Gliclazide: Difference between revisions
No edit summary |
|||
| Line 29: | Line 29: | ||
== Form and Composition: == |
== Form and Composition: == |
||
Each modified-release tablet contains 30 mg of gliclazide. |
Each modified-release tablet contains 30 mg of gliclazide. |
||
Not marketed in the United States. |
|||
== Indication: == |
== Indication: == |
||
Revision as of 12:55, 12 June 2007
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.221 |
| Chemical and physical data | |
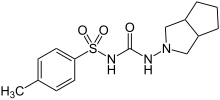
| Formula | C15H21N3O3S |
| Molar mass | 323.412 g/mol g·mol−1 |
Gliclazide is an oral hypoglycemic (anti-diabetic drug) and is classified as a sulfonylurea. It is marketed as Diamicron MR®. DIAMICRON MR is also distributed as: Diabeton MR, Diamicron 30mg, Diamicron LM 30mg, Diamicron MR 30 mg, Diamicron Uno 30mg, Dianormax MR, Diaprel MR and Uni Diamicron.
Form and Composition:
Each modified-release tablet contains 30 mg of gliclazide.
Not marketed in the United States.
Indication:
Type 2 diabetes.
Dosage:
30 to 120 mg depending on response, once daily with breakfast, including in elderly patients or those with mild-to-moderate renal failure
Properties:
Hypoglycemic sulfonylurea, restoring first peak of insulin secretion, increasing insulin sensitivity. Glycemia-independent hemovascular effects, antioxidant effect. No active circulating metabolites.
Contraindications:
type 1 diabetes, hypersensitivity to sulfonylureas, severe renal or hepatic failure, pregnancy and lactation, miconazole coprescription.
Interactions:
Hyperglycemic action may be caused by danazol, chlorpromazine, glucocorticoids, progestogens, ß-2 agonists. Its hypoglycemic action may be potentiated by phenylbutazone, alcohol, fluconazole, ß-blockers, possibly ACE inhibitors.
Adverse effects:
Hypoglycemia, gastrointestinal disturbance (reported), skin reactions (rare), hematological disorders (rare), hepatic enzyme rises (exceptional).
Overdosage:
Possible severe hypoglycemia requiring urgent IV glucose and monitoring.
