Transparency and translucency: Difference between revisions
m clean up, Replaced: → (4), | → |, using AWB |
|||
| Line 7: | Line 7: | ||
Some materials allow much of the light that falls on them to be transmitted through without being [[reflected]] or [[refracted]]; such materials are called optically transparent. Chemically pure window [[glass]] and clean [[water]] are examples of this. Most liquids and aqueous solutions are highly transparent. Absence of structural defects (voids, cracks, etc.) and molecular structure of most liquids are mostly responsible for excellent optical transmission. |
Some materials allow much of the light that falls on them to be transmitted through without being [[reflected]] or [[refracted]]; such materials are called optically transparent. Chemically pure window [[glass]] and clean [[water]] are examples of this. Most liquids and aqueous solutions are highly transparent. Absence of structural defects (voids, cracks, etc.) and molecular structure of most liquids are mostly responsible for excellent optical transmission. |
||
Materials which do not allow the transmission of light are called [[opaque]]. Many such substances have a [[chemical composition]] which includes what are referred to as [[absorption]] centers. Many substances are selective in their absorption of [[white light]] [[frequencies]]. They absorb certain portions of the [[visible spectrum]], while reflecting others. The frequencies of the spectrum which are not absorbed are either reflected back or transmitted for our physical observation. This is what gives rise to [[color]]. The attenuation of light of all frequencies and wavelengths is due to the combined mechanisms of [[absorption]] and [[ |
Materials which do not allow the transmission of light are called [[opaque]]. Many such substances have a [[chemical composition]] which includes what are referred to as [[absorption]] centers. Many substances are selective in their absorption of [[white light]] [[frequencies]]. They absorb certain portions of the [[visible spectrum]], while reflecting others. The frequencies of the spectrum which are not absorbed are either reflected back or transmitted for our physical observation. This is what gives rise to [[color]]. The attenuation of light of all frequencies and wavelengths is due to the combined mechanisms of [[absorption]] and [[Light scattering in liquids and solids|scattering]]. |
||
<ref>{{cite book|author=Fox, M.|title=Optical Properties of Solids|publisher=Oxford University Press, USA|year= 2002}}</ref> |
<ref>{{cite book|author=Fox, M.|title=Optical Properties of Solids|publisher=Oxford University Press, USA|year= 2002}}</ref> |
||
[[Image:Opacity Translucency Transparency.svg|thumb|200px|right|Comparisons of 1. [[opacity (optics)|opacity]], 2. translucency, and 3. transparency; behind each panel is a star]] |
[[Image:Opacity Translucency Transparency.svg|thumb|200px|right|Comparisons of 1. [[opacity (optics)|opacity]], 2. translucency, and 3. transparency; behind each panel is a star]] |
||
==Introduction== |
==Introduction== |
||
| Line 22: | Line 22: | ||
* Crystalline structure: How [[close-packed]] its atoms or molecules are, and whether or not the atoms or molecules exhibit the ''long-range order'' evidenced in crystalline solids. |
* Crystalline structure: How [[close-packed]] its atoms or molecules are, and whether or not the atoms or molecules exhibit the ''long-range order'' evidenced in crystalline solids. |
||
* Glassy structure: [[ |
* Glassy structure: [[Light scattering in liquids and solids|Scattering]] centers include fluctuations in density and/or composition. |
||
* Microstructure: [[ |
* Microstructure: [[Light scattering in liquids and solids|Scattering]] centers include internal surfaces such as grains, grain boundaries, and microscopic pores. |
||
''In [[ |
''In [[Light scattering in liquids and solids|light scattering]], the most critical factor is the length scale of any or all of these structural features relative to the wavelength of the light being scattered.'' |
||
==Nature of light== |
==Nature of light== |
||
| Line 37: | Line 37: | ||
<ref>{{cite book|author=Giancoli, D.C.|title=Physics for Scientists and Engineers|publisher=Prentice Hall|year=1988}}</ref> |
<ref>{{cite book|author=Giancoli, D.C.|title=Physics for Scientists and Engineers|publisher=Prentice Hall|year=1988}}</ref> |
||
The simplest representation of a beam of light is through the use of the [[light ray]]. The most important properties of the light ray are that it contains no [[mass]] and that it travels along a straight line. Light rays interact with the materials ([[liquids]] and [[solids]]) in several different ways; it is [[absorbed]], [[reflected]] or [[transmitted]] by the object. In the case of reflection, the interaction depends on the [[physical]] and [[chemical]] [[properties]] of the substance. If the materials surface is perfectly smooth (e.g. a |
The simplest representation of a beam of light is through the use of the [[light ray]]. The most important properties of the light ray are that it contains no [[mass]] and that it travels along a straight line. Light rays interact with the materials ([[liquids]] and [[solids]]) in several different ways; it is [[absorbed]], [[reflected]] or [[transmitted]] by the object. In the case of reflection, the interaction depends on the [[physical]] and [[chemical]] [[properties]] of the substance. If the materials surface is perfectly smooth (e.g. a [[mirror]]), rays of light collectively undergo total reflection (or [[specular reflection]]), leaving the surface of the glass at a particular [[angle]] and all in a [[parallel line]] with each other. |
||
==Light scattering== |
==Light scattering== |
||
| Line 43: | Line 43: | ||
[[Image:Reflection angles.svg|thumb|left| Specular reflection]] |
[[Image:Reflection angles.svg|thumb|left| Specular reflection]] |
||
[[Image:Diffuse reflection.PNG|thumb|right|300px|Diffuse reflection]] |
[[Image:Diffuse reflection.PNG|thumb|right|300px|Diffuse reflection]] |
||
Rough and irregular surfaces cause light rays to be reflected in many random directions. This type of reflection is called “[[diffuse reflection]]”, and is typically characterized by wide variety of reflection angles. Most of the objects visible to the naked eye are identified via diffuse reflection. Another term commonly used for this type of reflection is “light scattering”. ''[[ |
Rough and irregular surfaces cause light rays to be reflected in many random directions. This type of reflection is called “[[diffuse reflection]]”, and is typically characterized by wide variety of reflection angles. Most of the objects visible to the naked eye are identified via diffuse reflection. Another term commonly used for this type of reflection is “light scattering”. ''[[Light scattering in liquids and solids|Light scattering]] from the surfaces of objects is our primary mechanism of physical observation.'' |
||
<ref name="z">{{cite book|author=Kerker, M.|title=The Scattering of Light|publisher=Academic, New York|year=1969}}</ref><ref name="y">{{cite journal |
<ref name="z">{{cite book|author=Kerker, M.|title=The Scattering of Light|publisher=Academic, New York|year=1969}}</ref><ref name="y">{{cite journal |
||
|author=Mandelstam, L.I. |
|author=Mandelstam, L.I. |
||
| Line 58: | Line 58: | ||
==Absorption of light in solids== |
==Absorption of light in solids== |
||
{| class="wikitable" width=400 align="right" style="text-align: center; margin: 0.5em auto; width: auto; margin-left:1em;" |
{| class="wikitable" width=400 align="right" style="text-align: center; margin: 0.5em auto; width: auto; margin-left:1em;" |
||
! colspan="3" style="background:#FFF;" |
! colspan="3" style="background:#FFF;"|[[Image:Linear visible spectrum.svg|center|200px|sRGB rendering of the spectrum of visible light]] |
||
|- |
|- |
||
![[Color]] |
![[Color]] |
||
| Line 188: | Line 188: | ||
Optical transparency in polycrystalline materials is limited by the amount of light which is scattered by their microstructural features. Light scattering depends on the wavelength of the light. Limits to spatial scales of visibility (using white light) therefore arise, depending on the frequency of the light wave and the physical dimension of the scattering center. For example, since visible light has a wavelength scale on the order of a micrometer, scattering centers will have dimensions on a similar spatial scale. Primary scattering centers in polycrystalline materials include microstructural defects such as pores and grain boundaries. The volume fraction of microscopic pores has to be less than 1% for high-quality optical transmission, that is the material density should be 99.99% of the theoretical crystalline density. In addition to pores, most of the interfaces in a typical metal or ceramic object are in the form of [[grain boundary|grain boundaries]] which separate tiny regions of crystalline order. When the size of the scattering center (or grain boundary) is reduced below the size of the wavelength of the light being scattered, the scattering no longer occurs to any significant extent. |
Optical transparency in polycrystalline materials is limited by the amount of light which is scattered by their microstructural features. Light scattering depends on the wavelength of the light. Limits to spatial scales of visibility (using white light) therefore arise, depending on the frequency of the light wave and the physical dimension of the scattering center. For example, since visible light has a wavelength scale on the order of a micrometer, scattering centers will have dimensions on a similar spatial scale. Primary scattering centers in polycrystalline materials include microstructural defects such as pores and grain boundaries. The volume fraction of microscopic pores has to be less than 1% for high-quality optical transmission, that is the material density should be 99.99% of the theoretical crystalline density. In addition to pores, most of the interfaces in a typical metal or ceramic object are in the form of [[grain boundary|grain boundaries]] which separate tiny regions of crystalline order. When the size of the scattering center (or grain boundary) is reduced below the size of the wavelength of the light being scattered, the scattering no longer occurs to any significant extent. |
||
In the formation of polycrystalline materials (metals and ceramics) the size of the crystalline grains is determined largely by the size of the crystalline particles present in the raw material during formation (or pressing) of the object. Moreover, the size of the grain boundaries scales directly with particle size. Thus a reduction of the original particle size well below the wavelength of visible light (about 1/15 of the light wavelength or roughly 600/15 = 40 |
In the formation of polycrystalline materials (metals and ceramics) the size of the crystalline grains is determined largely by the size of the crystalline particles present in the raw material during formation (or pressing) of the object. Moreover, the size of the grain boundaries scales directly with particle size. Thus a reduction of the original particle size well below the wavelength of visible light (about 1/15 of the light wavelength or roughly 600/15 = 40 nm) eliminates much of light scattering, resulting in a translucent or even transparent material. |
||
Computer modeling of light transmission through translucent ceramic alumina has shown that microscopic pores trapped near grain boundaries cause act as primary scattering centers. The volume fraction of porosity had to be reduced below 1% for high-quality optical transmission (99.99 percent of theoretical density). This goal has been readily accomplished and amply demonstrated in laboratories and research facilities worldwide using the emerging chemical processing methods encompassed by the methods of [[sol-gel]] chemistry and [[nanotechnology]]. |
Computer modeling of light transmission through translucent ceramic alumina has shown that microscopic pores trapped near grain boundaries cause act as primary scattering centers. The volume fraction of porosity had to be reduced below 1% for high-quality optical transmission (99.99 percent of theoretical density). This goal has been readily accomplished and amply demonstrated in laboratories and research facilities worldwide using the emerging chemical processing methods encompassed by the methods of [[sol-gel]] chemistry and [[nanotechnology]]. |
||
Revision as of 07:19, 11 August 2009

In the field of optics, transparency is the physical property of allowing light to pass through a material. The opposite property is opacity. Transparent materials are clear (i.e. they can be seen through). Translucent materials allow light to pass through them only diffusely (i.e. they cannot be seen through clearly).
Thus, when light encounters a material, it can interact with it in several different ways. These interactions depend on the nature of the light (its wavelength, frequency, energy, etc.) and the nature of the material. Light waves interact with an object primarily by either reflection or transmission. A secondary response is refraction.
Some materials allow much of the light that falls on them to be transmitted through without being reflected or refracted; such materials are called optically transparent. Chemically pure window glass and clean water are examples of this. Most liquids and aqueous solutions are highly transparent. Absence of structural defects (voids, cracks, etc.) and molecular structure of most liquids are mostly responsible for excellent optical transmission.
Materials which do not allow the transmission of light are called opaque. Many such substances have a chemical composition which includes what are referred to as absorption centers. Many substances are selective in their absorption of white light frequencies. They absorb certain portions of the visible spectrum, while reflecting others. The frequencies of the spectrum which are not absorbed are either reflected back or transmitted for our physical observation. This is what gives rise to color. The attenuation of light of all frequencies and wavelengths is due to the combined mechanisms of absorption and scattering. [1]

Introduction
With regards to the absorption of light, primary material considerations include:
- At the electronic level, absorption in the ultraviolet and visible (UV-Vis) portions of the spectrum depends on whether the electron orbitals are spaced (or "quantized") such that they can absorb a quantum of light (or photon) of a specific frequency. For example, in most glasses, electrons have no available energy levels above them in range of that associated with visible light. Thus, there is no absorption in pure (undoped) glasses, making them ideal transparent materials for windows in buildings.
- At the atomic or molecular level, physical absorption in the infrared portion of the spectrum depends on the frequencies of atomic or molecular vibrations or chemical bonds.
With regards to the scattering of light, primary material considerations include:
- Crystalline structure: How close-packed its atoms or molecules are, and whether or not the atoms or molecules exhibit the long-range order evidenced in crystalline solids.
- Glassy structure: Scattering centers include fluctuations in density and/or composition.
- Microstructure: Scattering centers include internal surfaces such as grains, grain boundaries, and microscopic pores.
In light scattering, the most critical factor is the length scale of any or all of these structural features relative to the wavelength of the light being scattered.
Nature of light


Radiant energy is energy which is propagated in the form of Electromagnetic waves. The type of light which we perceive through our optical sensors (eyes) is referred to as white light, and it is composed of a range of colors (ROYGB: red, orange, yellow, green, blue) over a range of wavelengths, or frequencies. Visible (white) light is only a small fraction of the entire spectrum of electromagnetic radiation. At the short end of that wavelength scale is invisible ultraviolet (UV) light. At even shorter wavelengths than UV are X-rays and gamma-rays. At the longer end of that spectrum is infrared (IR) light, which is used for night vision and other heat-seeking devices. At longer wavelengths than infrared are microwaves (radar), and radio / television waves.
Electromagnetic radiation is classified according to the frequency (or wavelength, which is inversely proportional to the frequency) of the light. This includes (in order of increasing frequency): radio waves, microwaves, terahertz radiation, infrared radiation, visible light, ultraviolet (UV) radiation, X-rays and gamma rays. Of these, radio waves have the longest wavelengths and gamma rays have the shortest. A small window of frequencies, called the visible (or white light) portion of the spectrum, is sensed by the naked eye of various organisms. [2]
The simplest representation of a beam of light is through the use of the light ray. The most important properties of the light ray are that it contains no mass and that it travels along a straight line. Light rays interact with the materials (liquids and solids) in several different ways; it is absorbed, reflected or transmitted by the object. In the case of reflection, the interaction depends on the physical and chemical properties of the substance. If the materials surface is perfectly smooth (e.g. a mirror), rays of light collectively undergo total reflection (or specular reflection), leaving the surface of the glass at a particular angle and all in a parallel line with each other.
Light scattering
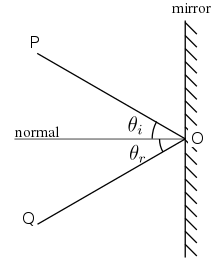
Rough and irregular surfaces cause light rays to be reflected in many random directions. This type of reflection is called “diffuse reflection”, and is typically characterized by wide variety of reflection angles. Most of the objects visible to the naked eye are identified via diffuse reflection. Another term commonly used for this type of reflection is “light scattering”. Light scattering from the surfaces of objects is our primary mechanism of physical observation. [3][4]
Light scattering in liquids and solids therefore depends on the wavelength of the light being scattered. Limits to spatial scales of visibility (using white light) therefore arise, depending on the frequency of the light wave and the physical dimension (or spatial scale) of the scattering center. For example, since visible light has a wavelength scale on the order of a micrometer (one millionth of a meter) scattering centers (or particles) as small as one micrometer have been observed directly in the light microscope (e.g. Brownian motion). [5] [6]
Absorption of light in solids
| Color | Wavelength | Frequency |
|---|---|---|
| violet | 380–450 nm | 668–789 THz |
| blue | 450–495 nm | 606–668 THz |
| green | 495–570 nm | 526–606 THz |
| yellow | 570–590 nm | 508–526 THz |
| orange | 590–620 nm | 484–508 THz |
| red | 620–750 nm | 400–484 THz |
When light strikes an object, it usually has not just a single frequency (or wavelength) but many. Objects have a tendency to selectively absorb, reflect or transmit light of certain frequencies. That is, one object might reflect green light while absorbing all other frequencies of visible light. Another object might selectively transmit blue light while absorbing all other frequencies of visible light. The manner in which visible light interacts with an object is dependent upon the frequency of the light, the nature of the atoms in the object, and often the nature of the electrons in the atoms of the object.
Some materials allow much of the light that falls on them to be transmitted through the material without being reflected. Materials that allow the transmission of light waves through them all called optically transparent. Chemically pure (undoped) window glass and clean river or spring water are prime examples of this.
Materials which do not allow the transmission of any light wave frequencies are called opaque. Such substances have a chemical composition which includes what are referred to as absorption centers. Most materials are composed of materials which are selective in their absorption of white light frequencies. Thus they absorb certain portions of the visible spectrum, while reflecting others. The frequencies of the spectrum which are not absorbed are either reflected back or transmitted for our physical observation. In the visible portion of the spectrum, this is what gives rise to color.[7][8]
Color centers are largely responsible for the appearance of specific wavelengths of visible light all around us. Moving from longer (0.7 micrometer) to shorter (0.4 micrometer) wavelengths: red, orange, yellow, green and blue (ROYGB) can all be identified by our senses in the appearance of color by the selective absorption of specific light wave frequencies (or wavelengths). Mechanisms of selective light wave absorption include:
- Electronic: Transitions in electron energy levels within the atom (e.g. pigments). These transitions are typically in the ultraviolet (UV) and/or visible portions of the spectrum.
- Vibrational: Resonance in atomic/molecular vibrational modes. These transitions are typically in the infrared portion of the spectrum.
UV-Vis: Electronic transitions
In electronic absorption, the frequency of the incoming light wave is at or near the energy levels of the electrons within the atoms which compose the substance. In this case, the electrons will absorb the energy of the light wave and increase their energy state, often moving outward from the nucleus of the atom into an outer shell or orbital.
The atoms that bind together to make the molecules of any particular substance contain a number of electrons (given by the atomic number Z in the periodic chart). Recall that all light waves are electromagnetic in origin. Thus they are affected strongly when coming into contact with negatively charged electrons in matter. When photons (individual packets of light energy) come in contact with the valence electrons of atom, one of several things can and will occur:
- An electron absorbs all of the energy of the photon and re-emits it with different color. This gives rise to luminescence, fluorescence and phosphorescence.
- An electron absorbs the energy of the photon and sends it back out the way it came in. This results in reflection or scattering.
- An electron cannot absorb the energy of the photon and the photon continues on its path. This results in transmission (provided no other absorption mechanisms are active).
- An electron selectively absorbs a portion of the photon, and the remaining frequencies are transmitted in the form of spectral color.
Most of the time, it is a combination of the above that happens to the light that hits an object. The electrons in different materials vary in the range of energy that they can absorb. Most glasses, for example, blocks ultraviolet (UV) light. What happens is the electrons in the glass absorb the energy of the photons in the UV range while ignoring the weaker energy of photons in the visible light spectrum.
Thus, when a material is illuminated, individual photons of light can make the valence electrons of an atom transition to a higher electronic energy level. The photon is destroyed in the process and the absorbed radiant energy is transformed to electric potential energy. Several things can happen then to the absorbed energy. as it may be re-emitted by the electron as radiant energy (in this case the overall effect is in fact a scattering of light), dissipated to the rest of the material (i.e. transformed into heat), or the electron can be freed from the atom (as in the photoelectric and Compton effects).
Infrared: Bond stretching
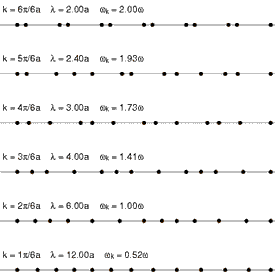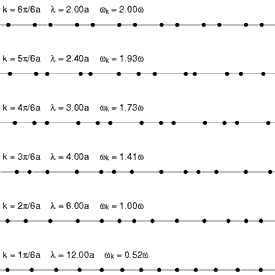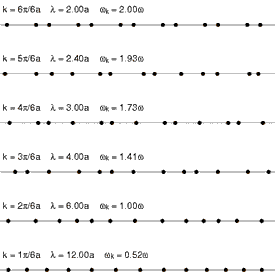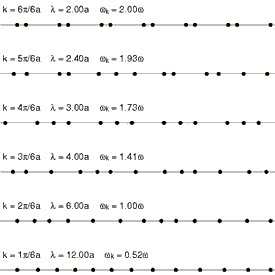
The primary physical mechanism for storing mechanical energy of motion in condensed matter is through heat, or thermal energy. Thermal energy manifests itself as energy of motion. Thus, heat is motion at the atomic and molecular levels. The primary mode of motion in crystalline substances is vibration. Any given atom will vibrate around some mean or average position within a crystalline structure, surrounded by its nearest neighbors. This vibration in 2-dimensions is equivalent to the oscillation of a clock’s pendulum. It swings back and forth symmetrically about some mean or average (vertical) position. Atomic and molecular vibrational frequencies may average on the order of 1012 cycles per second (Hertz).
When a light wave of a given frequency strikes a material with particles having the same or (resonant) vibrational frequencies, then those particles will absorb the energy of the light wave and transform it into thermal energy of vibrational motion. Since different atoms and molecules have different natural frequencies of vibration, they will selectively absorb different frequencies (or portions of the spectrum) of infrared light. Reflection and transmission of light waves occur because the frequencies of the light waves do not match the natural resonant frequencies of vibration of the objects. When infrared light of these frequencies strikes an object, the energy is reflected or transmitted.
If the object is transparent, then the light waves are passed on to neighboring atoms through the bulk of the material and re-emitted on the opposite side of the object. Such frequencies of light waves are said to be transmitted.[9] [10]
Transparency in insulators
An object may be not transparent either because it reflects the incoming light or because it absorbs the incoming light. Almost all solids reflect a part and absorb a part of the incoming light.
When light falls onto a block of metal, it encounters atoms that are tightly packed in a regular lattice and a "sea of electrons" moving randomly between the atoms. [11] In metals, most of these are non-bonding electrons (or free electrons) as opposed to the bonding electrons typically found in covalently bonded or ionically bonded non-metallic (insulating) solids. As a result of these electrons, most of the incoming light in metals is reflected back, which is why we see a shiny metal surface.
Thus, most insulators (or dielectric materials) are held together by ionic bonds. This class of materials includes all ceramics and glasses. These materials do not have free conduction electrons, and the bonding electrons reflect only a small fraction of the incident wave. The remaining frequencies (or wavelengths) are free to propagate (or be transmitted).
A dielectric also reflects a part of the incoming light. If it does not contain light-absorbent molecules (pigments, dyes, colorants), it is usually transparent to the spectrum of visible light. Color centers (or dye molecules, or "dopants") in a dielectric absorb a portion of the incoming light wave. The remaining frequencies (or wavelengths) are free to be reflected or transmitted. This is how colored glass is produced.
Most liquids and aqueous solutions are highly transparent. For example, water, cooking oil, rubbing alcohol, air, natural gas, are all clear. Absence of structural defects (voids, cracks, etc.) and molecular structure of most liquids are chiefly responsible for their excellent optical transmission. The ability of liquids to "heal" internal defects via viscous flow is one of the reasons why some fibrous materials (e.g. paper or fabric) increase their apparent transparency when wetted. The liquid fills up numerous voids making the material more structurally homogeneous.
Optical waveguides


Optically transparent materials focus on the response of a material to incoming light waves of a range of wavelengths. Guided light wave transmission via frequency selective waveguides involves the emerging field of fiber optics and the ability of certain glassy compositions as a transmission medium for a range of frequencies simultaneously (multimode optical fiber) with little or no interference between competing wavelengths or frequencies. This resonant mode of energy and data transmission via electromagnetic (light) wave propagation is relatively lossless.
An optical fiber is a cylindrical dielectric waveguide that transmits light along its axis by the process of total internal reflection. The fiber consists of a core surrounded by a cladding layer. To confine the optical signal in the core, the refractive index of the core must be greater than that of the cladding. The refractive index is the parameter reflecting the speed of light in a material. (Refractive index is the ratio of the speed of light in a vacuum to the speed of light in a given medium. The refractive index of a vacuum is therefore 1). The larger the refractive index, the more slowly light travels in that medium. Typical values for core and cladding of an optical fiber are 1.48 and 1.46, respectively.
When light traveling in a dense medium hits a boundary at a steep angle, the light will be completely reflected. This effect, called total internal reflection, is used in optical fibers to confine light in the core. Light travels along the fiber bouncing back and forth off of the boundary. Because the light must strike the boundary with an angle greater than the critical angle, only light that enters the fiber within a certain range of angles will be propagated. This range of angles is called the acceptance cone of the fiber. The size of this acceptance cone is a function of the refractive index difference between the fiber's core and cladding. Optical waveguides are used as components in integrated optical circuits (e.g. combined with lasers or light-emitting diodes, LEDs) or as the transmission medium in local and long haul optical communication systems.
Mechanisms of attenuation

Attenuation in fiber optics, also known as transmission loss, is the reduction in intensity of the light beam (or signal) with respect to distance traveled through a transmission medium. Attenuation coefficients in fiber optics usually use units of dB/km through the medium due to the relatively high quality of transparency of modern optical transmission media. The medium is usually a fiber of silica glass that confines the incident light beam to the inside. Attenuation is an important factor limiting the transmission of a signal across large distances. Thus, much research has gone into both limiting the attenuation and maximizing the amplification of the optical signal.
Attenuation is caused primarily by scattering and absorption. The scattering of light is caused by molecular level irregularities (compositional fluctuations) in the glass structure. This same phenomenon is seen as one of the limiting factors in the transparency of infrared missile domes. Further attenuation is caused by light absorbed by residual materials, such as metals or water ions, within the fiber core and inner cladding. In optical fiber, light leakage due to bending, splices, connectors, or other outside forces are other factors resulting in attenuation. [12][13]
Multi-phonon absorption
The design of any optically transparent device requires the selection of materials based upon knowledge of their properties and limitations. The lattice absorption characteristics observed at the lower frequency regions (mid-infrared to far-infrared wavelength range) define the long-wavelength transparency limit of the material. They are the result of the interactive coupling between the motions of thermally induced vibrations of the constituent atoms and molecules of the solid lattice and the incident light wave radiation. Hence, all materials are bounded by limiting regions of absorption caused by atomic and molecular vibrations (bond-stretching)in the far-infrared spectral region (>10 µm).
The concepts of temperature and thermal equilibrium associated with ionic solids are based on individual atoms and molecules in the system possessing vibrational motion. The frequencies of the normal modes of a system are known as its natural frequencies or resonant frequencies. These thermal vibrational modes are associated with atomic and molecular displacements, producing both longitudinal and transverse waves of atomic and molecular displacement.
In the longitudinal (or acoustic) mode, the displacement of particles from their positions of equilibrium coincides with the propagation direction of the wave. Mechanical longitudinal waves have been also referred to as compression waves. For transverse (or optical) modes, individual particles move perpendicular to the propagation of the wave.
As the rules of quantum mechanics apply to all the different vibrational modes in the solid, the lattice pulsates as a complete assembly in discrete energy steps, or thermal phonons. A phonon is a quantized mode of vibration occurring in a rigid crystal lattice. The study of phonons is an important part of solid state physics, because phonons play a major role in many of the physical properties of solids, including a material's thermal and electrical conductivity.
The phonon is related to both the frequency of vibration and the temperature. If the temperature is raised, the amplitude of vibration increases. The concept of the phonon is therefore considered as the quantum of lattice vibrational energy onto which is superimposed a complex pattern of standing and/or traveling waves that represent changes in temperature. If the solid is at a uniform temperature, the standing wave concept is adequate as the phonon vibrations are uniformly distributed.
Multi-phonon absorption occurs when two or more phonons simultaneously interact to produce electric dipole moments with which the incident radiation may couple. These dipoles can absorb energy from the incident radiation, reaching a maximum coupling with the radiation when the frequency is equal to the vibrational mode of the molecular dipole (e.g. Si-O bond in quartz) in the far-infrared spectral region.
All of the resonant absorption processes involved in an optically transparent material can be explained by the same common principle. At particular frequencies, the incident radiation is allowed to propagate through the lattice producing the observed transparency. Other frequencies however, are forbidden when the incident radiation is at resonance with any of the properties of the lattice material (e.g. molecular vibrational frequencies), and as such are transferred as thermal energy, exciting the atoms or electrons.
In order that a mode of vibration can absorb, a mechanism for coupling the vibrational motion to the electromagnetic radiation must exist. Transfer of electromagnetic radiation from the incident medium to the material is in the form of a couple, where the lattice vibration produces an oscillating dipole moment which can be driven by the oscillating electric field of the light wave, or radiation. Thus, the energy absorbed from the light wave will be converted into vibrational motion of the molecules.
Light scattering in ceramics
Optical transparency in polycrystalline materials is limited by the amount of light which is scattered by their microstructural features. Light scattering depends on the wavelength of the light. Limits to spatial scales of visibility (using white light) therefore arise, depending on the frequency of the light wave and the physical dimension of the scattering center. For example, since visible light has a wavelength scale on the order of a micrometer, scattering centers will have dimensions on a similar spatial scale. Primary scattering centers in polycrystalline materials include microstructural defects such as pores and grain boundaries. The volume fraction of microscopic pores has to be less than 1% for high-quality optical transmission, that is the material density should be 99.99% of the theoretical crystalline density. In addition to pores, most of the interfaces in a typical metal or ceramic object are in the form of grain boundaries which separate tiny regions of crystalline order. When the size of the scattering center (or grain boundary) is reduced below the size of the wavelength of the light being scattered, the scattering no longer occurs to any significant extent.
In the formation of polycrystalline materials (metals and ceramics) the size of the crystalline grains is determined largely by the size of the crystalline particles present in the raw material during formation (or pressing) of the object. Moreover, the size of the grain boundaries scales directly with particle size. Thus a reduction of the original particle size well below the wavelength of visible light (about 1/15 of the light wavelength or roughly 600/15 = 40 nm) eliminates much of light scattering, resulting in a translucent or even transparent material.
Computer modeling of light transmission through translucent ceramic alumina has shown that microscopic pores trapped near grain boundaries cause act as primary scattering centers. The volume fraction of porosity had to be reduced below 1% for high-quality optical transmission (99.99 percent of theoretical density). This goal has been readily accomplished and amply demonstrated in laboratories and research facilities worldwide using the emerging chemical processing methods encompassed by the methods of sol-gel chemistry and nanotechnology. [14] [15] [16] [17] [18]
Applications
Transparent ceramics have recently acquired a high degree of interest and notoriety, the basic applications being high energy lasers, transparent armor windows, nose cones for heat seeking missiles, radiation detectors for non-destructive testing, high energy physics, space exploration, security and medical imaging applications.
The development of transparent panel products will have other potential advanced applications including high strength, impact-resistant materials that can be used for domestic windows and skylights. Perhaps more important is that walls and other applications will have improved overall strength, especially for high-shear conditions found in high seismic and wind exposures. If the expected improvements in mechanical properties bear out, the traditional limits seen on glazing areas in today's building codes could quickly become outdated if the window area actually contributes to the shear resistance of the wall.
Currently available infrared transparent materials typically exhibit a trade-off between optical performance, mechanical strength and price. For example, sapphire (crystalline alumina) is very strong, but it is expensive and lacks full transparency throughout the 3-5 micrometer mid-infrared range. Yttria is fully transparent from 3-5 micrometers, but lacks sufficient strength, hardness, and thermal shock resistance for high-performance aerospace applications. Not surprisingly, a combination of these two materials in the form of the yttrium aluminium garnet (YAG) is one of the top performers in the field
See also
References
- ^ Fox, M. (2002). Optical Properties of Solids. Oxford University Press, USA.
- ^ Giancoli, D.C. (1988). Physics for Scientists and Engineers. Prentice Hall.
- ^ Kerker, M. (1969). The Scattering of Light. Academic, New York.
- ^ Mandelstam, L.I. (1926). "Light Scattering by Inhomogeneous Media". Zh. Russ. Fiz-Khim. Ova. 58: 381.
- ^ van de Hulst, H.C. (1981). Light scattering by small particles. New York: Dover. ISBN 0486642283.
- ^ Bohren, C.F. and Huffmann, D.R. (1983). Absorption and scattering of light by small particles. New York: Wiley-Interscience.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Simmons, J. and Potter, K.S. (2000). "Optical Materials (Academic Press, NY)". J. Chem. Phys.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Uhlmann, D.R.; et al. (1991). Optical Properties of Glass. Amer. Ceram. Soc.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Gunzler, H. and Gremlich, H. (2002). IR Spectroscopy: An Introduction. Wiley-VCH, Verlag, Germany.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Stuart, B. (2004). Infrared Spectroscopy: Fundamentals and Applications. John Wiley & Sons, Ltd.
- ^ Mott, N.F. and Jones, H. Theory of the Properties of Metals and Alloys. Clarendon Press, Oxford (1936) Dover Publications (1958).
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Smith, R.G. (1972). "Optical power handling capacity of low loss optical fibers as determined by stimulated Raman and Brillouin scattering". Appl. Opt. 11: 2489. doi:10.1364/AO.11.002489.
- ^ Archibald, P.S. and Bennett, H.E. (1978). "Scattering from infrared missile domes". Opt. Eng. 17: 647.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yoldas, B.E., Monolithic glass formation by chemical polymerization, J. Mat. Sci., Vol.14, p.1843 (1979)
- ^ Prochazka,, S. and Klug, S.J., Infrared-Transparent Mullite Ceramic, J. Am. Ceram. Soc., Vol.66, p.874 (1983)
- ^ Ikesue, A., et al., Fabrication and Optical Properties of High Performance Polycrystalline Ceramics of Solid State Lasers, J. Am. Ceram. Soc, Vol. 78, p. 1033 (1995), Polycrystalline Lasers, Optical Materials, Vol. 19, p.183 (2002)
- ^ Barnakov, Y.A., et al., The Progress Towards Transparent Ceramics Fabrication, Proc. SPIE, Vol. 6552, p.111 (2007)
- ^ Yamashita, I., et al., Transparent Ceramics, J. Am. Ceram. Soc., Vol. 91, p.813 (2008)
Further reading
- Electrodynamics of continuous media, Landau, L. D., Lifshits. E.M. and Pitaevskii, L.P., (Pergamon Press, Oxford, 1984)
- Laser Light Scattering: Basic Principles and Practice Chu, B., 2nd Edn. (Academic Press, New York 1992)
- Solid State Laser Engineering, W. Koechner (Springer-Verlag, New York, 1999)
- Introduction to Chemical Physics, J.C. Slater (McGraw-Hill, New York, 1939)
- Modern Theory of Solids, F. Seitz, (McGraw-Hill, New York, 1940)
- Modern Aspects of the Vitreous State, J.D.MacKenzie, Ed. (Butterworths, London, 1960)

