Ovalbumin
| Ovalbumin | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | ? | ||||||
| UniProt | P01012 | ||||||
| |||||||
Ovalbumin (abbreviated OVA[1]) is the main protein found in egg white, making up approximately 55% of the total protein.[2] Ovalbumin displays sequence and three-dimensional homology to the serpin superfamily, but unlike most serpins it is not a serine protease inhibitor.[3] The function of ovalbumin is unknown, although it is presumed to be a storage protein.[4]
Research
[edit]Ovalbumin is an important protein in several different areas of research, including:
- general studies of protein structure and properties (because it is available in large quantities).
- studies of serpin structure and function (the fact that ovalbumin does not inhibit proteases means that by comparing its structure with that of inhibitory serpins, the structural characteristics required for inhibition can be determined).
- proteomics (chicken egg ovalbumin is commonly used as a molecular weight marker for calibrating electrophoresis gels).
- immunology (commonly used to stimulate an allergic reaction in test subjects; e.g., established model allergen for airway hyper-responsiveness, AHR).
(For in vivo and in vitro studies based on ovalbumin it is important that the endotoxin content is less than 1 EU/mg.)[citation needed][needs context]
Structure
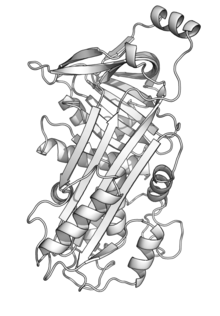
[edit]The ovalbumin protein of chickens consists of 385 amino acids, its relative molecular mass is 42.7 kDa,[5] and it adopts a serpin-like structure.[6] Ovalbumin also has several modifications, including N-terminal acetylation (G1), phosphorylation (S68, S344), and glycosylation (N292).[5] It has three isoforms, A1, A2, and A3, which vary based on the number of bound phosphate residues.[7] It is secreted from the cell, targeted by an internal signal sequence (residues 21–47), rather than the N-terminal signal sequence commonly found in other secreted proteins. Ovalbumin's signal sequence is not cleaved off, but remains as part of the mature protein.[8]
Change upon heating
[edit]When heated, ovalbumin undergoes a conformational change from its soluble, serpin structure into an insoluble all-β-sheet structure with exposed hydrophobic regions. This causes the protein to aggregate and cause the solidification associated with cooked egg white.[9]
See also
[edit]References
[edit]- ^ Sano K, Haneda K, Tamura G, Shirato K (June 1999). "Ovalbumin (OVA) and Mycobacterium tuberculosis bacilli cooperatively polarize anti-OVA T-helper (Th) cells toward a Th1-dominant phenotype and ameliorate murine tracheal eosinophilia". American Journal of Respiratory Cell and Molecular Biology. 20 (6): 1260–7. doi:10.1165/ajrcmb.20.6.3546. PMID 10340945. S2CID 22811888.
- ^ Sugino H, Nitoda T, Juneja LR (1996-12-13). "Chapter 2: General Chemical Composition of Hen Eggs". In Yamamoto T, Juneja LR, Hatta H, Kim M (eds.). Hen eggs. Boca Raton, FL: CRC Press. ISBN 978-0-8493-4005-5.
- ^ Hu HY, Du HN (April 2000). "Alpha-to-beta structural transformation of ovalbumin: heat and pH effects". Journal of Protein Chemistry. 19 (3): 177–83. doi:10.1023/A:1007099502179. PMID 10981809. S2CID 82745511.
- ^ Gettins PG (December 2002). "Serpin structure, mechanism, and function". Chemical Reviews. 102 (12): 4751–804. doi:10.1021/cr010170. PMID 12475206.
- ^ a b Nisbet AD, Saundry RH, Moir AJ, Fothergill LA, Fothergill JE (April 1981). "The complete amino-acid sequence of hen ovalbumin". European Journal of Biochemistry. 115 (2): 335–45. doi:10.1111/j.1432-1033.1981.tb05243.x. PMID 7016535.
- ^ Stein PE, Leslie AG, Finch JT, Carrell RW (October 1991). "Crystal structure of uncleaved ovalbumin at 1.95 A resolution". Journal of Molecular Biology. 221 (3): 941–59. doi:10.1016/0022-2836(91)80185-W. PMID 1942038.
- ^ Sugimoto, Yasushi (April 1999). "Ovalbumin in Developing Chicken Eggs Migrates from Egg White to Embryonic Organs while Changing Its Conformation and Thermal Stability*". Journal of Biological Chemistry. 274 (16).
- ^ Robinson A, Meredith C, Austen BM (July 1986). "Isolation and properties of the signal region from ovalbumin". FEBS Letters. 203 (2): 243–6. doi:10.1016/0014-5793(86)80751-7. PMID 3732511. S2CID 10064866.
- ^ Hu HY, Du HN (April 2000). "Alpha-to-beta structural transformation of ovalbumin: heat and pH effects". Journal of Protein Chemistry. 19 (3): 177–83. doi:10.1023/A:1007099502179. PMID 10981809. S2CID 82745511.
External links
[edit]- Ovalbumin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
