Diabetes: Difference between revisions
| [pending revision] | [pending revision] |
Split "Terminology". Some goes to form the lead in Classification and some goes to form the lead in History. |
WebHamster (talk | contribs) →Type 2 diabetes: recent news of surgery aiding the "cure" of T2 diabetes |
||
| Line 219: | Line 219: | ||
===Type 2 diabetes=== |
===Type 2 diabetes=== |
||
Type 2 diabetes can be cured by [[gastric bypass surgery]] in 80-100% of severely obese patients, and in some non-obese patients, usually within days after surgery. This is not an effect of weight loss, since it occurs long before weight loss.<ref>{{cite journal |author=Rubino F, Gagner M |title=Potential of surgery for curing type 2 diabetes mellitus |journal=Ann. Surg. |volume=236 |issue=5 |pages=554-9 |year=2002 |pmid=12409659 |doi=10.1097/01.SLA.0000032951.37471.80}}</ref> After gastric bypass surgery for obesity, the death rate from all causes is reduced by up to 40%<ref>{{cite journal |author=Sjöström L, Narbro K, Sjöström CD, ''et al'' |title=Effects of bariatric surgery on mortality in Swedish obese subjects |journal=N. Engl. J. Med. |volume=357 |issue=8 |pages=741-52 |year=2007 |pmid=17715408 |doi=10.1056/NEJMoa066254}}</ref> |
Type 2 diabetes can be cured by [[gastric bypass surgery]] in 80-100% of severely obese patients, and in some non-obese patients, usually within days after surgery. This is not an effect of weight loss, since it occurs long before weight loss.<ref>{{cite journal |author=Rubino F, Gagner M |title=Potential of surgery for curing type 2 diabetes mellitus |journal=Ann. Surg. |volume=236 |issue=5 |pages=554-9 |year=2002 |pmid=12409659 |doi=10.1097/01.SLA.0000032951.37471.80}}</ref> After gastric bypass surgery for obesity, the death rate from all causes is reduced by up to 40%<ref>{{cite journal |author=Sjöström L, Narbro K, Sjöström CD, ''et al'' |title=Effects of bariatric surgery on mortality in Swedish obese subjects |journal=N. Engl. J. Med. |volume=357 |issue=8 |pages=741-52 |year=2007 |pmid=17715408 |doi=10.1056/NEJMoa066254}}</ref> |
||
An article in ''[[New Scientist]]'' magazine <ref>{{Citation | last= Vasonconcelos | first= Alberto | publication-date= 2007-09-01 | title= Could type 2 diabetes be reversed using surgery? | periodical= [[New Scientist]] | issue= 2619 | pages= 11-13 | url= http://www.newscientist.com/channel/health/mg19526193.100-could-type-2-diabetes-be-reversed-using-surgery.html | accessdate= 2007-09-26}}.</ref> reports that a team of Italian, Brazilian and French doctors <ref>{{cite book | last = Cohen | first = Dr Ricardo V. | coauthors = Dr Carlos A. Schiavon, Dr José S. Pinheiro, Dr Jose Luiz Correa, Dr Francesco Rubino | title = Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases | origdate = 2007-09-01 | doi = 10.1016/j.soard.2007.01.009 }}</ref> have discovered that [[duodenum|duodenal exclusion]] surgery can result in patient's type 2 diabetes "vanishing". The doctors believe that the duodenum is responsible for releasing a form of "molecular signal" that results in a cell's insulin resistance. The article also reports that several other medical teams in [[Mexico]], [[Peru]], [[Dominican Republic]] and [[India]] have also reported similar findings (though their reports have not yet been published). Clinical trials are now starting in [[China]], [[Japan]], the [[United States of America|US]], [[Italy]] and [[Belgium]]. A proposal has already been submitted to the American Association of Clinical Endocrinologists (AACE). The AACE comment that they are studying the evidence but have so far not reached a decision. |
|||
==Prevention== |
==Prevention== |
||
Revision as of 14:57, 26 September 2007
| Diabetes | |
|---|---|
| Specialty | Diabetology, endocrinology |
Diabetes mellitus, often simply diabetes (IPA pronunciation: [daɪəˈbiːtiːz] or often [ˌdaɪəˈbiːtəs]), is a syndrome characterized by disordered metabolism and inappropiate hyperglycemia (high blood sugar) resulting either from low levels of the hormone insulin secretion or to a combination of resistance to insulin's effects and inadequate insulin secretion to compensate. An international expert committee recommended the use of the terms "type 1 and type 2 diabetes".[1]
The characteristic symptoms are polyuria (excessive urine production), polydipsia (thirst and increased fluid intake) and blurred vision; these symptoms may be absent if the blood sugar is only mildly elevated.
The World Health Organization recognizes three main forms of diabetes mellitus: type 1, type 2, and gestational diabetes (occurring during pregnancy),[2] which have similar signs, symptoms, and consequences, but different causes and population distributions. Ultimately, all forms are due to the beta cells of the pancreas being unable to produce sufficient insulin to prevent hyperglycemia.[3] Type 1 diabetes is usually due to autoimmune destruction of the pancreatic beta cells, which produce insulin. Type 2 diabetes is characterized by insulin resistance in target tissues, but some impairment of beta cell function is necessary for its development. Gestational diabetes is similar to type 2 diabetes, in that it involves insulin resistance; the hormones of pregnancy can cause insulin resistance in women genetically predisposed to developing this condition.
Gestational diabetes typically resolves with delivery of the child, however types 1 and 2 diabetes are incurable chronic conditions but have been treatable since insulin became medically available in 1921. Type 1 diabetes, in which insulin is not secreted by the pancreas, is directly treatable only with injected or inhaled insulin, although dietary and other lifestyle adjustments are part of management. Type II may be managed with a combination of dietary treatment, tablets and injections and, frequently, insulin supplementation. While insulin was originally produced from natural sources such as porcine pancreas, most insulin used today is produced through genetic engineering, either as a direct copy of human insulin, or human insulin with modified molecules that provide different onset and duration of action. Insulin can also be delivered continuously by a pump surgically embedded under the skin.
Diabetes can cause many complications. Acute complications (hypoglycemia, ketoacidosis or nonketotic hyperosmolar coma) may occur if the disease is not adequately controlled. Serious long-term complications include cardiovascular disease (doubled risk), chronic renal failure (diabetic nephropathy is the main cause of dialysis in developed world adults), retinal damage (which can lead to blindness and is the most significant cause of adult blindness in the non-elderly in the developed world), nerve damage (of several kinds), and microvascular damage, which may cause erectile dysfunction (impotence) and poor healing. Poor healing of wounds, particularly of the feet, can lead to gangrene, which may require amputation — the leading cause of non-traumatic amputation in adults in the developed world. Adequate treatment of diabetes, as well as increased emphasis on blood pressure control and lifestyle factors (such as not smoking and keeping a healthy body weight), may improve the risk profile of most aforementioned complications.
Classification
The term diabetes, without qualification, usually refers to diabetes mellitus, which is associated with excessive sweet urine, but there are several rarer conditions also named diabetes. The most common of these is diabetes insipidus in which the urine is not sweet (insipidus meaning "without taste" in Latin); it can be caused by either kidney (nephrogenic DI) or pituitary gland (central DI) damage.
The principle two idiopathic forms of diabetes mellitus are know as types 1 and 2. The term "type 1 diabetes" has universally replaced several former terms, including childhood-onset diabetes, juvenile diabetes, and insulin-dependent diabetes (IDDM). Likewise, the term "type 2 diabetes" has replaced several former terms, including adult-onset diabetes, obesity-related diabetes, and non-insulin-dependent diabetes (NIDDM). Beyond these two types, there is no agreed-upon standard nomenclature. Various sources have defined "type 3 diabetes" as, among others, gestational diabetes,[4] insulin-resistant type 1 diabetes (or "double diabetes"), type 2 diabetes which has progressed to require injected insulin, and latent autoimmune diabetes of adults (or LADA or "type 1.5" diabetes[5]). There is also maturity onset diabetes of the young (MODY) which is a single gene disorder with strong family history that presents as type 2 diabetes before 30 years of age.
Type 1 diabetes mellitus
Type 1 diabetes mellitus is characterized by loss of the insulin-producing beta cells of the islets of Langerhans in the pancreas, leading to a deficiency of insulin. The main cause of this beta cell loss is a T-cell mediated autoimmune attack.[3] There is no known preventative measure that can be taken against type 1 diabetes, which comprises up to 10% of diabetes mellitus cases in North America and Europe (though this varies by geographical location). Most affected people are otherwise healthy and of a healthy weight when onset occurs. Sensitivity and responsiveness to insulin are usually normal, especially in the early stages. Type 1 diabetes can affect children or adults but was traditionally termed "juvenile diabetes" because it represents a majority of cases of diabetes affecting children.
The principal treatment of type 1 diabetes, even from the earliest stages, is replacement of insulin combined with careful monitoring of blood glucose levels using blood testing monitors. Without insulin, ketosis and diabetic ketoacidosis can develop and coma or death will result. Emphasis is also placed on lifestyle adjustments (diet and exercise) though these cannot reverse the loss. Apart from the common subcutaneous injections, it is also possible to deliver insulin by a pump, which allows continuous infusion of insulin 24 hours a day at preset levels, and the ability to program doses (a bolus) of insulin as needed at meal times. An inhaled form of insulin, Exubera, was approved by the FDA in January 2006.[6]
Type 1 treatment must be continued indefinitely. Treatment does not impair normal activities, if sufficient awareness, appropriate care, and discipline in testing and medication is taken. The average glucose level for the type 1 patient should be as close to normal (80–120 mg/dl, 4–6 mmol/l) as possible. Some physicians suggest up to 140–150 mg/dl (7-7.5 mmol/l) for those having trouble with lower values, such as frequent hypoglycemic events. Values above 200 mg/dl (10 mmol/l) are often accompanied by discomfort and frequent urination leading to dehydration. Values above 300 mg/dl (15 mmol/l) usually require immediate treatment and may lead to ketoacidosis. Low levels of blood glucose, called hypoglycemia, may lead to seizures or episodes of unconsciousness.
Type 2 diabetes mellitus
Type 2 diabetes mellitus is due to insulin resistance or reduced insulin sensitivity, combined with reduced insulin secretion. The defective responsiveness of body tissues to insulin almost certainly involves the insulin receptor in cell membranes. In the early stage the predominant abnormality is reduced insulin sensitivity, characterized by elevated levels of insulin in the blood. At this stage hyperglycemia can be reversed by a variety of measures and medications that improve insulin sensitivity or reduce glucose production by the liver. As the disease progresses the impairment of insulin secretion worsens, and therapeutic replacement of insulin often becomes necessary.
There are numerous theories as to the exact cause and mechanism in type 2 diabetes. Central obesity (fat concentrated around the waist in relation to abdominal organs, but not subcutaneous fat) is known to predispose individuals for insulin resistance. Abdominal fat is especially active hormonally, secreting a group of hormones called adipokines that may possibly impair glucose tolerance. Obesity is found in approximately 55% of patients diagnosed with type 2 diabetes.[7] Other factors include aging (about 20% of elderly patients in North America have diabetes) and family history (type 2 is much more common in those with close relatives who have had it). In the last decade, type 2 diabetes has increasingly begun to affect children and adolescents, likely in connection with the increased prevalence of childhood obesity seen in recent decades in some places.[8]
Type 2 diabetes may go unnoticed for years because visible symptoms are typically mild, non-existent or sporadic, and usually there are no ketoacidotic episodes. However, severe long-term complications can result from unnoticed type 2 diabetes, including renal failure due to diabetic nephropathy, vascular disease (including coronary artery disease), vision damage due to diabetic retinopathy, loss of sensation or pain due to diabetes neuropathy, and liver damage from non-alcoholic steatohepatitis.
Type 2 diabetes is usually first treated by increasing physical activity, decreasing carbohydrate intake, and losing weight. These can restore insulin sensitivity even when the weight loss is modest, for example around 5 kg (10 to 15 lb), most especially when it is in abdominal fat deposits. It is sometimes possible to achieve long-term, satisfactory glucose control with these measures alone. However, the underlying tendency to insulin resistance is not lost, and so attention to diet, exercise, and weight loss must continue. The usual next step, if necessary, is treatment with oral antidiabetic drugs. Insulin production is initially only moderately impaired in type 2 diabetes, so oral medication (often used in various combinations) can be used to improve insulin production (e.g., sulfonylureas), to regulate inappropriate release of glucose by the liver and attenuate insulin resistance to some extent (e.g., metformin), and to substantially attenuate insulin resistance (e.g., thiazolidinediones). According to one study, overweight patients treated with metformin compared with diet alone, had relative risk reductions of 32% for any diabetes endpoint, 42% for diabetes related death and 36% for all cause mortality and stroke.[9] Eventually, it is not uncommon for beta cell insulin secretion to cease altogether. At this point, insulin therapy is necessary to maintain normal or near normal glucose levels.
Gestational diabetes
Gestational diabetes mellitus (GDM) resembles type 2 diabetes in several respects, involving a combination of inadequate insulin secretion and responsiveness. It occurs in about 2%–5% of all pregnancies and may improve or disappear after delivery. Gestational diabetes is fully treatable but requires careful medical supervision throughout the pregnancy. About 20%–50% of affected women develop type 2 diabetes later in life.
Even though it may be transient, untreated gestational diabetes can damage the health of the fetus or mother. Risks to the baby include macrosomia (high birth weight), congenital cardiac and central nervous system anomalies, and skeletal muscle malformations. Increased fetal insulin may inhibit fetal surfactant production and cause respiratory distress syndrome. Hyperbilirubinemia may result from red blood cell destruction. In severe cases, perinatal death may occur, most commonly as a result of poor placental profusion due to vascular impairment. Induction may be indicated with decreased placental function. A cesarean section may be performed if there is marked fetal distress or an increased risk of injury associated with macrosomia, such as shoulder dystocia.
Other types
There are several rare causes of diabetes mellitus that do not fit into type 1, type 2, or gestational diabetes; attempts to classify them remain controversial. Genetic mutations (autosomal or mitochondrial) can lead to defects in beta cell function. Abnormal insulin action may also been genetically determined in some cases. Any disease that causes extensive damage to the pancreas may lead to diabetes (for example, chronic pancreatitis and cystic fibrosis). Diseases associated with excessive secretion of insulin-antagonistic hormones can cause diabetes (which is typically resolved once the hormone excess is removed). Many drugs impair insulin secretion and some toxins damage pancreatic beta cells. The ICD-10 (1992) diagnostic entity, malnutrition-related diabetes mellitus (MRDM or MMDM, ICD-10 code E12), was deprecated by the World Health Organization when the current taxonomy was introduced in 1999.Cite error: The opening <ref> tag is malformed or has a bad name (see the help page).
Signs and symptoms
The classical triad of diabetes symptoms is polyuria, polydipsia and polyphagia, which are, respectively, frequent urination; increased thirst and consequent increased fluid intake; and increased appetite. Symptoms may develop quite rapidly (weeks or months) in type 1 diabetes, particularly in children. However, in type 2 diabetes the symptoms develop much more slowly and may be subtle or completely absent. Type 1 diabetes may also cause weight loss (despite normal or increased eating) and irreducible fatigue. These symptoms can also manifest in type 2 diabetes in patients whose diabetes is poorly controlled.
When the glucose concentration in the blood is raised beyond the renal threshold, reabsorption of glucose in the proximal renal tubuli is incomplete, and part of the glucose remains in the urine (glycosuria). This increases the osmotic pressure of the urine and inhibits the resorption of water by the kidney, resulting in increased urine production (polyuria) and increased fluid loss. Lost blood volume will be replaced osmotically from water held in body cells, causing dehydration and increased thirst.
Prolonged high blood glucose causes glucose absorption, which leads to changes in the shape of the lenses of the eyes, resulting in vision changes. Blurred vision is a common complaint leading to a diabetes diagnosis; type 1 should always be suspected in cases of rapid vision change whereas type 2 is generally more gradual, but should still be suspected.
Patients (usually with type 1 diabetes) may also present with diabetic ketoacidosis (DKA), an extreme state of metabolic dysregulation characterized by the smell of acetone on the patient's breath; a rapid, deep breathing known as Kussmaul breathing; polyuria; nausea; vomiting and abdominal pain; and any of many altered states of consciousness or arousal (such as hostility and mania or, equally, confusion and lethargy). In severe DKA, coma may follow, progressing to death. Diabetic ketoacidosis is a medical emergency and requires hospital admission.
A rarer but equally severe possibility is hyperosmolar nonketotic state, which is more common in type 2 diabetes and is mainly the result of dehydration due to loss of body water. Often, the patient has been drinking extreme amounts of sugar-containing drinks, leading to a vicious circle in regard to the water loss.
Genetics
Both type 1 and type 2 diabetes are at least partly inherited. Type 1 diabetes appears to be triggered by some (mainly viral) infections, or in a less common group, by stress or environmental exposure (such as exposure to certain chemicals or drugs). There is a genetic element in individual susceptibility to some of these triggers which has been traced to particular HLA genotypes (i.e., the genetic "self" identifiers relied upon by the immune system). However, even in those who have inherited the susceptibility, type 1 diabetes mellitus seems to require an environmental trigger. A small proportion of people with type 1 diabetes carry a mutated gene that causes maturity onset diabetes of the young (MODY).
There is a stronger inheritance pattern for type 2 diabetes. Those with first-degree relatives with type 2 have a much higher risk of developing type 2, increasing with the number of those relatives. Concordance among monozygotic twins is close to 100%, and about 25% of those with the disease have a family history of diabetes. Candidate genes include KCNJ11 (potassium inwardly rectifying channel, subfamily J, member 11), which encodes the islet ATP-sensitive potassium channel Kir6.2, and TCF7L2 (transcription factor 7–like 2), which regulates proglucagon gene expression and thus the production of glucagon-like peptide-1.[3] Moreover, obesity (which is an independent risk factor for diabetes) is strongly inherited.[10]
Various hereditary conditions may feature diabetes, for example myotonic dystrophy and Friedreich's ataxia. Wolfram's syndrome is an autosomal recessive neurodegenerative disorder that first becomes evident in childhood. It consists of diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, hence the acronym DIDMOAD.[11]
Pathophysiology
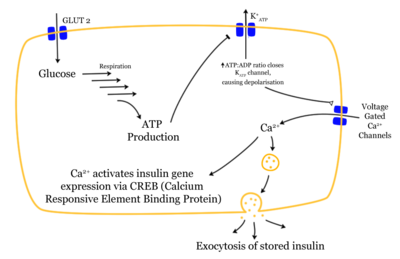
Insulin is the principal hormone that regulates uptake of glucose from the blood into most cells (primarily muscle and fat cells, but not central nervous system cells). Therefore deficiency of insulin or the insensitivity of its receptors plays a central role in all forms of diabetes mellitus.
Much of the carbohydrate in food is converted within a few hours to the monosaccharide glucose, the principal carbohydrate found in blood and used by the body as fuel. Some carbohydrates are not converted. Notable examples include fruit sugar (fructose) that is usable as cellular fuel, but it is not converted to glucose and does not participate in the insulin/glucose metabolic regulatory mechanism; additionally, the carbohydrate cellulose (though it is actually many glucose molecules in long chains) is not converted to glucose, as humans and many animals have no digestive pathway capable of handling cellulose.
Insulin is released into the blood by beta cells (β-cells) in the pancreas in response to rising levels of blood glucose after eating. Insulin is used by about two-thirds of the body's cells to absorb glucose from the blood for use as fuel, for conversion to other needed molecules, or for storage. Insulin is also the principal control signal for conversion of glucose to glycogen for internal storage in liver and muscle cells. Reduced glucose levels result both in the reduced release of insulin from the beta cells and in the reverse conversion of glycogen to glucose when glucose levels fall. Glucose thus recovered by the liver re-enters the bloodstream; muscle cells lack the necessary export mechanism.
Higher insulin levels increase many anabolic ("building up") processes such as cell growth and duplication, protein synthesis, and fat storage. Insulin is the principal signal in converting many of the bidirectional processes of metabolism from a catabolic to an anabolic direction, and vice versa. In particular, it is the trigger for entering or leaving ketosis (the fat burning metabolic phase).
If the amount of insulin available is insufficient, if cells respond poorly to the effects of insulin (insulin insensitivity or resistance), or if the insulin itself is defective, then glucose will not be handled properly by those body cells that require it or it will be stored appropriately in the liver and muscles. The net effect is persistent high levels of blood glucose, poor protein synthesis, and other metabolic derangements, such as acidosis.
Diagnosis
The diagnosis of type 1 diabetes, and many cases of type 2, is usually prompted by recent-onset symptoms of excessive urination (polyuria) and excessive thirst (polydipsia), often accompanied by weight loss. These symptoms typically worsen over days to weeks; about a quarter of people with new type 1 diabetes have developed some degree of diabetic ketoacidosis by the time the diabetes is recognized. The diagnosis of other types of diabetes is usually made in other ways. These include ordinary health screening; detection of hyperglycemia during other medical investigations; and secondary symptoms such as vision changes or unexplainable fatigue. Diabetes is often detected when a person suffers a problem that is frequently caused by diabetes, such as a heart attack, stroke, neuropathy, poor wound healing or a foot ulcer, certain eye problems, certain fungal infections, or delivering a baby with macrosomia or hypoglycemia.
Diabetes mellitus is characterized by recurrent or persistent hyperglycemia, and is diagnosed by demonstrating any one of the following:Cite error: The opening <ref> tag is malformed or has a bad name (see the help page).
- fasting plasma glucose level at or above 126 mg/dL (7.0 mmol/l).
- plasma glucose at or above 200 mg/dL (11.1 mmol/l) two hours after a 75 g oral glucose load as in a glucose tolerance test.
- random plasma glucose at or above 200 mg/dL (11.1 mmol/l).
A positive result, in the absence of clinical symptoms of diabetes, should be confirmed by another of the above-listed methods on a different day. Most physicians prefer to measure a fasting glucose level because of the ease of measurement and the considerable time commitment of formal glucose tolerance testing, which takes two hours to complete. According to the current definition, two fasting glucose measurements above 126 mg/dL (7.0 mmol/l) is considered diagnostic for diabetes mellitus.
Patients with fasting glucose levels between 110 and 125 mg/dL (6.1 and 7.0 mmol/l) are considered to have impaired fasting glycemia. Patients with plasma glucose at or above 140 mg/dL or 7.8 mmol/l two hours after a 75 g oral glucose load are considered to have impaired glucose tolerance. Of these two pre-diabetic states, the latter in particular is a major risk factor for progression to full-blown diabetes mellitus as well as cardiovascular disease.
While not used for diagnosis, an elevated level of glucose irreversibly bound to hemoglobin (termed glycosylated hemoglobin or HbA1c) of 6.0% or higher (the 2003 revised U.S. standard) is considered abnormal by most labs; HbA1c is primarily used as a treatment-tracking test reflecting average blood glucose levels over the preceding 90 days (approximately). However, some physicians may order this test at the time of diagnosis to track changes over time. The current recommended goal for HbA1c in patients with diabetes is <7.0%, which is considered good glycemic control, although some guidelines are stricter (<6.5%). People with diabetes who have HbA1c levels within this range have a significantly lower incidence of complications from diabetes, including retinopathy and diabetic nephropathy.[12]
Screening
Diabetes screening is recommended for many people at various stages of life, and for those with any of several risk factors. The screening test varies according to circumstances and local policy, and may be a random blood glucose test, a fasting blood glucose test, a blood glucose test two hours after 75 g of glucose, or an even more formal glucose tolerance test. Many healthcare providers recommend universal screening for adults at age 40 or 50, and often periodically thereafter. Earlier screening is typically recommended for those with risk factors such as obesity, family history of diabetes, high-risk ethnicity (Mestizo, Native American, African American, Pacific Island, and South Asian ancestry).
Many medical conditions are associated with diabetes and warrant screening. A partial list includes: high blood pressure, elevated cholesterol levels, coronary artery disease, past gestational diabetes, polycystic ovary syndrome, chronic pancreatitis, fatty liver, hemochromatosis, cystic fibrosis, several mitochondrial neuropathies and myopathies, myotonic dystrophy, Friedreich's ataxia, some of the inherited forms of neonatal hyperinsulinism. The risk of diabetes is higher with chronic use of several medications, including high-dose glucocorticoids, some chemotherapy agents (especially L-asparaginase), as well as some of the antipsychotics and mood stabilizers (especially phenothiazines and some atypical antipsychotics).
History
The term diabetes (Greek: διαβήτης) was coined by Aretaeus of Cappadocia. It is derived from the Greek word διαβαίνειν, diabaínein that literally means "passing through," or "siphon", a reference to one of diabetes' major symptoms—excessive urine production. In 1675, Thomas Willis added the word mellitus, from the Latin meaning "honey", a reference to the sweet taste of the urine. This sweet taste had been noticed in urine by the ancient Greeks, Chinese, Egyptians, and Indians. In 1776, Matthew Dobson confirmed that the sweet taste was because of an excess of a kind of sugar in the urine and blood of people with diabetes.[13]
The ancient Indians tested for diabetes by observing whether ants were attracted to a person's urine, and called the ailment "sweet urine disease" (Madhumeha). The Korean, Chinese, and Japanese words for diabetes are based on the same ideographs (糖尿病) which mean "sugar urine disease".
Although diabetes has been recognized since antiquity, and treatments of various efficacy have been known in various regions since the Middle Ages, and in legend for much longer, pathogenesis of diabetes has only been understood experimentally since about 1900.[14] The discovery of a role for the pancreas in diabetes is generally ascribed to Joseph von Mering and Oskar Minkowski, who in 1889 found that dogs whose pancreas was removed developed all the signs and symptoms of diabetes and died shortly afterwards.[15] In 1910, Sir Edward Albert Sharpey-Schafer suggested that people with diabetes were deficient in a single chemical that was normally produced by the pancreas—he proposed calling this substance insulin, from the Latin insula, meaning island, in reference to the insulin-producing islets of Langerhans in the pancreas.[14]
The endocrine role of the pancreas in metabolism, and indeed the existence of insulin, was not further clarified until 1921, when Sir Frederick Grant Banting and Charles Herbert Best repeated the work of Von Mering and Minkowski, and went further to demonstrate they could reverse induced diabetes in dogs by giving them an extract from the pancreatic islets of Langerhans of healthy dogs.[16] Banting, Best, and colleagues (especially the chemist Collip) went on to purify the hormone insulin from bovine pancreases at the University of Toronto. This led to the availability of an effective treatment—insulin injections—and the first patient was treated in 1922. For this, Banting and laboratory director MacLeod received the Nobel Prize in Physiology or Medicine in 1923; both shared their Prize money with others in the team who were not recognized, in particular Best and Collip. Banting and Best made the patent available without charge and did not attempt to control commercial production. Insulin production and therapy rapidly spread around the world, largely as a result of this decision.
The distinction between what is now known as type 1 diabetes and type 2 diabetes was first clearly made by Sir Harold Percival (Harry) Himsworth, and published in January 1936.[17]
Despite the availability of treatment, diabetes has remained a major cause of death. For instance, statistics reveal that the cause-specific mortality rate during 1927 amounted to about 47.7 per 100,000 population in Malta.[18]
Other landmark discoveries include:[14]
- identification of the first of the sulfonylureas in 1942
- reintroduction of the use of biguanides for Type 2 diabetes in the late 1950s. The initial phenformin was withdrawn worldwide (in the U.S. in 1977) due to its potential for sometimes fatal lactic acidosis and metformin was first marketed in France in 1979, but not until 1994 in the US.
- the determination of the amino acid sequence of insulin (by Sir Frederick Sanger, for which he received a Nobel Prize)
- the radioimmunoassay for insulin, as discovered by Rosalyn Yalow and Solomon Berson (gaining Yalow the 1977 Nobel Prize in Physiology or Medicine)[19]
- the three-dimensional structure of insulin (PDB: 2INS)
- Dr Gerald Reaven's identification of the constellation of symptoms now called metabolic syndrome in 1988
- demonstration that intensive glycemic control in type 1 diabetes reduces chronic side effects more as glucose levels approach 'normal' in a large longitudinal study,[20] and also in type 2 diabetics in other large studies
- identification of the first thiazolidinedione as an effective insulin sensitizer during the 1990s
Complications
The complications of diabetes are far less common and less severe in people who have well-controlled blood sugar levels.[21][22] In fact, the better the control, the lower the risk of complications. Hence, patient education, understanding, and participation is vital. Healthcare professionals treating diabetes also often attempt to address health issues that may accelerate the deleterious effects of diabetes. These include smoking (stopping), elevated cholesterol levels (control or reduction with diet, exercise or medication), obesity (even modest weight loss can be beneficial), high blood pressure (exercise or medication if needed), and lack of regular exercise.
Acute complications
- Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is an acute, dangerous complication and is always a medical emergency. Lack of insulin causes the liver to turn fat into ketone bodies, a fuel mainly for the brain. Large concentration of ketone bodies in the blood decreases the blood's pH, leading to most of the symptoms of DKA. On presentation at hospital, the patient in DKA is typically dehydrated and breathing both fast and deeply. Abdominal pain is common and may be severe. The level of consciousness is typically normal until late in the process, when lethargy (dulled or reduced level of alertness or consciousness) may progress to coma. Ketoacidosis can become severe enough to cause hypotension, shock, and death. Prompt proper treatment usually results in full recovery, though death can result from inadequate treatment, delayed treatment or from a variety of complications. Ketoacidosis occurs in type 1 and type 2 but is much more common in type 1.
- Nonketotic hyperosmolar coma
While not generally progressing to coma, this hyperosmolar nonketotic state (HNS) is another acute problem associated with diabetes mellitus. It has many symptoms in common with DKA, but an entirely different cause, and requires different treatment. In anyone with very high blood glucose levels (usually considered to be above 300 mg/dl (16 mmol/l)), water will be osmotically drawn out of cells into the blood. The kidneys will also be "dumping" glucose into the urine, resulting in concomitant loss of water, and causing an increase in blood osmolality. If fluid is not replaced (by mouth or intravenously), the osmotic effect of high glucose levels combined with the loss of water will eventually result in very high serum osmolality (i.e. dehydration). The body's cells will become progressively dehydrated as water is taken from them and excreted. Electrolyte imbalances are also common, and dangerous. This combination of changes, especially if prolonged, will result in symptoms of lethargy (dulled or reduced level of alertness or consciousness) and may progress to coma. As with DKA urgent medical treatment is necessary, especially volume replacement. This is the 'diabetic coma' which more commonly occurs in type 2 diabetics.
- Hypoglycemia
Hypoglycemia, or abnormally low blood glucose, is a complication of several diabetes treatments. It may develop if the glucose intake does not cover the treatment. The patient may become agitated, sweaty, and have many symptoms of sympathetic activation of the autonomic nervous system resulting in feelings similar to dread and immobilized panic. Consciousness can be altered, or even lost, in extreme cases, leading to coma and/or seizures, or even brain damage and death. In patients with diabetes, this can be caused by several factors, such as too much or incorrectly timed insulin, too much exercise or incorrectly timed exercise (exercise decreases insulin requirements) or not enough food (actually an insufficient amount of glucose-producing carbohydrates in food). In most cases, hypoglycemia is treated with sugary drinks or food. In severe cases, an injection of glucagon (a hormone with the opposite effects of insulin) or an intravenous infusion of glucose is used for treatment, but usually only if the person is unconscious. In hospital, intravenous dextrose is often used.
- Diabetic foot
Persons with poorly controlled diabetes often heal slowly, even from small cuts, abrasions, blisters, or separated callus (corns). The underlying cause of this healing problem is impaired circulation, which in diabetics is usually adequate to support normal tissue function but which may be inadequate for the additional circulation required to support tissue healing. In such cases, the damage, if unnoticed, left untreated, or failing to heal, can result in an infection. The resulting infection, in extreme cases, can necessitate to amputation.
Chronic complications
- Vascular disease
Chronic elevation of blood glucose level leads to damage of blood vessels (angiopathy). The endothelial cells lining the blood vessels take in more glucose than normal, since they don't depend on insulin. They then form more surface glycoproteins than normal, and cause the basement membrane to grow thicker and weaker. In diabetes, the resulting problems are grouped under "microvascular disease" (due to damage to small blood vessels) and "macrovascular disease" (due to damage to the arteries).
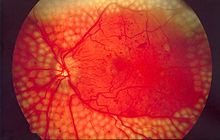
The damage to small blood vessels leads to a microangiopathy, which can cause one or more of the following:
- Diabetic retinopathy, growth of friable and poor-quality new blood vessels in the retina as well as macular edema (swelling of the macula), which can lead to severe vision loss or blindness. Retinal damage (from microangiopathy) makes it the most common cause of blindness among non-elderly adults in the US.
- Diabetic neuropathy, abnormal and decreased sensation, usually in a 'glove and stocking' distribution starting with the feet but potentially in other nerves, later often fingers and hands. When combined with damaged blood vessels this can lead to diabetic foot (see below). Other forms of diabetic neuropathy may present as mononeuritis or autonomic neuropathy. Diabetic amyotrophy is muscle weakness due to neuropathy.
- Diabetic nephropathy, damage to the kidney which can lead to chronic renal failure, eventually requiring dialysis. Diabetes mellitus is the most common cause of adult kidney failure worldwide in the developed world.
Macrovascular disease leads to cardiovascular disease, to which accelerated atherosclerosis is a contributor:
- Coronary artery disease, leading to angina or myocardial infarction ("heart attack")
- Stroke (mainly the ischemic type)
- Peripheral vascular disease, which contributes to intermittent claudication (exertion-related leg and foot pain) as well as diabetic foot.
- Diabetic myonecrosis ('muscle wasting')
Diabetic foot, often due to a combination of neuropathy and arterial disease, may cause skin ulcer and infection and, in serious cases, necrosis and gangrene. It is the most common cause of adult amputation, usually of toes and or feet, in the developed world.
Carotid artery stenosis does not occur more often in diabetes, and there appears to be a lower prevalence of abdominal aortic aneurysm. However, diabetes does cause higher morbidity, mortality and operative risks with these conditions.[23]
Treatment and management
Diabetes mellitus is currently a chronic disease, without a cure, and medical emphasis must necessarily be on managing/avoiding possible short-term as well as long-term diabetes-related problems. There is an exceptionally important role for patient education, dietetic support, sensible exercise, self glucose monitoring, with the goal of keeping both short-term blood glucose levels, and long term levels as well, within acceptable bounds. Careful control is needed to reduce the risk of long term complications. This can be achieved with combinations of diet, exercise and weight loss (type 2), various oral diabetic drugs (type 2 only), and insulin use (type 1 and increasingly for type 2 not responding to oral medication). In addition, given the associated higher risks of cardiovascular disease, lifestyle modifications should be undertaken to control blood pressure[24] and cholesterol by exercising more, smoking cessation, consuming an appropriate diet, wearing diabetic socks, and if necessary, taking any of several drugs to reduce pressure.
In countries using a general practitioner system, such as the United Kingdom, care may take place mainly outside hospitals, with hospital-based specialist care used only in case of complications, difficult blood sugar control, or research projects. In other circumstances, general practitioners and specialists share care of a patient in a team approach. Optometrists, podiatrists/chiropodists, dietitians, physiotherapists, clinical nurse specialists (eg, Certified Diabetes Educators), or nurse practitioners may jointly provide multidisciplinary expertise. In countries where patients must provide their own health care, the impact of out-of-pocket costs of diabetic care can be high. In addition to the medications and supplies needed, patients are often advised to receive regular consultation from a physician (eg, at least every three months).
Curing diabetes
Type 1 diabetes
There is no practical cure now for type 1 diabetes. The fact that type 1 diabetes is due to the failure of one of the cell types of a single organ with a relatively simple function (i.e. the failure of the islets of Langerhans) has led to the study of several possible schemes to cure this form diabetes mostly by replacing the pancreas or just the beta cells.[25] Only those type 1 diabetics who have received a kidney-pancreas transplant (when they have developed diabetic nephropathy) and become insulin-independent may now be considered "cured" from their diabetes. Still, they generally remain on long-term immunosuppressive drugs and there is a possibility that the immune system will mount a host versus graft response against the transplanted organ.[25]
Transplants of exogenous beta cells have been performed experimentally in both mice and humans, but this measure is not yet practical in regular clinical practice. Thus far, like any such transplant, it has provoked an immune reaction and long-term immunosuppressive drugs will be needed to protect the transplanted tissue.[26] An alternative technique has been proposed to place transplanted beta cells in a semi-permeable container, isolating and protecting them from the immune system. Stem cell research has also been suggested as a potential avenue for a cure since it may permit regrowth of Islet cells which are genetically part of the treated individual, thus perhaps eliminating the need for immuno-suppressants.[25] A 2007 trial of 15 newly diagnosed patients with type 1 diabetes treated with stem cells raised from their own bone marrow after immune suppression showed that the majority did not require any insulin treatment for prolonged periods of time.[27]
Microscopic or nanotechnological approaches are under investigation as well, in one proposed case with implanted stores of insulin metered out by a rapid response valve sensitive to blood glucose levels. At least two approaches have been demonstrated in vitro. These are, in some sense, closed-loop insulin pumps.
Type 2 diabetes
Type 2 diabetes can be cured by gastric bypass surgery in 80-100% of severely obese patients, and in some non-obese patients, usually within days after surgery. This is not an effect of weight loss, since it occurs long before weight loss.[28] After gastric bypass surgery for obesity, the death rate from all causes is reduced by up to 40%[29]
An article in New Scientist magazine [30] reports that a team of Italian, Brazilian and French doctors [31] have discovered that duodenal exclusion surgery can result in patient's type 2 diabetes "vanishing". The doctors believe that the duodenum is responsible for releasing a form of "molecular signal" that results in a cell's insulin resistance. The article also reports that several other medical teams in Mexico, Peru, Dominican Republic and India have also reported similar findings (though their reports have not yet been published). Clinical trials are now starting in China, Japan, the US, Italy and Belgium. A proposal has already been submitted to the American Association of Clinical Endocrinologists (AACE). The AACE comment that they are studying the evidence but have so far not reached a decision.
Prevention
Type 1 diabetes risk is known to depend upon a genetic predisposition based on HLA types (particularly types DR3 and DR4), an unknown environmental trigger, and an uncontrolled autoimmune response which attacks the insulin producing beta cells.[32] Research from the 1980s suggested that breastfeeding decreased the risk,[33]; various other nutritional risk factors are being studied, but few have a strong link with the development of type 1 diabetes.[34]
Type 2 diabetes risk can be reduced in many cases by making changes in diet and increasing physical activity.[35][36] A review article by the American Diabetes Association[37] recommends maintaining a healthy weight, getting at least 2½ hours of exercise per week (marathon intensity or duration is not needed; a brisk sustained walk appears sufficient), have a modest fat intake, and eating a good amount of fiber and whole grains. Magnesium may play a significant role in preventing Type 2 diabetes.[38] Although they do not recommend alcohol consumption as a preventative, they note that moderate alcohol intake (at or below one ounce of alcohol per day depending on body mass) may reduce the risk. They state that there is not enough consistent evidence that eating foods of low glycemic index is helpful, but nutritious, low glycemic-index (low carbohydrate) foods are encouraged.
Some studies have shown delayed progression to diabetes in predisposed patients through the use of metformin,[36] rosiglitazone,[39] or valsartan.[40] In patients on hydroxychloroquine for rheumatoid arthritis, incidence of diabetes was reduced by 77%.[41] Breastfeeding might also be correlated with the prevention of type 2 of the disease in mothers.[42]
As of late 2006, although there are many claims of nutritional cures, there is no credible demonstration for any. In addition, despite claims by some that vaccinations (eg, as for childhood diseases) may cause diabetes, there are no studies proving any such connection.
Aging
According to the American Diabetes Association, approximately 18.3% (8.6 million) of Americans age 60 and older have diabetes. [43] Diabetes mellitus prevalence increases with age, and the numbers of older persons with diabetes are expected to grow as the elderly population increases in number. The National Health and Nutrition Examination Survey (NHANES III) demonstrated that, in the population over 65 years old, 18% to 20% have diabetes, with 40% having either diabetes or its precursor form of impaired glucose tolerance.[44]
The way diabetes is managed changes with age. Insulin production decreases because of the age-related impairment of pancreatic beta cells. Insulin resistance increases due to the loss of lean tissue and the accumulation of fat, particularly intra-abdominal fat, and the decreased tissue sensitivity to insulin. Glucose tolerance progressively declines with age, and there is a high prevalence of type 2 diabetes and postchallenge hyperglycemia in the older population.[44] Age-related glucose intolerance in humans is often accompanied by insulin resistance, but circulating insulin levels are similar to those of younger people. [45]
Researchers and clinicians agree that treatment goals for older patient with diabetes need to be individualized and take into account health status, as well as life expectancy, level of dependence, and willingness to adhere to a treatment regimen. [46] Following evaluation, one of two levels of care can be recommended: symptom-preventing care or aggressive care. The decision is made jointly by the patient and the primary caregiver. [47]
Public health and policy
The 1989 Declaration of St Vincent was the result of international efforts to improve the care accorded to those with diabetes. Doing so is important both in terms of quality of life and life expectancy but also economically - expenses to diabetes have been shown to be a major drain on health- and productivity-related resources for healthcare systems and governments.
Several countries established more and less successful national diabetes programmes to improve treatment of the disease.[48]
Epidemiology and statistics
In 2006, according to the World Health Organization, at least 171 million people worldwide suffer from diabetes. Its incidence is increasing rapidly, and it is estimated that by the year 2030, this number will double. Diabetes mellitus occurs throughout the world, but is more common (especially type 2) in the more developed countries. The greatest increase in prevalence is, however, expected to occur in Asia and Africa, where most patients will likely be found by 2030. The increase in incidence of diabetes in developing countries follows the trend of urbanization and lifestyle changes, perhaps most importantly a "Western-style" diet. This has suggested an environmental (i.e., dietary) effect, but there is little understanding of the mechanism(s) at present, though there is much speculation, some of it most compellingly presented.
Diabetes is in the top 10, and perhaps the top 5, of the most significant diseases in the developed world, and is gaining in significance there and elsewhere (see big killers).
For at least 20 years, diabetes rates in North America have been increasing substantially. In 2005 there are about 20.8 million people with diabetes in the United States alone. According to the American Diabetes Association, there are about 6.2 million people undiagnosed and about 41 million people that would be considered prediabetic.[49] However, the criteria for diagnosing diabetes in the USA means that it is more readily diagnosed than in some other countries. The Centers for Disease Control has termed the change an epidemic. The National Diabetes Information Clearinghouse estimates that diabetes costs $132 billion in the United States alone every year. About 5%–10% of diabetes cases in North America are type 1, with the rest being type 2. The fraction of type 1 in other parts of the world differs; this is likely due to both differences in the rate of type 1 and differences in the rate of other types, most prominently type 2. Most of this difference is not currently understood. The American Diabetes Association point out the 2003 assessment of the National Center for Chronic Disease Prevention and Health Promotion (Centers for Disease Control and Prevention) that 1 in 3 Americans born after 2000 will develop diabetes in their lifetime.[50][49]
See also
References
- ^ L M Tierney, S J McPhee, M A Papadakis (2002). Current medical Diagnosis & Treatment. International edition. New York: Lange Medical Books/McGraw-Hill. p. 1203. ISBN 0-07-137688-7.
- ^ World Health Organisation Department of Noncommunicable Disease Surveillance (1999). "Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications" (PDF).
- ^ a b c Rother, KI (2007). "Diabetes Treatment — Bridging the Divide". N Engl J Med. 356 (15): 1499–1501.
- ^ "Other "types" of diabetes". American Diabetes Association. August 25, 2005.
- ^ "Diseases: Johns Hopkins Autoimmune Disease Research Center". Retrieved 2007-09-23.
- ^ "FDA Approves First Ever Inhaled Insulin Combination Product for Treatment of Diabetes". Retrieved 2007-09-09.
- ^ Eberhart, MS (November 19, 2004). "Prevalence of Overweight and Obesity Among Adults with Diagnosed Diabetes --- United States, 1988--1994 and 1999--2002". Morbidity and Mortality Weekly Report. 53 (45). Centers for Disease Control and Prevention: 1066–1068. Retrieved 2007-03-11.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Arlan Rosenbloom, Janet H Silverstein (2003). Type 2 Diabetes in Children and Adolescents: A Clinician's Guide to Diagnosis, Epidemiology, Pathogenesis, Prevention, and Treatment. American Diabetes Association,U.S. p. 1. ISBN 978-1580401555.
- ^ "Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group". Lancet. 352 (9131): 854–65. 1998. PMID 9742977.
- ^ Walley AJ, Blakemore AI, Froguel P (2006). "Genetics of obesity and the prediction of risk for health". Hum. Mol. Genet. 15 Spec No 2: R124-30. doi:10.1093/hmg/ddl215. PMID 16987875.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barrett TG (2001). "Mitochondrial diabetes, DIDMOAD and other inherited diabetes syndromes". Best Pract. Res. Clin. Endocrinol. Metab. 15 (3): 325–43. doi:10.1053/beem.2001.0149. PMID 11554774.
- ^ Genuth S (2006). "Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes". Endocr Pract. 12 Suppl 1: 34–41. PMID 16627378.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dobson, M. (1776). "Nature of the urine in diabetes". Medical Observations and Inquiries. 5: 298–310.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b c Patlak M (2002). "New weapons to combat an ancient disease: treating diabetes". FASEB J. 16 (14): 1853. PMID 12468446.
- ^ Von Mehring J, Minkowski O. (1890). "Diabetes mellitus nach pankreasexstirpation". Arch Exp Pathol Pharmakol. 26: 371–387.
- ^ Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA (1922). "Pancreatic extracts in the treatment of diabetes mellitus". Canad Med Assoc J. 12: 141–146.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Himsworth (1936). "Diabetes mellitus: its differentiation into insulin-sensitive and insulin-insensitive types". Lancet. i: 127–130.
- ^ Department of Health (Malta), 1897–1972:Annual Reports.
- ^ Yalow RS, Berson SA (1960). "Immunoassay of endogenous plasma insulin in man". J. Clin. Invest. 39: 1157–75. PMID 13846364.
- ^ "The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group". N Engl J Med. 329 (14): 977–86. 1993. PMID 8366922.
- ^ Nathan DM, Cleary PA, Backlund JY; et al. (2005). "Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes". N. Engl. J. Med. 353 (25): 2643–53. doi:10.1056/NEJMoa052187. PMID 16371630.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med 1995;122:561-8. PMID 7887548.
- ^ Weiss J, Sumpio B (2006). "Review of prevalence and outcome of vascular disease in patients with diabetes mellitus". Eur J Vasc Endovasc Surg. 31 (2): 143–50. PMID 16203161.
- ^ Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412-9. PMID 10938049.
- ^ a b c Vinik AI, Fishwick DT, Pittenger G (2004). "Advances in diabetes for the millennium: toward a cure for diabetes". MedGenMed : Medscape general medicine. 6 (3 Suppl): 12. PMID 15647717.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shapiro AM, Ricordi C, Hering BJ; et al. (2006). "International trial of the Edmonton protocol for islet transplantation". N. Engl. J. Med. 355 (13): 1318–30. doi:10.1056/NEJMoa061267. PMID 17005949.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Voltarelli, JC (2007). "Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus". JAMA. 297 (14): 1568–76. PMID 17426276.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rubino F, Gagner M (2002). "Potential of surgery for curing type 2 diabetes mellitus". Ann. Surg. 236 (5): 554–9. doi:10.1097/01.SLA.0000032951.37471.80. PMID 12409659.
- ^ Sjöström L, Narbro K, Sjöström CD; et al. (2007). "Effects of bariatric surgery on mortality in Swedish obese subjects". N. Engl. J. Med. 357 (8): 741–52. doi:10.1056/NEJMoa066254. PMID 17715408.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Vasonconcelos, Alberto (2007-09-01), "Could type 2 diabetes be reversed using surgery?", New Scientist, no. 2619, pp. 11–13, retrieved 2007-09-26.
- ^ Cohen, Dr Ricardo V. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. doi:10.1016/j.soard.2007.01.009.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|origdate=ignored (|orig-date=suggested) (help) - ^ Daneman D (2006). "Type 1 diabetes". Lancet. 367 (9513): 847–58. PMID 16530579.
- ^ Borch-Johnsen K, Joner G, Mandrup-Poulsen T, Christy M, Zachau-Christiansen B, Kastrup K, Nerup J (1984). "Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus. A hypothesis". Lancet. 2 (8411): 1083–6. PMID 6150150.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Virtanen S, Knip M (2003). "Nutritional risk predictors of beta cell autoimmunity and type 1 diabetes at a young age". Am J Clin Nutr. 78 (6): 1053–67. PMID 14668264.
- ^ Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson J, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle T, Uusitupa M, Tuomilehto J (2006). "Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study". Lancet. 368 (9548): 1673–9. PMID 17098085.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Knowler W, Barrett-Connor E, Fowler S, Hamman R, Lachin J, Walker E, Nathan D (2002). "Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin". N Engl J Med. 346 (6): 393–403. PMID 11832527.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ American Diabetes Association (2006). "Nutrition Recommendations and Interventions for Diabetes–2006". Diabetes Care. 29: 2140–57.
- ^ van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR (2006). "Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women". Diabetes Care. 29 (10): 2238–43. doi:10.2337/dc06-1014. PMID 17003299.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gerstein H, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman R (2006). "Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial". Lancet. 368 (9541): 1096–105. PMID 16997664.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kjeldsen SE, Julius S, Mancia G, McInnes GT, Hua T, Weber MA, Coca A, Ekman S, Girerd X, Jamerson K, Larochelle P, Macdonald TM, Schmieder RE, Schork MA, Stolt P, Viskoper R, Widimsky J, Zanchetti A; for the VALUE Trial Investigators (2006). "Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high-risk hypertensive patients: the VALUE trial". J Hypertens. 24 (7): 1405–1412. PMID 16794491.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wasko MC, Hubert HB, Lingala VB; et al. (2007). "Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis". JAMA. 298 (2): 187–93. doi:10.1001/jama.298.2.187. PMID 17622600.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB (2005). "Duration of lactation and incidence of type 2 diabetes". JAMA. 294 (20): 2601–10. PMID 16304074.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Seniors and Diabetes". Elderly And Diabetes - Diabetes and Seniors. LifeMed Media. 2006. Retrieved 2007-05-14.
- ^ a b Harris MI, Flegal KM, Cowie CC; et al. (1998). "Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994". Diabetes Care. 21 (4): 518–24. PMID 9571335.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Annette M. Chang and Jeffrey B. Halter (2003). "Aging and insulin secretion". AJP - Endocrinology and Metabolism. Retrieved 2007-05-14.
- ^ "Diabetes and Aging". Diabetes Dateline. National Institute of Diabetes and Digestive and Kidney Diseases. 2002. Retrieved 2007-05-14.
- ^ Kenneth L. Minaker (2006). "Treatment and Management of Diabetes Mellitus". Treatment of Diabetes - Geriatric Medicine. Armenian Health Network, Health.am. Retrieved 2007-05-14.
- ^ Dubois, HFW and Bankauskaite, V (2005). "Type 2 diabetes programmes in Europe" (PDF). Euro Observer. 7 (2): 5–6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b American Diabetes Association (2005). "Total Prevalence of Diabetes & Pre-diabetes". Retrieved 2006-03-17.
- ^ Narayan K, Boyle J, Thompson T, Sorensen S, Williamson D (2003). "Lifetime risk for diabetes mellitus in the United States". JAMA. 290 (14): 1884–90. PMID 14532317.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- American Diabetes Association
- Diabetes Australia-NSW
- Canadian Diabetes Association
- Diet, Nutrition and the prevention of chronic diseases (including diabetes) by a Joint WHO/FAO Expert consultation (2003)
- Centers for Disease Control Diabetes Section
- Diabetes UK
- Diabetes Health Institute
- Diabetes Institute for Immunology and Transplantion
- The Iacocca Foundation
- The Immunology of Diabetes Society
- International Diabetes Federation
- Juvenile Diabetes Research Foundation
- MedlinePlus Diabetes from the U.S. National Library of Medicine
- National Diabetes Education Program
- National Diabetes Information Clearinghouse
- World Health Organization fact sheet on diabetes
- World Health Organization—The Diabetes Programme
This template is no longer used; please see Template:Endocrine pathology for a suitable replacement
