied. This molecule is produced by osteoblasts and other cells (e.g. lymphocytes), and stimulates RANK (receptor activator of nuclear factor κB). Osteoprotegerin (OPG) binds RANKL before it has an opportunity to bind to RANK, and hence suppresses its ability to increase bone resorption. RANKL, RANK and OPG are closely related to tumor necrosis factor and its receptors. The role of the wnt signalling pathway is recognized but less well understood. Local production of eicosanoids and interleukins is thought to participate in the regulation of bone turnover, and excess or reduced production of these mediators may underlie the development of osteoporosis.[9]
Trabecular bone (or cancellous bone) is the sponge-like bone in the ends of long bones and vertebrae. Cortical bone is the hard outer shell of bones and the middle of long bones. Because osteoblasts and osteoclasts inhabit the surface of bones, trabecular bone is more active, more subject to bone turnover, to remodeling. Not only is bone density decreased, but the microarchitecture of bone is disrupted. The weaker spicules of trabecular bone break ("microcracks"), and are replaced by weaker bone. Common osteoporotic fracture sites, the wrist, the hip and the spine, have a relatively high trabecular bone to cortical bone ratio. These areas rely on trabecular bone for strength, and therefore the intense remodeling causes these areas to degenerate most when the remodeling is imbalanced.[citation needed] Around the ages of 30-35, cancellous or trabecular bone loss begins. Women may lose as much as 50%, while men lose about 30%.[1]
Diagnosis
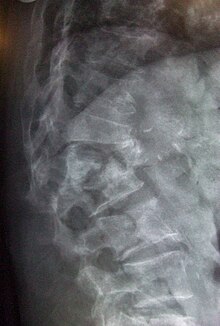

The diagnosis of osteoporosis can be made using conventional radiography and by measuring the bone mineral density (BMD).[44] The most popular method of measuring BMD is dual energy x-ray absorptiometry (DXA or DEXA). In addition to the detection of abnormal BMD, the diagnosis of osteoporosis requires investigations into potentially modifiable underlying causes; this may be done with blood tests. Depending on the likelihood of an underlying problem, investigations for cancer with metastasis to the bone, multiple myeloma, Cushing's disease and other above-mentioned causes may be performed.
Conventional radiography
Conventional radiography is useful, both by itself and in conjunction with CT or MRI, for detecting complications of osteopenia (reduced bone mass; pre-osteoporosis), such as fractures; for differential diagnosis of osteopenia; or for follow-up examinations in specific clinical settings, such as soft tissue calcifications, secondary hyperparathyroidism, or osteomalacia in renal osteodystrophy. However, radiography is relatively insensitive to detection of early disease and requires a substantial amount of bone loss (about 30%) to be apparent on x-ray images.
The main radiographic features of generalized osteoporosis are cortical thinning and increased radiolucency. Frequent complications of osteoporosis are vertebral fractures for which spinal radiography can help considerably in diagnosis and follow-up. Vertebral height measurements can objectively be made using plain-film x-rays by using several methods such as height loss together with area reduction, particularly when looking at vertical deformity in T4-L4, or by determining a spinal fracture index that takes into account the number of vertebrae involved. Involvement of multiple vertebral bodies leads to kyphosis of the thoracic spine, obvious to the clinician as "dowager's hump."
Clinical decision rule
A number of clinical decision rules have been created to predict the risk of osteoporotic fractures. The QFracture score was developed in 2009 and is based on age, BMI, smoking status, alcohol use, rheumatoid arthritis, cardiovascular disease, type 2 diabetes, asthma, use of tricyclic antidepressants or corticosteroids, liver disease, and a history of falls in men. In women hormone replacement therapy, parental history of osteoporosis, gastrointestinal malabsorption, and menopausal symptoms are also taken into account.[45] A website is available to help apply this score.[46]
Dual energy X-ray absorptiometry
Dual energy X-ray absorptiometry (DXA, formerly DEXA) is considered the gold standard for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the bone mineral density is less than or equal to 2.5 standard deviations below that of a young adult reference population. This is translated as a T-score. The World Health Organization has established the following diagnostic guidelines:[2][14]
- T-score -1.0 or greater is "normal"
- T-score between -1.0 and -2.5 is "low bone mass" (or "osteopenia")
- T-score -2.5 or below is osteoporosis
When there has also been an osteoporotic fracture (also termed "low trauma-fracture" or "fragility fracture"), defined as one that occurs as a result of a fall from a standing height, the term "severe or established" osteoporosis is used.[2]
The International Society for Clinical Densitometry takes the position that a diagnosis of osteoporosis in men under 50 years of age should not be made on the basis of densitometric criteria alone. It also states that for pre-menopausal women, Z-scores (comparison with age group rather than peak bone mass) rather than T-scores should be used, and that the diagnosis of osteoporosis in such women also should not be made on the basis of densitometric criteria alone.[47]
Biomarkers
Chemical biomarkers are a useful tool in detecting bone degradation. The enzyme cathepsin K breaks down type-I collagen protein, an important constituent in bones. Prepared antibodies can recognize the resulting fragment, called a neoepitope, as a way to diagnose osteoporosis.[48] Increased urinary excretion of C-telopeptides, a type-I collagen breakdown product, also serves as a biomarker for osteoporosis.[49]
| Condition | Calcium | Phosphate | Alkaline phosphatase | Parathyroid hormone | Comments |
|---|---|---|---|---|---|
| Osteopenia | unaffected | unaffected | normal | unaffected | decreased bone mass |
| Osteopetrosis | unaffected | unaffected | elevated | unaffected [citation needed] | thick dense bones also known as marble bone |
| Osteomalacia and rickets | decreased | decreased | elevated | elevated | soft bones |
| Osteitis fibrosa cystica | elevated | decreased | elevated | elevated | brown tumors |
| Paget's disease of bone | unaffected | unaffected | variable (depending on stage of disease) | unaffected | abnormal bone architecture |
Other measuring tools
Quantitative computed tomography differs from DXA in that it gives separate estimates of BMD for trabecular and cortical bone and reports precise volumetric mineral density in mg/cm3 rather than BMD's relative Z score. Among QCT's advantages: it can be performed at axial and peripheral sites, can be calculated from existing CT scans without a separate radiation dose, is sensitive to change over time, can analyze a region of any size or shape, excludes irrelevant tissue such as fat, muscle, and air, and does not require knowledge of the patient's subpopulation in order to create a clinical score (e.g. the Z-score of all females of a certain age). Among QCT's disadvantages: it requires a high radiation dose compared to DXA, CT scanners are large and expensive, and because its practice has been less standardized than BMD, its results are more operator-dependent. Peripheral QCT has been introduced to improve upon the limitations of DXA and QCT.[44]
Quantitative ultrasound has many advantages in assessing osteoporosis. The modality is small, no ionizing radiation is involved, measurements can be made quickly and easily, and the cost of the device is low compared with DXA and QCT devices. The calcaneus is the most common skeletal site for quantitative ultrasound assessment because it has a high percentage of trabecular bone that is replaced more often than cortical bone, providing early evidence of metabolic change. Also, the calcaneus is fairly flat and parallel, reducing repositioning errors. The method can be applied to children, neonates, and preterm infants, just as well as to adults. Once microimaging tools to examine specific aspects of bone quality are developed, it is expected that quantitative ultrasound will be increasingly used in clinical practice.[44]
Screening
The U.S. Preventive Services Task Force (USPSTF) recommended in 2011 that all women 65 years of age or older should be screened with bone densitometry.[50] They recommend screening women of any age with increased risk factors that puts them at risk equivalent to a 65 year old without additional risk factors.[50] The most significant risk factors is lower body weight (weight < 70 kg), with less evidence for history of smoking or family history. There was insufficient evidence to make recommendations about the optimal intervals for repeated screening and the appropriate age to stop screening. Clinical prediction rules are available to guide selection of women ages 60–64 for screening. The Osteoporosis Risk Assessment Instrument (ORAI) may be the most sensitive.[51]
The USPSTF concludes that the harm versus benefit of screening for osteoporosis in men of any age is unknown.[50] Others have however claimed that screening may be cost effective in those 80 to 85 years of age.[52]
Prevention
Methods to prevent osteoporosis include changes of lifestyle. However, there are medications that can be used for prevention as well. As a different concept there are osteoporosis ortheses which help to prevent spine fractures and support the building up of muscles.[citation needed] Fall prevention can help prevent osteoporosis complications. There is some evidence for hip protectors specifically among those who are in care homes.[53]
Lifestyle
Lifestyle prevention of osteoporosis is in many aspects inversions from potentially modifiable risk factors. As tobacco smoking and unsafe alcohol intake have been linked with osteoporosis, smoking cessation and moderation of alcohol intake are commonly recommended in the prevention of osteoporosis. Many other risk factors, some modifiable and others non modifiable such as genetic may be involved in osteoporosis.[54]
Achieving a higher peak bone mass through exercise and proper nutrition during adolescence is important for the prevention of osteoporosis. Exercise and nutrition throughout the rest of the life delays bone degeneration. Jogging, walking, or stair climbing at 70-90% of maximum effort three times per week, along with 1,500 mg of calcium per day, increased bone density of the lumbar (lower) spine by 5% over nine months. Individuals already diagnosed with osteopenia or osteoporosis should discuss their exercise program with their physician to avoid fractures.[55]
Nutrition
Calcium and calcium plus vitamin D are effective in preventing osteoporosis.[56] People at risk for osteoporosis (e.g. steroid use) are generally treated with vitamin D and calcium supplements and often with bisphosphonates. Vitamin D supplementation alone does not prevent fractures, and needs to be combined with calcium.[57][58] People who are taking proton pump inhibitors or H2 blockers do not absorb calcium carbonate well; calcium citrate is the supplement of choice in this population.[59] In renal disease, more active forms of Vitamin D such as cholecalciferol or (1,25-dihydroxycholecalciferol or calcitriol which is the main biologically active form of vitamin D) is used, as the kidney cannot adequately generate calcitriol from calcidiol (25-hydroxycholecalciferol) which is the storage form of vitamin D.In vitamin D assays, vitamin D2 (ergocalitrol) is not accurately measured, therefore vitamin D3 (cholecalciferol) is recommended for supplementation.[59]
Medication
Just as for treatment, bisphosphonate can be used in cases of very high risk. Other medicines prescribed for prevention of osteoporosis include raloxifene, a selective estrogen receptor modulator (SERM).
Estrogen replacement therapy remains a good treatment for prevention of osteoporosis but, at this time, is not recommended unless there are other indications for its use as well. There is uncertainty and controversy about whether estrogen should be recommended in women in the first decade after the menopause.
In hypogonadal men testosterone has been shown to give improvement in bone quantity and quality, but, as of 2008, there are no studies of the effects on fractures or in men with a normal testosterone level.[35]
Management
Lifestyle changes are an important aspect of treatment and there are several medications used to treat osteoporosis, depending on gender. Aerobics, weight bearing, and resistance exercises all maintain or increase BMD in postmenopausal women.[60] A major problem is gaining long-term adherence to therapy from people with osteoporosis with half not taking their medications and most discontinuing within one year.[61]
Medications
Most bisphosphonates are effective in preventing fracture of the vertebra, non-vertebral bones and hips[62] when taken for three to four years.[63] They however have not been compared directly to each other therefore it is not known if one is better than the others.[62] Fracture risk reduction is between 25 and 70% depending on the bone involved.[62] There are concerns of atypical femoral fractures and osteonecrosis of the jaw with long term use however these risk are low.[62] With evidence of little benefit when used for more than three to five years and in light of the potential adverse events it may be appropriate to stop treatment after this point in time in some.[63]
Teriparatide ( a recombinant parathyroid hormone ) has been shown to be effective in treatment of women with postmenopausal osteoporosis.[64] There is also some evidence that strontium ranelate is effective in decreasing the risk of vertebral and non vertebral fractures in postmenopausal osteoporotic women.[65] Hormone replacement therapy while effective for osteoporosis is only recommended in women who also have menopausal symptoms.[62] Raloxifene while effective in decreasing vertebral fractures does not effect the risk of non vertebral fracture.[62] And while it reduces the risk of breast cancer it increases the risk of blood clots and strokes.[62] Denosumab is also effective for preventing osteoporotic fractures.[62] In hypogonadal men testosterone has been shown to improvement bone quantity and quality, but, there are no studies looking at its effect on fracture risk or in men with a normal testosterone level.[35]
Nutrition
Calcium and vitamin D decrease the risk of non-vertebral fractures in those with postmenopausal osteoporosis by approximately 18%.[62] High intake of vitamin D reduces fractures in the elderly.[56][66] Vitamin K prevents bone loss and/or fractures in those with postmenopausal osteoporosis,[67]
Prognosis
| WHO category | Age 50-64 | Age > 64 | Overall |
|---|---|---|---|
| Normal | 5.3 | 9.4 | 6.6 |
| Osteopenia | 11.4 | 19.6 | 15.7 |
| Osteoporosis | 22.4 | 46.6 | 40.6 |
Although osteoporosis patients have an increased mortality rate due to the complications of fracture, it is rarely lethal.
Hip fractures can lead to decreased mobility and an additional risk of numerous complications (such as deep venous thrombosis and/or pulmonary embolism, pneumonia). The 6-month mortality rate following hip fracture is approximately 13.5%, and a substantial proportion (almost 13%) of people who have suffered a hip fracture need total assistance to mobilize after a hip fracture.[69]
Vertebral fractures, while having a smaller impact on mortality, can lead to severe chronic pain of neurogenic origin, which can be hard to control, as well as deformity. Though rare, multiple vertebral fractures can lead to such severe hunch back (kyphosis) that the resulting pressure on internal organs can impair one's ability to breathe.
Apart from risk of death and other complications, osteoporotic fractures are associated with a reduced health-related quality of life.[70]
Epidemiology
Osteoporosis affects 55% of Americans aged 50 and above. Of these, approximately 80% are women.[71] It is estimated[citation needed] that 1 in 3 women and 1 in 12 men over the age of 50 worldwide have osteoporosis. It is responsible for millions of fractures annually, mostly involving the lumbar vertebrae, hip, and wrist. Fragility fractures of ribs are also common in men.
Hip fractures
Hip fractures are responsible for the most serious consequences of osteoporosis. In the United States, more than 250,000 hip fractures annually are attributable to osteoporosis.[72] It is estimated that a 50-year-old white woman has a 17.5% lifetime risk of fracture of the proximal femur. The incidence of hip fractures increases each decade from the sixth through the ninth for both women and men for all populations. The highest incidence is found among men and women ages 80 or older.[73]
Vertebral fractures
Between 35-50% of all women over 50 had at least one vertebral fracture. In the United States, 700,000 vertebral fractures occur annually, but only about a third are recognized. In a series of 9704 of women aged 68.8 on average studied for 15 years, 324 had already suffered a vertebral fracture at entry into the study; 18.2% developed a vertebral fracture, but that risk rose to 41.4% in women who had a previous vertebral fracture.[74]
Wrist
In the United States, 250,000 wrist fractures annually are attributable to osteoporosis.[72] Wrist fractures are the third most common type of osteoporotic fractures. The lifetime risk of sustaining a Colles' fracture is about 16% for white women. By the time women reach age 70, about 20% have had at least one wrist fracture.[73]
Rib fractures
Fragility fractures of the ribs are common in men as young as age thirty-five on. These are often overlooked as signs of osteoporosis as these men are often physically active and suffer the fracture in the course of physical activity. An example would be as a result of falling while water skiing or jet skiing. However, a quick test of the individual's testosterone level following the diagnosis of the fracture will readily reveal whether that individual might be at risk.
History
The link between age-related reductions in bone density and fracture risk goes back at least to Astley Cooper, and the term "osteoporosis" and recognition of its pathological appearance is generally attributed to the French pathologist Jean Lobstein.[75] The American endocrinologist Fuller Albright linked osteoporosis with the postmenopausal state.[76] Bisphosponates, which revolutionized the treatment of osteoporosis, were discovered in the 1960s.[77]
References
- ^ a b c d Brian K Alldredge; Koda-Kimble, Mary Anne; Young, Lloyd Y.; Wayne A Kradjan; B. Joseph Guglielmo (2009). Applied therapeutics: the clinical use of drugs. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 101–3. ISBN 0-7817-6555-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c WHO (1994). "Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group". World Health Organization technical report series. 843: 1–129. PMID 7941614.
- ^ Old, JL (2004). "Vertebral compression fractures in the elderly". American Family Physician. 69 (1): 111–116. PMID 14727827. Retrieved 31 March 2011.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kim DH, Vaccaro AR (2006). "Osteoporotic compression fractures of the spine; current options and considerations for treatment". The spine journal : official journal of the North American Spine Society. 6 (5): 479–87. doi:10.1016/j.spinee.2006.04.013. PMID 16934715.
- ^ Susan Ott. "Fracture Risk Calculator". Retrieved 2009-11-03.
- ^ Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ (2007). "Will my patient fall?". JAMA. 297 (1): 77–86. doi:10.1001/jama.297.1.77. PMID 17200478.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Waugh, EJ (2009 Jan). "Risk factors for low bone mass in healthy 40-60 year old women: a systematic review of the literature". Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 20 (1): 1–21. doi:10.1007/s00198-008-0643-x. PMID 18523710.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Melton LJ (2003). "Epidemiology worldwide". Endocrinol. Metab. Clin. North Am. 32 (1): 1–13, v. doi:10.1016/S0889-8529(02)00061-0. PMID 12699289.
- ^ a b c d e f Raisz L (2005). "Pathogenesis of osteoporosis: concepts, conflicts, and prospects". J Clin Invest. 115 (12): 3318–25. doi:10.1172/JCI27071. PMC 1297264. PMID 16322775.
- ^ Ojo F, Al Snih S, Ray LA, Raji MA, Markides KS (2007). "History of fractures as predictor of subsequent hip and nonhip fractures among older Mexican Americans". Journal of the National Medical Association. 99 (4): 412–8. PMC 2569658. PMID 17444431.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Poole KE, Compston JE (2006). "Osteoporosis and its management". BMJ. 333 (7581): 1251–6. doi:10.1136/bmj.39050.597350.47. PMC 1702459. PMID 17170416.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Berg KM, Kunins HV, Jackson JL; et al. (2008). "Association between alcohol consumption and both osteoporotic fracture and bone density". Am J Med. 121 (5): 406–18. doi:10.1016/j.amjmed.2007.12.012. PMC 2692368. PMID 18456037.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Nieves JW (1 May 2005). "Osteoporosis: the role of micronutrients". Am J Clin Nutr. 81 (5): 1232S–1239S. PMID 15883457.
- ^ a b c d e f g h i j k l m n WHO Scientific Group on the Prevention and Management of Osteoporosis (2000 : Geneva, Switzerland) (2003). "Prevention and management of osteoporosis : report of a WHO scientific group" (pdf). Retrieved 2007-05-31.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ Wong PK, Christie JJ, Wark JD (2007). "The effects of smoking on bone health". Clin. Sci. 113 (5): 233–41. doi:10.1042/CS20060173. PMID 17663660.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jasminka Z. Ilich, PhD, RD and Jane E Kerstetter, PhD, RD (2000). "Nutrition in Bone Health Revisited: A Story Beyond Calcium". Journal of the American College of Nutrition. 19 (6): 715–737. PMID 11194525.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Abelow BJ, Holford TL and Insogna KL (1992). "Cross-cultural association between dietary animal protein and hip fracture: a hypothesis". Calcified tissue international. 50 (1): 14–18. doi:10.1007/BF00297291. PMID 1739864.
- ^ Hegsted M, Schuette SA, Zemel MB and Linkswiler HM (1981). "Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake". The Journal of nutrition. 111 (3): 553–562. PMID 7205408.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kerstetter JE and Allen LH (1990). "Dietary protein increases urinary calcium" (PDF). Journal of Nutrition. 120 (1): 134–6. PMID 2406396.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21102327, please use {{cite journal}} with
|pmid=21102327instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16373952, please use {{cite journal}} with
|pmid=16373952instead. - ^ Shapses SA, Riedt CS (1 June 2006). "Bone, body weight, and weight reduction: what are the concerns?". J. Nutr. 136 (6): 1453–6. PMID 16702302.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20975110, please use {{cite journal}} with
|pmid=20975110instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15048548, please use {{cite journal}} with
|pmid=15048548instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8370698, please use {{cite journal}} with
|pmid=8370698instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9383270, please use {{cite journal}} with
|pmid=9383270instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10953900, please use {{cite journal}} with
|pmid=10953900instead. - ^ Staessen J, Roels H, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R (1999). "Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group". Lancet. 353 (9159): 1140–4. doi:10.1016/S0140-6736(98)09356-8. PMID 10209978.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP (2006). "Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study". Am. J. Clin. Nutr. 84 (4): 936–42. PMID 17023723.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ American Academy of Pediatrics Committee on School Health (2004). "Soft drinks in schools". Pediatrics. 113 (1 Pt 1): 152–4. doi:10.1542/peds.113.1.152. PMID 14702469.
- ^ Feskanich D, Willett WC, Stampfer MJ, Colditz GA (1996). "Protein consumption and bone fractures in women". Am. J. Epidemiol. 143 (5): 472–79. PMID 8610662.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kerstetter JE, O'Brien KO, Insogna KL (2003). "Dietary protein, calcium metabolism, and skeletal homeostasis revisited". Am. J. Clin. Nutr. 78 (3 Suppl): 584S–592S. PMID 12936953.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Simonelli, C; et al. (2006). "ICSI Health Care Guideline: Diagnosis and Treatment of Osteoporosis, 5th edition" (PDF). Institute for Clinical Systems Improvement. Retrieved 2008-04-08.
{{cite web}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ a b c d e f g h i j k l Kohlmeier, Lynn Kohlmeier (1998). "Osteoporosis - Risk Factors, Screening, and Treatment". Medscape Portals. Retrieved 2008-05-11. [dead link]
- ^ a b c d Ebeling PR (2008). "Clinical practice. Osteoporosis in men". N Engl J Med. 358 (14): 1474–82. doi:10.1056/NEJMcp0707217. PMID 18385499.
- ^ Bone and Tooth Society of Great Britain, National Osteoporosis Society, Royal College of Physicians (2003). Glucocorticoid-induced Osteoporosis (PDF). London, UK: Royal College of Physicians of London. ISBN 1-86016-173-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Gourlay M, Franceschini N, Sheyn Y (2007). "Prevention and treatment strategies for glucocorticoid-induced osteoporotic fractures". Clin Rheumatol. 26 (2): 144–53. doi:10.1007/s10067-006-0315-1. PMID 16670825.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Petty SJ, O'Brien TJ, Wark JD (2007). "Anti-epileptic medication and bone health". Osteoporosis international. 18 (2): 129–42. doi:10.1007/s00198-006-0185-z. PMID 17091219.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ruiz-Irastorza G, Khamashta MA, Hughes GR (2002). "Heparin and osteoporosis during pregnancy: 2002 update". Lupus. 11 (10): 680–82. doi:10.1191/0961203302lu262oa. PMID 12413068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF (2006). "Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2". Arch. Intern. Med. 166 (2): 241–46. doi:10.1001/archinte.166.2.241. PMID 16432096.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yang YX, Lewis JD, Epstein S, Metz DC (2006). "Long-term proton pump inhibitor therapy and risk of hip fracture". JAMA. 296 (24): 2947–53. doi:10.1001/jama.296.24.2947. PMID 17190895.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Murphy CE, Rodgers PT (2007). "Effects of thiazolidinediones on bone loss and fracture". Ann Pharmacother. 41 (12): 2014–18. doi:10.1345/aph.1K286. PMID 17940125.
- ^ Frost HM, Thomas CC. Bone Remodeling Dynamics. Springfield, IL: 1963.
- ^ a b c Guglielmi G, Scalzo G. Imaging tools transform diagnosis of osteoporosis. Diagnostic Imaging Europe. 2010;26(May):7-11.
- ^ "Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores -- Hippisley-Cox and Coupland 339: b4229 -- BMJ".
- ^ "www.qfracture.org".
- ^ Leib ES, Lewiecki EM, Binkley N, Hamdy RC (2004). "Official positions of the International Society for Clinical Densitometry". J Clin Densitom. 7 (1): 1799. doi:10.1385/JCD:7:1:1. PMID 14742881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) quoted in: "Diagnosis of osteoporosis in men, premenopausal women, and children" - ^ Yasuda, Y (2005). "The role of cathepsins in osteoporosis and arthritis: Rationale for the design of new therapeutics". Advanced Drug Delivery Reviews. 57 (7): 973–993. doi:10.1016/j.addr.2004.12.013. PMID 15876399.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Meunier, Pierre (1998). Osteoporosis: Diagnosis and Management. London: Taylor and Francis. ISBN 1-85317-412-2.
- ^ a b c U.S. Preventive Services Task, Force (2011-03-01). "Screening for osteoporosis: U.S. preventive services task force recommendation statement". Annals of internal medicine. 154 (5): 356–64. doi:10.1059/0003-4819-154-5-201103010-00307. PMID 21242341.
- ^ Martínez-Aguilà D, Gómez-Vaquero C, Rozadilla A, Romera M, Narváez J, Nolla JM (2007). "Decision rules for selecting women for bone mineral density testing: application in postmenopausal women referred to a bone densitometry unit". J. Rheumatol. 34 (6): 1307–12. PMID 17552058.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schousboe JT, Taylor BC, Fink HA; et al. (2007). "Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men". JAMA. 298 (6): 629–37. doi:10.1001/jama.298.6.629. PMID 17684185.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Kasturi, GC (2011 Jun). "Osteoporosis: nonpharmacologic management". PM & R : the journal of injury, function, and rehabilitation. 3 (6): 562–72. doi:10.1016/j.pmrj.2010.12.014. PMID 21478069.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Davis S, Oliver A, Goeckeritz B, Sachdeva A (2010). "All about osteoporosis: a comprehensive analysis". Journal of Musculoskeletal Medicine. 27 (4): 149–153.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ (1988). "Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women". Ann. Intern. Med. 108 (6): 824–28. PMID 3259410.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A (2007). "Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis". Lancet. 370 (9588): 657–66. doi:10.1016/S0140-6736(07)61342-7. PMID 17720017.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sahota, O. (2009). "Reducing the risk of fractures with calcium and vitamin D: The combination is more effective than vitamin D alone". BMJ. 339: b5492. doi:10.1136/bmj.b5492.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ DIPART (vitamin D Individual Patient Analysis of Randomized Trials (2010). "Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe". BMJ. 340: b5463. doi:10.1136/bmj.b5463. PMC 2806633. PMID 20068257.
- ^ a b Rosen, Hillel N. Calcium and vitamin D supplementation in osteoporosis. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2010.
- ^ Bonaiuti D, Shea B, Iovine R; et al. (2002). Bonaiuti, Donatella (ed.). "Exercise for preventing and treating osteoporosis in postmenopausal women". Cochrane database of systematic reviews (Online) (3): CD000333. doi:10.1002/14651858.CD000333. PMID 12137611.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Davis S, Sachdeva A, Goeckeritz B, Oliver A (2010). "Approved treatments for osteoporosis and what's in the pipeline". Drug Benefit Trends. 22 (4): 121–124.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i Body, JJ (2011 Nov-Dec). "How to manage postmenopausal osteoporosis?". Acta clinica Belgica. 66 (6): 443–7. PMID 22338309.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Whitaker, Marcea (9 May 2012). "Bisphosphonates for Osteoporosis — Where Do We Go from Here?". New England Journal of Medicine: 120509140014000. doi:10.1056/NEJMp1202619.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Han, SL (2012 Feb). "Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: meta-analysis of randomised controlled trials". International journal of clinical practice. 66 (2): 199–209. doi:10.1111/j.1742-1241.2011.02837.x. PMID 22257045.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ O'Donnell, S (2006 Jul 19). "Strontium ranelate for preventing and treating postmenopausal osteoporosis". Cochrane database of systematic reviews (Online) (3): CD005326. doi:10.1002/14651858.CD005326.pub2. PMID 16856092.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005). "Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials". JAMA. 293 (18): 2257–64. doi:10.1001/jama.293.18.2257. PMID 15886381.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cockayne, S (2006). "Vitamin K and the Prevention of Fractures: Systematic Review and Meta-analysis of Randomized Controlled Trials". Archives of Internal Medicine. 166 (12): 1256–1261. doi:10.1001/archinte.166.12.1256. PMID 16801507.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007). "Low bone mineral density and fracture burden in postmenopausal women". CMAJ. 177 (6): 575–80. doi:10.1503/cmaj.070234. PMC 1963365. PMID 17846439.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hannan EL, Magaziner J, Wang JJ; et al. (2001). "Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes". JAMA. 285 (21): 2736–42. doi:10.1001/jama.285.21.2736. PMID 11386929.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES (2006). "Impact of recent fracture on health-related quality of life in postmenopausal women". J. Bone Miner. Res. 21 (6): 809–16. doi:10.1359/jbmr.060301. PMID 16753011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002.
- ^ a b Riggs, B.L.; Melton, Lj 3.r.d. (2005). "The worldwide problem of osteoporosis: insights afforded by epidemiology". Bone. 17 (5 Suppl): 505S–511S. doi:10.1016/8756-3282(95)00258-4. PMID 8573428.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ a b "MerckMedicus Modules: Osteoporosis - Epidemiology". Merck & Co., Inc. Archived from the original on 2007-12-28. Retrieved 2008-06-13.
- ^ Cauley JA, Hochberg MC, Lui LY; et al. (2007). "Long-term Risk of Incident Vertebral Fractures". JAMA. 298 (23): 2761–67. doi:10.1001/jama.298.23.2761. PMID 18165669.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Lobstein JGCFM. Lehrbuch der pathologischen Anatomie. Stuttgart: Bd II, 1835.
- ^ Albright F, Bloomberg E, Smith PH (1940). "Postmenopausal osteoporosis". Trans. Assoc. Am. Physicians. 55: 298–305.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Patlak M (2001). "Bone builders: the discoveries behind preventing and treating osteoporosis". FASEB J. 15 (10): 1677E–E. doi:10.1096/fj.15.10.1677e. PMID 11481214.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
