Matter: Difference between revisions
improved choice of words |
m →External links: Added a link to a quiz on Matter. |
||
| Line 1,076: | Line 1,076: | ||
* [http://imagine.gsfc.nasa.gov/docs/ask_astro/answers/970213.html NASA on superfluid core of neutron star] |
* [http://imagine.gsfc.nasa.gov/docs/ask_astro/answers/970213.html NASA on superfluid core of neutron star] |
||
* [http://profmattstrassler.com/articles-and-posts/particle-physics-basics/mass-energy-matter-etc/matter-and-energy-a-false-dichotomy/ Matter and Energy: A False Dichotomy] - Conversations About Science with Theoretical Physicist Matt Strassler |
* [http://profmattstrassler.com/articles-and-posts/particle-physics-basics/mass-energy-matter-etc/matter-and-energy-a-false-dichotomy/ Matter and Energy: A False Dichotomy] - Conversations About Science with Theoretical Physicist Matt Strassler |
||
* [http://www.cognibloom.com/question_banks/321-matter A quiz on Matter] |
|||
{{Nature nav}} |
{{Nature nav}} |
||
Revision as of 05:13, 12 January 2014
    | |
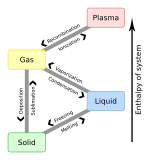
| Matter is usually classified into three classical states, with plasma sometimes added as a fourth state. From top to bottom: quartz (solid), water (liquid), nitrogen dioxide (gas), and a plasma globe (plasma). |
Matter is a poorly defined term in science (see definitions below). The term often refers to a substance (often a particle) that has rest mass. Matter is also used loosely as a general term for the substance that makes up all observable physical objects.[1][2]
All objects we see with the naked eye are composed of atoms. This atomic matter is in turn made up of interacting subatomic particles—usually a nucleus of protons and neutrons, and a cloud of orbiting electrons.[3][4] Typically, science considers these composite particles matter because they have both rest mass and volume. By contrast, massless particles, such as photons, are not considered matter, because they have neither rest mass nor volume. However, not all particles with rest mass have a classical volume, since fundamental particles such as quarks and leptons (sometimes equated with matter) are considered "point particles" with no effective size or volume. Nevertheless, quarks and leptons together make up "ordinary matter," and their interactions contribute to the effective volume of the composite particles that make up ordinary matter.
Matter commonly exists in four states (or phases): solid, liquid and gas, and plasma. However, advances in experimental techniques have revealed other previously theoretical phases, such as Bose–Einstein condensates and fermionic condensates. A focus on an elementary-particle view of matter also leads to new phases of matter, such as the quark–gluon plasma.[5] For much of the history of the natural sciences people have contemplated the exact nature of matter. The idea that matter was built of discrete building blocks, the so-called particulate theory of matter, was first put forward by the Greek philosophers Leucippus (~490 BC) and Democritus (~470–380 BC).[6]
Albert Einstein showed[7] that ultimately all matter is capable of being converted to energy (known as mass-energy equivalence) by the famous formula E = mc2, where E is the energy of a piece of matter of mass m, times c2 the speed of light squared. As the speed of light is 299,792,458 metres per second (186,282 mi/s), a relatively small amount of matter may be converted to a large amount of energy. An example is that positrons and electrons (matter) may transform into photons (non-matter). However, although matter may be created or destroyed in such processes, neither the quantity of mass or energy change during the process.
Matter should not be confused with mass, as the two are not quite the same in modern physics.[8] For example, mass is a conserved quantity, which means that its value is unchanging through time, within closed systems. However, matter is not conserved in such systems, although this is not obvious in ordinary conditions on Earth, where matter is approximately conserved. Still, special relativity shows that matter may disappear by conversion into energy, even inside closed systems, and it can also be created from energy, within such systems. However, because mass (like energy) can neither be created nor destroyed, the quantity of mass and the quantity of energy remain the same during a transformation of matter (which represents a certain amount of energy) into non-material (i.e., non-matter) energy. This is also true in the reverse transformation of energy into matter.
Different fields of science use the term matter in different, and sometimes incompatible, ways. Some of these ways are based on loose historical meanings, from a time when there was no reason to distinguish mass and matter. As such, there is no single universally-agreed scientific meaning of the word "matter." Scientifically, the term "mass" is well-defined, but "matter" is not. Sometimes in the field of physics "matter" is simply equated with particles that exhibit rest mass (i.e., that cannot travel at the speed of light), such as quarks and leptons. However, in both physics and chemistry, matter exhibits both wave-like and particle-like properties, the so-called wave–particle duality.[9][10][11]
Definition
Common definition

The common definition of matter is anything that has both mass and volume (occupies space).[12][13] For example, a car would be said to be made of matter, as it occupies space, and has mass.
The observation that matter occupies space goes back to antiquity. However, an explanation for why matter occupies space is recent, and is argued to be a result of the Pauli exclusion principle.[14][15] Two particular examples where the exclusion principle clearly relates matter to the occupation of space are white dwarf stars and neutron stars, discussed further below.
Relativity
In the context of relativity, mass is not an additive quantity, in the sense that one can add the rest masses of particles in a system to get the total rest mass of the system.[1] Thus, in relativity usually a more general view is that it is not the sum of rest masses, but the energy–momentum tensor that quantifies the amount of matter. This tensor gives the rest mass for the entire system. "Matter" therefore is sometimes considered as anything that contributes to the energy–momentum of a system, that is, anything that is not purely gravity.[16][17] This view is commonly held in fields that deal with general relativity such as cosmology. But in this view, light and other types of insubstantial energy may be part of matter.
The reason for this is that in this definition, electromagnetic radiation (such as light) as well as the energy of electromagnetic fields contributes to the mass of systems, and therefore appears to add matter to them. For example, light radiation (or thermal radiation) trapped inside a box would contribute to the mass of the box, as would any kind of energy inside the box, including the kinetic energy of particles held by the box. Nevertheless, isolated individual particles of light (photons) and the isolated kinetic energy of massive particles, are normally not considered to be matter.
A difference between matter and mass therefore may seem to arise when single particles are examined. In such cases, the mass of single photons is zero. For particles with rest mass, such as leptons and quarks, isolation of the particle in a frame where it is not moving, removes its kinetic energy.
A source of definition difficulty in relativity arises from two definitions of mass in common use, one of which is formally equivalent to total energy (and is thus observer-dependent), and the other of which is referred to as rest mass or invariant mass and is independent of the observer. Only the latter type of mass is loosely equated with matter (since it can be weighed). However, energies which contribute to the first type of mass may be weighed also in special circumstances, such as when trapped in a system with no net momentum (as in the box example above). Thus, a photon with no mass may add mass to a system in which it is trapped. Since such mass is measured as part of ordinary matter in complex systems, the "matter" status of "massless particles" becomes unclear in such systems. These problems contribute to the lack of a rigorous definition of matter in science, although mass is easier to define as the total stress-energy above (this is also what is weighed on a scale, and what is the source of gravity).
Atoms definition
A definition of "matter" based on its physical and chemical structure is: matter is made up of atoms.[18] As an example, deoxyribonucleic acid molecules (DNA) are matter under this definition because they are made of atoms. This definition can extend to include charged atoms and molecules, so as to include plasmas (gases of ions) and electrolytes (ionic solutions), which are not obviously included in the atoms definition. Alternatively, one can adopt the protons, neutrons, and electrons definition.
Protons, neutrons and electrons definition
A definition of "matter" more fine-scale than the atoms and molecules definition is: matter is made up of what atoms and molecules are made of, meaning anything made of positively charged protons, neutral neutrons, and negatively charged electrons.[19] This definition goes beyond atoms and molecules, however, to include substances made from these building blocks that are not simply atoms or molecules, for example white dwarf matter—typically, carbon and oxygen nuclei in a sea of degenerate electrons. At a microscopic level, the constituent "particles" of matter such as protons, neutrons, and electrons obey the laws of quantum mechanics and exhibit wave–particle duality. At an even deeper level, protons and neutrons are made up of quarks and the force fields (gluons) that bind them together (see Quarks and leptons definition below).
Quarks and leptons definition

As seen in the above discussion, many early definitions of what can be called ordinary matter were based upon its structure or building blocks. On the scale of elementary particles, a definition that follows this tradition can be stated as: ordinary matter is everything that is composed of elementary fermions, namely quarks and leptons.[20][21] The connection between these formulations follows.
Leptons (the most famous being the electron), and quarks (of which baryons, such as protons and neutrons, are made) combine to form atoms, which in turn form molecules. Because atoms and molecules are said to be matter, it is natural to phrase the definition as: ordinary matter is anything that is made of the same things that atoms and molecules are made of. (However, notice that one also can make from these building blocks matter that is not atoms or molecules.) Then, because electrons are leptons, and protons, and neutrons are made of quarks, this definition in turn leads to the definition of matter as being quarks and leptons, which are the two types of elementary fermions. Carithers and Grannis state: Ordinary matter is composed entirely of first-generation particles, namely the [up] and [down] quarks, plus the electron and its neutrino.[21] (Higher generations particles quickly decay into first-generation particles, and thus are not commonly encountered.[22])
This definition of ordinary matter is more subtle than it first appears. All the particles that make up ordinary matter (leptons and quarks) are elementary fermions, while all the force carriers are elementary bosons.[23] The W and Z bosons that mediate the weak force are not made of quarks or leptons, and so are not ordinary matter, even if they have mass.[24] In other words, mass is not something that is exclusive to ordinary matter.
The quark–lepton definition of ordinary matter, however, identifies not only the elementary building blocks of matter, but also includes composites made from the constituents (atoms and molecules, for example). Such composites contain an interaction energy that holds the constituents together, and may constitute the bulk of the mass of the composite. As an example, to a great extent, the mass of an atom is simply the sum of the masses of its constituent protons, neutrons and electrons. However, digging deeper, the protons and neutrons are made up of quarks bound together by gluon fields (see dynamics of quantum chromodynamics) and these gluons fields contribute significantly to the mass of hadrons.[25] In other words, most of what composes the "mass" of ordinary matter is due to the binding energy of quarks within protons and neutrons.[26] For example, the sum of the mass of the three quarks in a nucleon is approximately 12.5 MeV/c2, which is low compared to the mass of a nucleon (approximately 938 MeV/c2).[22][27] The bottom line is that most of the mass of everyday objects comes from the interaction energy of its elementary components.
Smaller building blocks issue
The Standard Model groups matter particles into three generations, where each generation consists of two quarks and two leptons. The first generation is the up and down quarks, the electron and the electron neutrino; the second includes the charm and strange quarks, the muon and the muon neutrino; the third generation consists of the top and bottom quarks and the tau and tau neutrino.[28] The most natural explanation for this would be that quarks and leptons of higher generations are excited states of the first generations. If this turns out to be the case, it would imply that quarks and leptons are composite particles, rather than elementary particles.[29]
Structure
In particle physics, fermions are particles that obey Fermi–Dirac statistics. Fermions can be elementary, like the electron—or composite, like the proton and neutron. In the Standard Model, there are two types of elementary fermions: quarks and leptons, which are discussed next.
Quarks
Quarks are particles of spin-1⁄2, implying that they are fermions. They carry an electric charge of −1⁄3 e (down-type quarks) or +2⁄3 e (up-type quarks). For comparison, an electron has a charge of −1 e. They also carry colour charge, which is the equivalent of the electric charge for the strong interaction. Quarks also undergo radioactive decay, meaning that they are subject to the weak interaction. Quarks are massive particles, and therefore are also subject to gravity.
| name | symbol | spin | electric charge (e) |
mass (MeV/c2) |
mass comparable to | antiparticle | antiparticle symbol |
|---|---|---|---|---|---|---|---|
| up-type quarks | |||||||
| up | u |
1⁄2 | +2⁄3 | 1.5 to 3.3 | ~ 5 electrons | antiup | u |
| charm | c |
1⁄2 | +2⁄3 | 1160 to 1340 | ~ 1 proton | anticharm | c |
| top | t |
1⁄2 | +2⁄3 | 169,100 to 173,300 | ~ 180 protons or ~ 1 tungsten atom |
antitop | t |
| down-type quarks | |||||||
| down | d |
1⁄2 | −1⁄3 | 3.5 to 6.0 | ~ 10 electrons | antidown | d |
| strange | s |
1⁄2 | −1⁄3 | 70 to 130 | ~ 200 electrons | antistrange | s |
| bottom | b |
1⁄2 | −1⁄3 | 4130 to 4370 | ~ 5 protons | antibottom | b |
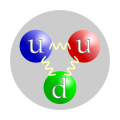
Baryonic matter
Baryons are strongly interacting fermions, and so are subject to Fermi-Dirac statistics. Amongst the baryons are the protons and neutrons, which occur in atomic nuclei, but many other unstable baryons exist as well. The term baryon usually refers to triquarks—particles made of three quarks. "Exotic" baryons made of four quarks and one antiquark are known as the pentaquarks, but their existence is not generally accepted.
Baryonic matter is the part of the universe that is made of baryons (including all atoms). This part of the universe does not include dark energy, dark matter, black holes or various forms of degenerate matter, such as compose white dwarf stars and neutron stars. Microwave light seen by Wilkinson Microwave Anisotropy Probe (WMAP), suggests that only about 4.6% of that part of the universe within range of the best telescopes (that is, matter that may be visible because light could reach us from it), is made of baryonic matter. About 23% is dark matter, and about 72% is dark energy.[31]

Degenerate matter
In physics, degenerate matter refers to the ground state of a gas of fermions at a temperature near absolute zero.[32] The Pauli exclusion principle requires that only two fermions can occupy a quantum state, one spin-up and the other spin-down. Hence, at zero temperature, the fermions fill up sufficient levels to accommodate all the available fermions—and in the case of many fermions, the maximum kinetic energy (called the Fermi energy) and the pressure of the gas becomes very large, and depends on the number of fermions rather than the temperature, unlike normal states of matter.
Degenerate matter is thought to occur during the evolution of heavy stars.[33] The demonstration by Subrahmanyan Chandrasekhar that white dwarf stars have a maximum allowed mass because of the exclusion principle caused a revolution in the theory of star evolution.[34]
Degenerate matter includes the part of the universe that is made up of neutron stars and white dwarfs.
Strange matter
Strange matter is a particular form of quark matter, usually thought of as a liquid of up, down, and strange quarks. It is contrasted with nuclear matter, which is a liquid of neutrons and protons (which themselves are built out of up and down quarks), and with non-strange quark matter, which is a quark liquid that contains only up and down quarks. At high enough density, strange matter is expected to be color superconducting. Strange matter is hypothesized to occur in the core of neutron stars, or, more speculatively, as isolated droplets that may vary in size from femtometers (strangelets) to kilometers (quark stars).
Two meanings of the term "strange matter"
In particle physics and astrophysics, the term is used in two ways, one broader and the other more specific.
- The broader meaning is just quark matter that contains three flavors of quarks: up, down, and strange. In this definition, there is a critical pressure and an associated critical density, and when nuclear matter (made of protons and neutrons) is compressed beyond this density, the protons and neutrons dissociate into quarks, yielding quark matter (probably strange matter).
- The narrower meaning is quark matter that is more stable than nuclear matter. The idea that this could happen is the "strange matter hypothesis" of Bodmer[35] and Witten.[36] In this definition, the critical pressure is zero: the true ground state of matter is always quark matter. The nuclei that we see in the matter around us, which are droplets of nuclear matter, are actually metastable, and given enough time (or the right external stimulus) would decay into droplets of strange matter, i.e. strangelets.
Leptons
Leptons are particles of spin-1⁄2, meaning that they are fermions. They carry an electric charge of −1 e (charged leptons) or 0 e (neutrinos). Unlike quarks, leptons do not carry colour charge, meaning that they do not experience the strong interaction. Leptons also undergo radioactive decay, meaning that they are subject to the weak interaction. Leptons are massive particles, therefore are subject to gravity.
| name | symbol | spin | electric charge (e) |
mass (MeV/c2) |
mass comparable to | antiparticle | antiparticle symbol |
|---|---|---|---|---|---|---|---|
| charged leptons[37] | |||||||
| electron | e− |
1⁄2 | −1 | 0.5110 | 1 electron | antielectron | e+ |
| muon | μ− |
1⁄2 | −1 | 105.7 | ~ 200 electrons | antimuon | μ+ |
| tau | τ− |
1⁄2 | −1 | 1,777 | ~ 2 protons | antitau | τ+ |
| neutrinos[38] | |||||||
| electron neutrino | ν e |
1⁄2 | 0 | < 0.000460 | < 1⁄1000 electron | electron antineutrino | ν e |
| muon neutrino | ν μ |
1⁄2 | 0 | < 0.19 | < 1⁄2 electron | muon antineutrino | ν μ |
| tau neutrino | ν τ |
1⁄2 | 0 | < 18.2 | < 40 electrons | tau antineutrino | ν τ |
Phases

In bulk[disambiguation needed], matter can exist in several different forms, or states of aggregation, known as phases,[40] depending on ambient pressure, temperature and volume.[41] A phase is a form of matter that has a relatively uniform chemical composition and physical properties (such as density, specific heat, refractive index, and so forth). These phases include the three familiar ones (solids, liquids, and gases), as well as more exotic states of matter (such as plasmas, superfluids, supersolids, Bose–Einstein condensates, ...). A fluid may be a liquid, gas or plasma. There are also paramagnetic and ferromagnetic phases of magnetic materials. As conditions change, matter may change from one phase into another. These phenomena are called phase transitions, and are studied in the field of thermodynamics. In nanomaterials, the vastly increased ratio of surface area to volume results in matter that can exhibit properties entirely different from those of bulk material, and not well described by any bulk phase (see nanomaterials for more details).
Phases are sometimes called states of matter, but this term can lead to confusion with thermodynamic states. For example, two gases maintained at different pressures are in different thermodynamic states (different pressures), but in the same phase (both are gases).
Antimatter
In particle physics and quantum chemistry, antimatter is matter that is composed of the antiparticles of those that constitute ordinary matter. If a particle and its antiparticle come into contact with each other, the two annihilate; that is, they may both be converted into other particles with equal energy in accordance with Einstein's equation E = mc2. These new particles may be high-energy photons (gamma rays) or other particle–antiparticle pairs. The resulting particles are endowed with an amount of kinetic energy equal to the difference between the rest mass of the products of the annihilation and the rest mass of the original particle-antiparticle pair, which is often quite large.
Antimatter is not found naturally on Earth, except very briefly and in vanishingly small quantities (as the result of radioactive decay, lightning or cosmic rays). This is because antimatter that came to exist on Earth outside the confines of a suitable physics laboratory would almost instantly meet the ordinary matter that Earth is made of, and be annihilated. Antiparticles and some stable antimatter (such as antihydrogen) can be made in tiny amounts, but not in enough quantity to do more than test a few of its theoretical properties.
There is considerable speculation both in science and science fiction as to why the observable universe is apparently almost entirely matter, and whether other places are almost entirely antimatter instead. In the early universe, it is thought that matter and antimatter were equally represented, and the disappearance of antimatter requires an asymmetry in physical laws called the charge parity (or CP symmetry) violation. CP symmetry violation can be obtained from the Standard Model,[42] but at this time the apparent asymmetry of matter and antimatter in the visible universe is one of the great unsolved problems in physics. Possible processes by which it came about are explored in more detail under baryogenesis.
Other types of matter

Ordinary matter, in the quarks and leptons definition, constitutes about 4% of the energy of the observable universe. The remaining energy is theorized to be due to exotic forms, of which 23% is dark matter[44][45] and 73% is dark energy.[46][47]

Dark matter
In astrophysics and cosmology, dark matter is matter of unknown composition that does not emit or reflect enough electromagnetic radiation to be observed directly, but whose presence can be inferred from gravitational effects on visible matter.[51][52] Observational evidence of the early universe and the big bang theory require that this matter have energy and mass, but is not composed of either elementary fermions (as above) OR gauge bosons. The commonly accepted view is that most of the dark-matter is non-baryonic in nature.[51] As such, it is composed of particles as yet unobserved in the laboratory. Perhaps they are supersymmetric particles,[53] which are not Standard Model particles, but relics formed at very high energies in the early phase of the universe and still floating about.[51]
Dark energy
In cosmology, dark energy is the name given to the antigravitating influence that is accelerating the rate of expansion of the universe. It is known not to be composed of known particles like protons, neutrons or electrons, nor of the particles of dark matter, because these all gravitate.[54][55]
Fully 70% of the matter density in the universe appears to be in the form of dark energy. Twenty-six percent is dark matter. Only 4% is ordinary matter. So less than 1 part in 20 is made out of matter we have observed experimentally or described in the standard model of particle physics. Of the other 96%, apart from the properties just mentioned, we know absolutely nothing.
— Lee Smolin: The Trouble with Physics, p. 16
Exotic matter
Exotic matter is a hypothetical concept of particle physics. It covers any material that violates one or more classical conditions or is not made of known baryonic particles. Such materials would possess qualities like negative mass or being repelled rather than attracted by gravity.
Historical development
Origins
The pre-Socratics were among the first recorded speculators about the underlying nature of the visible world. Thales (c. 624 BC–c. 546 BC) regarded water as the fundamental material of the world. Anaximander (c. 610 BC–c. 546 BC) posited that the basic material was wholly characterless or limitless: the Infinite (apeiron). Anaximenes (flourished 585 BC, d. 528 BC) posited that the basic stuff was pneuma or air. Heraclitus (c. 535–c. 475 BC) seems to say the basic element is fire, though perhaps he means that all is change. Empedocles (c. 490–430 BC) spoke of four elements of which everything was made: earth, water, air, and fire.[56] Meanwhile, Parmenides argued that change does not exist, and Democritus argued that everything is composed of minuscule, inert bodies of all shapes called atoms, a philosophy called atomism. All of these notions had deep philosophical problems.[57]
Aristotle (384 BC – 322 BC) was the first to put the conception on a sound philosophical basis, which he did in his natural philosophy, especially in Physics book I.[58] He adopted as reasonable suppositions the four Empedoclean elements, but added a fifth, aether. Nevertheless these elements are not basic in Aristotle's mind. Rather they, like everything else in the visible world, are composed of the basic principles matter and form.
The word Aristotle uses for matter, ὑλη (hyle or hule), can be literally translated as wood or timber, that is, "raw material" for building.[59] Indeed, Aristotle's conception of matter is intrinsically linked to something being made or composed. In other words, in contrast to the early modern conception of matter as simply occupying space, matter for Aristotle is definitionally linked to process or change: matter is what underlies a change of substance.
For example, a horse eats grass: the horse changes the grass into itself; the grass as such does not persist in the horse, but some aspect of it—its matter—does. The matter is not specifically described (e.g., as atoms), but consists of whatever persists in the change of substance from grass to horse. Matter in this understanding does not exist independently (i.e., as a substance), but exists interdependently (i.e., as a "principle") with form and only insofar as it underlies change. It can be helpful to conceive of the relationship of matter and form as very similar to that between parts and whole. For Aristotle, matter as such can only receive actuality from form; it has no activity or actuality in itself, similar to the way that parts as such only have their existence in a whole (otherwise they would be independent wholes).
Early modernity
René Descartes (1596–1650) originated the modern conception of matter. He was primarily a geometer. Instead of, like Aristotle, deducing the existence of matter from the physical reality of change, Descartes arbitrarily postulated matter to be an abstract, mathematical substance that occupies space:
So, extension in length, breadth, and depth, constitutes the nature of bodily substance; and thought constitutes the nature of thinking substance. And everything else attributable to body presupposes extension, and is only a mode of extended
— René Descartes, Principles of Philosophy[60]
For Descartes, matter has only the property of extension, so its only activity aside from locomotion is to exclude other bodies:[61] this is the mechanical philosophy. Descartes makes an absolute distinction between mind, which he defines as unextended, thinking substance, and matter, which he defines as unthinking, extended substance.[62] They are independent things. In contrast, Aristotle defines matter and the formal/forming principle as complementary principles that together compose one independent thing (substance). In short, Aristotle defines matter (roughly speaking) as what things are actually made of (with a potential independent existence), but Descartes elevates matter to an actual independent thing in itself.
The continuity and difference between Descartes' and Aristotle's conceptions is noteworthy. In both conceptions, matter is passive or inert. In the respective conceptions matter has different relationships to intelligence. For Aristotle, matter and intelligence (form) exist together in an interdependent relationship, whereas for Descartes, matter and intelligence (mind) are definitionally opposed, independent substances.[63]
Descartes' justification for restricting the inherent qualities of matter to extension is its permanence, but his real criterion is not permanence (which equally applied to color and resistance), but his desire to use geometry to explain all material properties.[64] Like Descartes, Hobbes, Boyle, and Locke argued that the inherent properties of bodies were limited to extension, and that so-called secondary qualities, like color, were only products of human perception.[65]
Isaac Newton (1643–1727) inherited Descartes' mechanical conception of matter. In the third of his "Rules of Reasoning in Philosophy," Newton lists the universal qualities of matter as "extension, hardness, impenetrability, mobility, and inertia."[66] Similarly in Optics he conjectures that God created matter as "solid, massy, hard, impenetrable, movable particles," which were "...even so very hard as never to wear or break in pieces."[67] The "primary" properties of matter were amenable to mathematical description, unlike "secondary" qualities such as color or taste. Like Descartes, Newton rejected the essential nature of secondary qualities.[68]
Newton developed Descartes' notion of matter by restoring to matter intrinsic properties in addition to extension (at least on a limited basis), such as mass. Newton's use of gravitational force, which worked "at a distance," effectively repudiated Descartes' mechanics, in which interactions happened exclusively by contact.[69]
Though Newton's gravity would seem to be a power of bodies, Newton himself did not admit it to be an essential property of matter. Carrying the logic forward more consistently, Joseph Priestley argued that corporeal properties transcend contact mechanics: chemical properties require the capacity for attraction.[69] He argued matter has other inherent powers besides the so-called primary qualities of Descartes, et al.[70]
Since Priestley's time, there has been a massive expansion in knowledge of the constituents of the material world (viz., molecules, atoms, subatomic particles), but there has been no further development in the definition of matter. Rather the question has been set aside. Noam Chomsky summarizes the situation that has prevailed since that time:
What is the concept of body that finally emerged?[...] The answer is that there is no clear and definite conception of body.[...] Rather, the material world is whatever we discover it to be, with whatever properties it must be assumed to have for the purposes of explanatory theory. Any intelligible theory that offers genuine explanations and that can be assimilated to the core notions of physics becomes part of the theory of the material world, part of our account of body. If we have such a theory in some domain, we seek to assimilate it to the core notions of physics, perhaps modifying these notions as we carry out this enterprise.
— Noam Chomsky, Language and problems of knowledge: the Managua lectures, p. 144[69]
So matter is whatever physics studies and the object of study of physics is matter: there is no independent general definition of matter, apart from its fitting into the methodology of measurement and controlled experimentation. In sum, the boundaries between what constitutes matter and everything else remains as vague as the demarcation problem of delimiting science from everything else.[71]
Late nineteenth and early twentieth centuries
In the 19th century, following the development of the periodic table, and of atomic theory, atoms were seen as being the fundamental constituents of matter; atoms formed molecules and compounds.[72]
The common definition in terms of occupying space and having mass is in contrast with most physical and chemical definitions of matter, which rely instead upon its structure and upon attributes not necessarily related to volume and mass. At the turn of the nineteenth century, the knowledge of matter began a rapid evolution.
Aspects of the Newtonian view still held sway. James Clerk Maxwell discussed matter in his work Matter and Motion.[73] He carefully separates "matter" from space and time, and defines it in terms of the object referred to in Newton's first law of motion.
However, the Newtonian picture was not the whole story. In the 19th century, the term "matter" was actively discussed by a host of scientists and philosophers, and a brief outline can be found in Levere.[74][further explanation needed] A textbook discussion from 1870 suggests matter is what is made up of atoms:[75]
Three divisions of matter are recognized in science: masses, molecules and atoms.
A Mass of matter is any portion of matter appreciable by the senses.
A Molecule is the smallest particle of matter into which a body can be divided without losing its identity.
An Atom is a still smaller particle produced by division of a molecule.
Rather than simply having the attributes of mass and occupying space, matter was held to have chemical and electrical properties. The famous physicist J. J. Thomson wrote about the "constitution of matter" and was concerned with the possible connection between matter and electrical charge.[76]
Later developments
There is an entire literature concerning the "structure of matter", ranging from the "electrical structure" in the early 20th century,[77] to the more recent "quark structure of matter", introduced today with the remark: Understanding the quark structure of matter has been one of the most important advances in contemporary physics.[78][further explanation needed] In this connection, physicists speak of matter fields, and speak of particles as "quantum excitations of a mode of the matter field".[9][10] And here is a quote from de Sabbata and Gasperini: "With the word "matter" we denote, in this context, the sources of the interactions, that is spinor fields (like quarks and leptons), which are believed to be the fundamental components of matter, or scalar fields, like the Higgs particles, which are used to introduced mass in a gauge theory (and that, however, could be composed of more fundamental fermion fields)."[79][further explanation needed]
The modern conception of matter has been refined many times in history, in light of the improvement in knowledge of just what the basic building blocks are, and in how they interact.
In the late 19th century with the discovery of the electron, and in the early 20th century, with the discovery of the atomic nucleus, and the birth of particle physics, matter was seen as made up of electrons, protons and neutrons interacting to form atoms. Today, we know that even protons and neutrons are not indivisible, they can be divided into quarks, while electrons are part of a particle family called leptons. Both quarks and leptons are elementary particles, and are currently seen as being the fundamental constituents of matter.[80]
These quarks and leptons interact through four fundamental forces: gravity, electromagnetism, weak interactions, and strong interactions. The Standard Model of particle physics is currently the best explanation for all of physics, but despite decades of efforts, gravity cannot yet be accounted for at the quantum-level; it is only described by classical physics (see quantum gravity and graviton).[81] Interactions between quarks and leptons are the result of an exchange of force-carrying particles (such as photons) between quarks and leptons.[82] The force-carrying particles are not themselves building blocks. As one consequence, mass and energy (which cannot be created or destroyed) cannot always be related to matter (which can be created out of non-matter particles such as photons, or even out of pure energy, such as kinetic energy). Force carriers are usually not considered matter: the carriers of the electric force (photons) possess energy (see Planck relation) and the carriers of the weak force (W and Z bosons) are massive, but neither are considered matter either.[83] However, while these particles are not considered matter, they do contribute to the total mass of atoms, subatomic particles, and all systems that contain them.[84][85]
Summary
The term "matter" is used throughout physics in a bewildering variety of contexts: for example, one refers to "condensed matter physics",[86] "elementary matter",[87] "partonic" matter, "dark" matter, "anti"-matter, "strange" matter, and "nuclear" matter. In discussions of matter and antimatter, normal matter has been referred to by Alfvén as koinomatter (Gk. common matter).[88] It is fair to say that in physics, there is no broad consensus as to a general definition of matter, and the term "matter" usually is used in conjunction with a specifying modifier.
See also
|
Antimatter
|
Cosmology
|
Dark matter
|
Philosophy Other
|
References
- ^ a b
R. Penrose (1991). "The mass of the classical vacuum". The Philosophy of Vacuum. Oxford University Press. p. 21. ISBN 0-19-824449-5.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ "Matter (physics)". McGraw-Hill's Access Science: Encyclopedia of Science and Technology Online. Retrieved 24 May 2009.
- ^ P. Davies (1992). The New Physics: A Synthesis. Cambridge University Press. p. 1. ISBN 0-521-43831-4.
- ^ G. 't Hooft (1997). In search of the ultimate building blocks. Cambridge University Press. p. 6. ISBN 0-521-57883-3.
- ^ "RHIC Scientists Serve Up "Perfect" Liquid" (Press release). Brookhaven National Laboratory. 18 April 2005. Retrieved 15 September 2009.
- ^ J. Olmsted, G.M. Williams (1996). Chemistry: The Molecular Science (2nd ed.). Jones & Bartlett. p. 40. ISBN 0-8151-8450-6.
- ^ Einstein, A. (1905). "Ist die Trägheit eines Körpers von seinem Energieinhalt abhängig?". Annalen der Physik. 18 (13): 639–643. Bibcode:1905AnP...323..639E. doi:10.1002/andp.19053231314Template:Inconsistent citations
{{cite journal}}: CS1 maint: postscript (link). See also the English translation. - ^ J. Mongillo (2007). Nanotechnology 101. Greenwood Publishing. p. 30. ISBN 0-313-33880-9.
- ^ a b P.C.W. Davies (1979). The Forces of Nature. Cambridge University Press. p. 116. ISBN 0-521-22523-X.
- ^ a b S. Weinberg (1998). The Quantum Theory of Fields. Cambridge University Press. p. 2. ISBN 0-521-55002-5.
- ^ M. Masujima (2008). Path Integral Quantization and Stochastic Quantization. Springer. p. 103. ISBN 3-540-87850-5.
- ^ S.M. Walker, A. King (2005). What is Matter?. Lerner Publications. p. 7. ISBN 0-8225-5131-4.
- ^ J.Kenkel, P.B. Kelter, D.S. Hage (2000). Chemistry: An Industry-based Introduction with CD-ROM. CRC Press. p. 2. ISBN 1-56670-303-4.
All basic science textbooks define matter as simply the collective aggregate of all material substances that occupy space and have mass or weight.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ K.A. Peacock (2008). The Quantum Revolution: A Historical Perspective. Greenwood Publishing Group. p. 47. ISBN 0-313-33448-X.
- ^ M.H. Krieger (1998). Constitutions of Matter: Mathematically Modeling the Most Everyday of Physical Phenomena. University of Chicago Press. p. 22. ISBN 0-226-45305-7.
- ^ S.M. Caroll (2004). Spacetime and Geometry. Addison Wesley. pp. 163–164. ISBN 0-8053-8732-3.
- ^
P. Davies (1992). The New Physics: A Synthesis. Cambridge University Press. p. 499. ISBN 0-521-43831-4.
Matter fields: the fields whose quanta describe the elementary particles that make up the material content of the Universe (as opposed to the gravitons and their supersymmetric partners).
- ^ G.F. Barker (1870). "Divisions of matter". A text-book of elementary chemistry: theoretical and inorganic. John F Morton & Co. p. 2. ISBN 978-1-4460-2206-1.
- ^ M. de Podesta (2002). Understanding the Properties of Matter (2nd ed.). CRC Press. p. 8. ISBN 0-415-25788-3.
- ^
B. Povh, K. Rith, C. Scholz, F. Zetsche, M. Lavelle (2004). "Part I: Analysis: The building blocks of matter". Particles and Nuclei: An Introduction to the Physical Concepts (4th ed.). Springer. ISBN 3-540-20168-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b B. Carithers, P. Grannis (1995). "Discovery of the Top Quark" (PDF). Beam Line. 25 (3). SLAC National Accelerator Laboratory: 4–16.
- ^ a b D. Green (2005). High PT physics at hadron colliders. Cambridge University Press. p. 23. ISBN 0-521-83509-7. Cite error: The named reference "Green" was defined multiple times with different content (see the help page).
- ^ L. Smolin (2007). The Trouble with Physics: The Rise of String Theory, the Fall of a Science, and What Comes Next. Mariner Books. p. 67. ISBN 0-618-91868-X.
- ^ The W boson mass is 80.398 GeV; see Figure 1 in C. Amsler et al. (Particle Data Group) (2008). "Review of Particle Physics: The Mass and Width of the W Boson" (PDF). Physics Letters B. 667: 1. Bibcode:2008PhLB..667....1P. doi:10.1016/j.physletb.2008.07.018.
- ^ I.J.R. Aitchison, A.J.G. Hey (2004). Gauge Theories in Particle Physics. CRC Press. p. 48. ISBN 0-7503-0864-8.
- ^
B. Povh, K. Rith, C. Scholz, F. Zetsche, M. Lavelle (2004). Particles and Nuclei: An Introduction to the Physical Concepts. Springer. p. 103. ISBN 3-540-20168-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ T. Hatsuda (2008). "Quark-gluon plasma and QCD". In H. Akai (ed.). Condensed matter theories. Vol. 21. Nova Publishers. p. 296. ISBN 1-60021-501-7.
- ^ K.W Staley (2004). "Origins of the Third Generation of Matter". The Evidence for the Top Quark. Cambridge University Press. p. 8. ISBN 0-521-82710-8.
- ^
Y. Ne'eman, Y. Kirsh (1996). The Particle Hunters (2nd ed.). Cambridge University Press. p. 276. ISBN 0-521-47686-0.
[T]he most natural explanation to the existence of higher generations of quarks and leptons is that they correspond to excited states of the first generation, and experience suggests that excited systems must be composite
- ^ C. Amsler et al. (Particle Data Group) (2008). "Reviews of Particle Physics: Quarks" (PDF). Physics Letters B. 667: 1. Bibcode:2008PhLB..667....1P. doi:10.1016/j.physletb.2008.07.018.
- ^ "Five Year Results on the Oldest Light in the Universe". NASA. 2008. Retrieved 2 May 2008.
- ^ H.S. Goldberg, M.D. Scadron (1987). Physics of Stellar Evolution and Cosmology. Taylor & Francis. p. 202. ISBN 0-677-05540-4.
- ^ H.S. Goldberg, M.D. Scadron (1987). Physics of Stellar Evolution and Cosmology. Taylor & Francis. p. 233. ISBN 0-677-05540-4.
- ^
J.-P. Luminet, A. Bullough, A. King (1992). Black Holes. Cambridge University Press. p. 75. ISBN 0-521-40906-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ A. Bodmer (1971). "Collapsed Nuclei". Physical Review D. 4 (6): 1601. Bibcode:1971PhRvD...4.1601B. doi:10.1103/PhysRevD.4.1601.
- ^ E. Witten (1984). "Cosmic Separation of Phases". Physical Review D. 30 (2): 272. Bibcode:1984PhRvD..30..272W. doi:10.1103/PhysRevD.30.272.
- ^ C. Amsler et al. (Particle Data Group) (2008). "Review of Particle Physics: Leptons" (PDF). Physics Letters B. 667: 1. Bibcode:2008PhLB..667....1P. doi:10.1016/j.physletb.2008.07.018.
- ^ C. Amsler et al. (Particle Data Group) (2008). "Review of Particle Physics: Neutrinos Properties" (PDF). Physics Letters B. 667: 1. Bibcode:2008PhLB..667....1P. doi:10.1016/j.physletb.2008.07.018.
- ^ S.R. Logan (1998). Physical Chemistry for the Biomedical Sciences. CRC Press. pp. 110–111. ISBN 0-7484-0710-3.
- ^ P.J. Collings (2002). "Chapter 1: States of Matter". Liquid Crystals: Nature's Delicate Phase of Matter. Princeton University Press. ISBN 0-691-08672-9.
- ^ D.H. Trevena (1975). "Chapter 1.2: Changes of phase". The Liquid Phase. Taylor & Francis. ISBN 978-0-85109-031-3.
- ^ National Research Council (US) (2006). Revealing the hidden nature of space and time. National Academies Press. p. 46. ISBN 0-309-10194-8.
- ^ J.P. Ostriker, P.J. Steinhardt (2003). "New Light on Dark Matter". Science. 300 (5627): 1909–13. arXiv:astro-ph/0306402. Bibcode:2003Sci...300.1909O. doi:10.1126/science.1085976. PMID 12817140.
- ^ K. Pretzl (2004). "Dark Matter, Massive Neutrinos and Susy Particles". Structure and Dynamics of Elementary Matter. Walter Greiner. p. 289. ISBN 1-4020-2446-0.
- ^ K. Freeman, G. McNamara (2006). "What can the matter be?". In Search of Dark Matter. Birkhäuser Verlag. p. 105. ISBN 0-387-27616-5.
- ^ J.C. Wheeler (2007). Cosmic Catastrophes: Exploding Stars, Black Holes, and Mapping the Universe. Cambridge University Press. p. 282. ISBN 0-521-85714-7.
- ^ J. Gribbin (2007). The Origins of the Future: Ten Questions for the Next Ten Years. Yale University Press. p. 151. ISBN 0-300-12596-8.
- ^ P. Schneider (2006). Extragalactic Astronomy and Cosmology. Springer. p. 4, Fig. 1.4. ISBN 3-540-33174-3.
- ^ T. Koupelis, K.F. Kuhn (2007). In Quest of the Universe. Jones & Bartlett Publishers. p. 492; Fig. 16.13. ISBN 0-7637-4387-9.
- ^
M.H. Jones, R.J. Lambourne, D.J. Adams (2004). An Introduction to Galaxies and Cosmology. Cambridge University Press. p. 21; Fig. 1.13. ISBN 0-521-54623-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c
D. Majumdar (2007). "Dark matter — possible candidates and direct detection". arXiv:hep-ph/0703310.
{{cite arXiv}}:|class=ignored (help) - ^
K.A. Olive (2003). "Theoretical Advanced Study Institute lectures on dark matter". arXiv:astro-ph/0301505.
{{cite arXiv}}:|class=ignored (help) - ^ K.A. Olive (2009). "Colliders and Cosmology". European Physical Journal C. 59 (2): 269–295. arXiv:0806.1208. Bibcode:2009EPJC...59..269O. doi:10.1140/epjc/s10052-008-0738-8.
- ^ J.C. Wheeler (2007). Cosmic Catastrophes. Cambridge University Press. p. 282. ISBN 0-521-85714-7.
- ^ L. Smolin (2007). The Trouble with Physics. Mariner Books. p. 16. ISBN 0-618-91868-X.
- ^ S. Toulmin, J. Goodfield (1962). The Architecture of Matter. University of Chicago Press. pp. 48–54.
- ^ Discussed by Aristotle in Physics, esp. book I, but also later; as well as Metaphysics I-II.
- ^ For a good explanation and elaboration, see R.J. Connell (1966). Matter and Becoming. Priory Press.
- ^
H.G. Liddell, R. Scott, J.M. Whiton (1891). A lexicon abridged from Liddell & Scott's Greek-English lexicon. Harper and Brothers. p. 725.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ R. Descartes (1644). "The Principles of Human Knowledge". Principles of Philosophy I. p. 53.
- ^ though even this property seems to be non-essential (Rene Descartes, Principles of Philosophy II [1644], “On the Principles of Material Things,” no. 4.)
- ^ R. Descartes (1644). "The Principles of Human Knowledge". Principles of Philosophy I. pp. 8, 54, 63.
- ^ D.L. Schindler (1986). "The Problem of Mechanism". In D.L. Schindler (ed.). Beyond Mechanism. University Press of America.
- ^ E.A. Burtt, Metaphysical Foundations of Modern Science (Garden City, NY: Doubleday and Company, 1954), 117-118.
- ^ J.E. McGuire and P.M. Heimann, "The Rejection of Newton's Concept of Matter in the Eighteenth Century," The Concept of Matter in Modern Philosophy ed. Ernan McMullin (Notre Dame: University of Notre Dame Press, 1978), 104-118 (105).
- ^ Isaac Newton, Mathematical Principles of Natural Philosophy, trans. A. Motte, revised by F. Cajori (Berkeley: University of California Press, 1934), pp. 398-400. Further analyzed by Maurice A. Finocchiaro, "Newton's Third Rule of Philosophizing: A Role for Logic in Historiography," Isis 65:1 (Mar. 1974), pp. 66-73.
- ^ Isaac Newton, Optics, Book III, pt. 1, query 31.
- ^ McGuire and Heimann, 104.
- ^ a b c N. Chomsky (1988). Language and problems of knowledge: the Managua lectures (2nd ed.). MIT Press. p. 144. ISBN 0-262-53070-8.
- ^ McGuire and Heimann, 113.
- ^ Nevertheless, it remains true that the mathematization regarded as requisite for a modern physical theory carries its own implicit notion of matter, which is very like Descartes', despite the demonstrated vacuity of the latter's notions.
- ^ M. Wenham (2005). Understanding Primary Science: Ideas, Concepts and Explanations (2nd ed.). Paul Chapman Educational Publishing. p. 115. ISBN 1-4129-0163-4.
- ^ J.C. Maxwell (1876). Matter and Motion. Society for Promoting Christian Knowledge. p. 18. ISBN 0-486-66895-9.
- ^ T.H. Levere (1993). "Introduction". Affinity and Matter: Elements of Chemical Philosophy, 1800–1865. Taylor & Francis. ISBN 2-88124-583-8.
- ^ G.F. Barker (1870). "Introduction". A Text Book of Elementary Chemistry: Theoretical and Inorganic. John P. Morton and Company. p. 2.
- ^ J.J. Thomson (1909). "Preface". Electricity and Matter. A. Constable.
- ^ O.W. Richardson (1914). "Chapter 1". The Electron Theory of Matter. The University Press.
- ^ M. Jacob (1992). The Quark Structure of Matter. World Scientific. ISBN 981-02-3687-5.
- ^ V. de Sabbata, M. Gasperini (1985). Introduction to Gravitation. World Scientific. p. 293. ISBN 9971-5-0049-3.
- ^
The history of the concept of matter is a history of the fundamental length scales used to define matter. Different building blocks apply depending upon whether one defines matter on an atomic or elementary particle level. One may use a definition that matter is atoms, or that matter is hadrons, or that matter is leptons and quarks depending upon the scale at which one wishes to define matter.
B. Povh, K. Rith, C. Scholz, F. Zetsche, M. Lavelle (2004). "Fundamental constituents of matter". Particles and Nuclei: An Introduction to the Physical Concepts (4th ed.). Springer. ISBN 3-540-20168-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ J. Allday (2001). Quarks, Leptons and the Big Bang. CRC Press. p. 12. ISBN 0-7503-0806-0.
- ^ B.A. Schumm (2004). Deep Down Things: The Breathtaking Beauty of Particle Physics. Johns Hopkins University Press. p. 57. ISBN 0-8018-7971-X.
- ^ See for example, M. Jibu, K. Yasue (1995). Quantum Brain Dynamics and Consciousness. John Benjamins Publishing Company. p. 62. ISBN 1-55619-183-9., B. Martin (2009). Nuclear and Particle Physics (2nd ed.). Wiley. p. 125. ISBN 0-470-74275-5. and K.W. Plaxco, M. Gross (2006). Astrobiology: A Brief Introduction. Johns Hopkins University Press. p. 23. ISBN 0-8018-8367-9.
- ^ P.A. Tipler, R.A. Llewellyn (2002). Modern Physics. Macmillan. pp. 89–91, 94–95. ISBN 0-7167-4345-0.
- ^
P. Schmüser, H. Spitzer (2002). "Particles". In L. Bergmann; et al. (eds.). Constituents of Matter: Atoms, Molecules, Nuclei. CRC Press. pp. 773 ff. ISBN 0-8493-1202-7.
{{cite book}}: Explicit use of et al. in:|editor=(help) - ^ P.M. Chaikin, T.C. Lubensky (2000). Principles of Condensed Matter Physics. Cambridge University Press. p. xvii. ISBN 0-521-79450-1.
- ^
W. Greiner (2003). W. Greiner, M.G. Itkis, G. Reinhardt, M.C. Güçlü (ed.). Structure and Dynamics of Elementary Matter. Springer. p. xii. ISBN 1-4020-2445-2.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ P. Sukys (1999). Lifting the Scientific Veil: Science Appreciation for the Nonscientist. Rowman & Littlefield. p. 87. ISBN 0-8476-9600-6.
Further reading
- Lillian Hoddeson, Michael Riordan, ed. (1997). The Rise of the Standard Model. Cambridge University Press. ISBN 0-521-57816-7.
- Timothy Paul Smith (2004). "The search for quarks in ordinary matter". Hidden Worlds. Princeton University Press. p. 1. ISBN 0-691-05773-7.
- Harald Fritzsch (2005). Elementary Particles: Building blocks of matter. World Scientific. p. 1. ISBN 981-256-141-2.
- Bertrand Russell (1992). "The philosophy of matter". A Critical Exposition of the Philosophy of Leibniz (Reprint of 1937 2nd ed.). Routledge. p. 88. ISBN 0-415-08296-X.
- Stephen Toulmin and June Goodfield, The Architecture of Matter (Chicago: University of Chicago Press, 1962).
- Richard J. Connell, Matter and Becoming (Chicago: The Priory Press, 1966).
- Ernan McMullin, The Concept of Matter in Greek and Medieval Philosophy (Notre Dame, IN: Univ. of Notre Dame Press, 1965).
- Ernan McMullin, The Concept of Matter in Modern Philosophy (Notre Dame, IN: University of Notre Dame Press, 1978).
External links
- Visionlearning Module on Matter
- Matter in the universe How much Matter is in the Universe?
- NASA on superfluid core of neutron star
- Matter and Energy: A False Dichotomy - Conversations About Science with Theoretical Physicist Matt Strassler
- A quiz on Matter