Amygdala: Difference between revisions
m Reverted edits by 65.79.245.15 (talk) to last revision by Logan (HG) |
|||
| Line 142: | Line 142: | ||
* [[BELBIC]] |
* [[BELBIC]] |
||
* [[List of regions in the human brain]] |
* [[List of regions in the human brain]] |
||
Nic snook likes big baby boys |
|||
==References== |
==References== |
||
Revision as of 17:16, 3 October 2011
| Amygdala | |
|---|---|
 Location of the amygdala in the human brain | |
 Subdivision of the amygdala | |
| Details | |
| Identifiers | |
| Latin | corpus amygdaloideum |
| MeSH | D000679 |
| NeuroNames | 237 |
| NeuroLex ID | birnlex_1241 |
| TA98 | A14.1.09.402 |
| TA2 | 5549 |
| FMA | 61841 |
| Anatomical terms of neuroanatomy | |
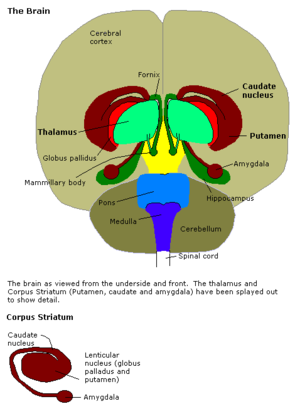
The amygdalae (/[invalid input: 'icon']əˈmɪɡdəliː/; singular: amygdala; also corpus amygdaloideum; Latin, from Greek αμυγδαλή, amygdalē, 'almond', 'tonsil', listed in the Gray's Anatomy as the nucleus amygdalæ)[1] are almond-shaped groups of nuclei located deep within the medial temporal lobes of the brain in complex vertebrates, including humans.[2] Shown in research to perform a primary role in the processing and memory of emotional reactions, the amygdalae are considered part of the limbic system.[3]
Anatomical subdivisions
The regions described as amygdala nuclei encompass several structures with distinct functional traits. Among these nuclei are the basolateral complex, the cortical nucleus, the medial nucleus, and the central nucleus. The basolateral complex can be further subdivided into the lateral, the basal, and the accessory basal nuclei.[3][4][5]

Anatomically, the amygdala[6] and more particularly, its central and medial nuclei,[7] have sometimes been classified as a part of the basal ganglia.
Connections
The amygdala sends impulses to the hypothalamus for activation of the sympathetic nervous system, to the thalamic reticular nucleus for increased reflexes, to the nuclei of the trigeminal nerve and the facial nerve, and to the ventral tegmental area, locus coeruleus, and laterodorsal tegmental nucleus for activation of dopamine, norepinephrine and epinephrine.[4]

The cortical nucleus is involved in the sense of smell and pheromone-processing. It receives input from the olfactory bulb and olfactory cortex. The lateral amygdalae, which send impulses to the rest of the basolateral complexes and to the centromedial nuclei, receive input from the sensory systems. The centromedial nuclei are the main outputs for the basolateral complexes, and are involved in emotional arousal in rats and cats.[4][5][8]
Emotional learning
In complex vertebrates, including humans, the amygdalae perform primary roles in the formation and storage of memories associated with emotional events. Research indicates that, during fear conditioning, sensory stimuli reach the basolateral complexes of the amygdalae, particularly the lateral nuclei, where they form associations with memories of the stimuli. The association between stimuli and the aversive events they predict may be mediated by long-term potentiation, a sustained enhancement of signalling between affected neurons.[3]
Memories of emotional experiences imprinted in reactions of synapses in the lateral nuclei elicit fear behavior through connections with the central nucleus of the amygdalae and the bed nuclei of the stria terminalis (BNST). The central nuclei are involved in the genesis of many fear responses, including freezing (immobility), tachycardia (rapid heartbeat), increased respiration, and stress-hormone release. Damage to the amygdalae impairs both the acquisition and expression of Pavlovian fear conditioning, a form of classical conditioning of emotional responses.[3]
The amygdalae are also involved in appetitive (positive) conditioning. It seems that distinct neurons respond to positive and negative stimuli, but there is no clustering of these distinct neurons into clear anatomical nuclei.[9] However, lesions of the central nucleus in the amygdala have been shown to reduce appetitive learning in rats. Lesions of the basolateral lesions do not exhibit the same effect.[10] Research like this indicates that different nuclei within the amygdala have different functions in appetitive conditioning.[11]
Memory modulation
The amygdala is also involved in the modulation of memory consolidation. Following any learning event, the long-term memory for the event is not formed instantaneously. Rather, information regarding the event is slowly assimilated into long-term (potentially life-long) storage over time, possibly via long-term potentiation. Recent studies suggest that, while the amygdala is not itself a long-term memory storage site, and learning can occur without it, one of its roles is to regulate memory consolidation in other brain regions.[12] Also, fear conditioning, a type of memory that is impaired following amygdala damage, is mediated in part by long-term potentiation.[13][14]
During the consolidation period, the memory can be modulated. In particular, it appears that emotional arousal following the learning event influences the strength of the subsequent memory for that event. Greater emotional arousal following a learning event enhances a person's retention of that event. Experiments have shown that administration of stress hormones to mice immediately after they learn something enhances their retention when they are tested two days later.[15]
The amygdalae, especially the basolateral nuclei, are involved in mediating the effects of emotional arousal on the strength of the memory for the event, as shown by many laboratories including that of James McGaugh. These laboratories have trained animals on a variety of learning tasks and found that drugs injected into the amygdala after training affect the animals' subsequent retention of the task. These tasks include basic classical conditioning tasks such as inhibitory avoidance, where a rat learns to associate a mild footshock with a particular compartment of an apparatus, and more complex tasks such as spatial or cued water maze, where a rat learns to swim to a platform to escape the water. If a drug that activates the amygdalae is injected into the amygdalae, the animals had better memory for the training in the task.[16] If a drug that inactivates the amygdalae is injected, the animals had impaired memory for the task.
Buddhist monks who do compassion meditation have been shown to modulate their amygdala, along with their temporoparietal junction and insula, during their practice.[17] In an fMRI study, more intensive insula activity was found in expert meditators than in novices.[18] Increased activity in the amygdala following compassion-oriented meditation may contribute to social connectedness.[19]
Amygdala activity at the time of encoding information correlates with retention for that information. However, this correlation depends on the relative "emotionalness" of the information. More emotionally-arousing information increases amygdalar activity, and that activity correlates with retention. Amygdala neurons show various types of oscillation during emotional arousal, such as theta activity. These synchronized neuronal events could promote synaptic plasticity (which is involved in memory retention) by increasing interactions between neocortical storage sites and temporal lobe structures involved in declarative memory.[20]
Research using Rorschach test blot 03 finds that the number of ‘‘unique responses’’ to this random figure links to larger sized amygdalae. The researchers note, "Since previous reports have indicated that unique responses were observed at higher frequency in the artistic population than in the nonartistic normal population, this positive correlation suggests that amygdalar enlargement in the normal population might be related to creative mental activity."[21]
Neuropsychological correlates of amygdala activity
Early research on primates provided explanations as to the functions of the amygdala, as well as a basis for further research. As early as 1888, rhesus monkeys with a lesioned temporal cortex (including the amygdala) were observed to have significant social and emotional deficits.[22] Heinrich Klüver and Paul Bucy later expanded upon this same observation by showing that large lesions to the anterior temporal lobe produced noticeable changes, including overreaction to all objects, hypoemotionality, loss of fear, hypersexuality, and hyperorality, a condition in which inappropriate objects are placed in the mouth. Some monkeys also displayed an inability to recognize familiar objects and would approach animate and inanimate objects indiscriminately, exhibiting a loss of fear towards the experimenters. This behavioral disorder was later named Klüver-Bucy syndrome accordingly.[23] Later studies served to focus on the amygdala specifically, as the temporal cortex encompasses a broad set of brain structures, making it difficult to find which ones specifically may have correlated with certain symptoms. Monkey mothers who had amygdala damage showed a reduction in maternal behaviors towards their infants, often physically abusing or neglecting them.[24] In 1981, researchers found that selective radio frequency lesions of the whole amygdala caused Klüver-Bucy Syndrome.[25]
With advances in neuroimaging technology such as MRI, neuroscientists have made significant findings concerning the amygdala in the human brain. A variety of data shows the amygdala has a substantial role in mental states, and is related to many psychological disorders. Some studies have shown children with anxiety disorders tend to have a smaller left amygdala. In the majority of the cases, there was an association between an increase in the size of the left amygdala with the use of SSRI's (antidepressant medication) or psychotherapy. The left amygdala has been linked to social anxiety, obsessive and compulsive disorders, and post traumatic stress, as well as more broadly to separation and general anxiety.[26] In a 2003 study, subjects with borderline personality disorder showed significantly greater left amygdala activity than normal control subjects. Some borderline patients even had difficulties classifying neutral faces or saw them as threatening.[27] Individuals with psychopathy show reduced autonomic responses, relative to comparison individuals, to instructed fear cues.[28] In 2006, researchers observed hyperactivity in the amygdala when patients were shown threatening faces or confronted with frightening situations. Patients with more severe social phobia showed a correlation with increased response in the amygdala.[29] Similarly, depressed patients showed exaggerated left amygdala activity when interpreting emotions for all faces, and especially for fearful faces. Interestingly, this hyperactivity was normalized when patients went on antidepressants.[30] By contrast, the amygdala has been observed to respond differently in people with bipolar disorder. A 2003 study found that adult and adolescent bipolar patients tended to have considerably smaller amygdala volumes and somewhat smaller hippocampal volumes.[31] Many studies have focused on the connections between the amygdala and autism.[32]
Studies in 2004 and 2006 showed that normal subjects exposed to images of frightened faces or faces of people from another race will show increased activity of the amygdala, even if that exposure is subliminal.[33][34] However, the amygdala is not necessary for the processing of fear-related stimuli, since persons in whom it is bilaterally damaged show rapid reactions to fearful faces, even in the absence of a functional amygdala.[35]
Recent research suggests that parasites, in particular toxoplasma, form cysts in the brain of rats, often taking up residence in the amygdala. This may provide clues as to how specific parasites may contribute to the development of disorders, including paranoia.[36]
Recent studies have suggested possible correlations between brain structure, including differences in hemispheric ratios and connection patterns in the amygdala, and sexual orientation. Homosexual men tend to exhibit more female-like patterns in the amygdala than do heterosexual males, just as homosexual females tend to show more male-like patterns in the amygdala than do heterosexual women. It is evident in humans that gender identity is programmed during fetal and neonatal development; however an individual's sexual orientation development in these early stages has not yet been determined.[37] It was observed that amygdala connections were more widespread from the left amygdala in homosexual males, as is also found in heterosexual females. Amygdala connections were more widespread from the right amygdala in homosexual females, as in heterosexual males.[37]
Social interaction
Amygdala volume correlates positively with both the size (the number of contacts a person has) and the complexity (the number of different groups to which a person belongs) of social networks.[38][39] Individuals with larger amygdalae had larger and more complex social networks. They were also better able to make accurate social judgments about other persons' faces.[40] It is hypothesized that larger amygdalae allow for greater emotional intelligence, enabling greater societal integration and cooperation with others.[41]
The amygdala processes reactions to violations concerning personal space. These reactions are absent in persons in whom the amygdala is damaged bilaterally.[42] Furthermore, the amygdala is found to be activated in fMRI when people observe that others are physically close to them, such as when a person being scanned knows that an experimenter is standing immediately next to the scanner, versus standing at a distance.[42]
Alcoholism and binge drinking
The amygdala appears to play a role in binge drinking, being damaged by repeated episodes of intoxication and withdrawal.[43] Alcoholism is associated with dampened activation in brain networks responsible for emotional processing, including the amygdala.[44] Protein kinase C-epsilon in the amygdala is important for regulating behavioral responses to morphine, ethanol, and controlling anxiety-like behavior. The protein is involved in controlling the function of other proteins and plays a role in development of the ability to consume a large amount of ethanol.[45][46]
Future studies
Future studies have been proposed to address the role of the amygdala in positive emotions, and the ways in which the amygdala networks with other brain regions.[47]
See also
References
- ^ amygdala - Definitions from Dictionary.com
- ^ University of Idaho College of Science (2004). "amygdala". Retrieved 2007-03-15.
- ^ a b c d Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, Habel U, Schneider F, Zilles K (2005). "Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps". Anat Embryol (Berl). 210 (5–6): 343–52. doi:10.1007/s00429-005-0025-5. PMID 16208455.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "rd" was defined multiple times with different content (see the help page). - ^ a b c Ben Best (2004). "The Amygdala and the Emotions". Retrieved 2007-03-15.
- ^ a b Solano-Castiella E, Anwander A, Lohmann G, Weiss M, Docherty C, Geyer S, Reimer E, Friederici AD, Turner R (2010). "Diffusion tensor imaging segments the human amygdala in vivo". Neuroimage. 49 (4): 2958–65. doi:10.1016/j.neuroimage.2009.11.027. PMID 19931398.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ See Amygdala in the BrainInfo database
- ^ Larry W. Swanson and Gorica D. Petrovich (1998). "What is the amygdala?". Trends in Neurosciences. 21 (8): 323–331. doi:10.1016/S0166-2236(98)01265-X.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Michael McDannald, Erin Kerfoot, Michela Gallagher, and Peter C. Holland (2005). "Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting". Behav Neurosci. 119 (1): 202–212. doi:10.1037/0735-7044.119.1.202. PMC 1255918. PMID 15727525.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Paton, Joseph (25 November 2005). "The primate amygdala represents the positive and negative value of visual stimuli during learning". Nature. 439 (7078): 865–870. doi:10.1038/nature04490. PMC 2396495. PMID 16482160.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Parkinson, John; Robbins, Trevor; Everitt, Barry (9 OCT 2008). (Document)Template:Inconsistent citations
{{cite document}}: Check date values in:|date=(help); Cite document requires|publisher=(help); Missing or empty|title=(help); Unknown parameter|accessdate=ignored (help)CS1 maint: postscript (link) - ^ See recent TINS article by Balleine and Killcross (2006)
- ^ Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Maren S.Trends Neurosci. 1999 Dec;22(12):561-7. Review.
- ^ Killcross S, Robbins T, Everitt B (1997). "Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala". Nature. 388 (6640): 377–80. doi:10.1038/41097. PMID 9237754.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Blair HT, Schafe GE, Bauer EP, Rodrigues SM, and Ledoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis for fear conditioning. Learn Mem 8: 229–242, 2001
- ^ "Researchers Prove A Single Memory Is Processed In Three Separate Parts Of The Brain" http://www.sciencedaily.com/releases/2006/02/060202182107.htm
- ^ Ferry B, Roozendaal B, McGaugh J (1999). "Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala". Biol Psychiatry. 46 (9): 1140–52. doi:10.1016/S0006-3223(99)00157-2. PMID 10560021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Cultivating compassion: Neuroscientific and behavioral approaches" a talk given by Richard J. Davidson found online at http://ccare.stanford.edu/node/25
- ^ Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ (2008) Regulation of the Neural Circuitry of Emotion by Compassion Meditation: Effects of Meditative Expertise. PLoS ONE 3(3): e1897. http://brainimaging.waisman.wisc.edu/~lutz/Lutz_al_Compassion_expertise_Plos_One_2008.pdf
- ^ Hutcherson, C.A., Seppala, E.M., & Gross, J.J. (2008). Loving-kindness meditation increases social connectedness. Emotion, 8(5), 720-724.http://spl.stanford.edu/pdfs/Hutcherson_08_2.pdf
- ^ Paré D., Collins D.R., Pelletier J.G. (2002). "Amygdala oscillations and the consolidation of emotional memories". Trends in Cognitive Sciences. 6 (7): 306–314. doi:10.1016/S1364-6613(02)01924-1. PMID 12110364.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Asari T, Konishi S, Jimura K, Chikazoe J, Nakamura N, Miyashita Y (2010). "Amygdalar enlargement associated with unique perception". Cortex. 46 (1): 94–99. doi:10.1016/j.cortex.2008.08.001. PMID 18922517.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brown, S. & Shafer, E. (1888). "An investigation into the functions of the occipital and temporal lobes of the monkey's brain". Philosophical Transactions of the Royal Society of London: Biological Sciences. 179: 303–327. doi:10.1098/rstb.1888.0011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kluver, H. & Bucy, P. (1939). "Preliminary analysis of function of the temporal lobe in monkeys". Archives of Neurology. 42: 979–1000.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bucher, K., Myersn, R., Southwick, C. (1970). "Anterior temporal cortex and maternal behaviour in monkey". Neurology. 20 (4): 415. PMID 4998075.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Aggleton, JP. & Passingham, RE. (1981). "Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta)". Journal of Comparative and Physiological Psychology. 95 (6): 961–977. doi:10.1037/h0077848. PMID 7320283.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://pn.psychiatryonline.org/content/40/9/37.full
- ^ Donegan; Sanislow, CA; Blumberg, HP; Fulbright, RK; Lacadie, C; Skudlarski, P; Gore, JC; Olson, IR; McGlashan, TH; et al. (2003). "Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation". Biological Psychiatry. 54 (11): 1284–1293. doi:10.1016/S0006-3223(03)00636-X. PMID 14643096.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ R. J. R. Blair (23 April 2008). "The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy". Philosophical Transactions of the Royal Society. 363 (1503): 2557–2565. doi:10.1098/rstb.2008.0027. PMC 2606709. PMID 18434283.
- ^ Studying Brain Activity Could Aid Diagnosis Of Social Phobia. Monash University. January 19, 2006.
- ^ Sheline; Barch, DM; Donnelly, JM; Ollinger, JM; Snyder, AZ; Mintun, MA; et al. (2001). "Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study". Biological Psychiatry. 50 (9): 651–658. doi:10.1016/S0006-3223(01)01263-X. PMID 11704071.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Blumberg; Kaufman, J; Martin, A; Whiteman, R; Zhang, JH; Gore, JC; Charney, DS; Krystal, JH; Peterson, BS; et al. (2003). "Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder". Arch Gen Psychiatry. 60 (12): 1201–8. doi:10.1001/archpsyc.60.12.1201. PMID 14662552.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Schultz RT (2005). "Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area". Int J Dev Neurosci. 23 (2–3): 125–41. doi:10.1016/j.ijdevneu.2004.12.012. PMID 15749240.
- ^ Williams, Leanne M. (2006). "Amygdala-prefrontal dissociation of subliminal and supraliminal fear". Human Brain Mapping. 27 (8): 652–661. doi:10.1002/hbm.20208. PMID 16281289.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Brain Activity Reflects Complexity Of Responses To Other-race Faces, Science Daily, 14 December 2004
- ^ Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R (2009). "Intact rapid detection of fearful faces in the absence of the amygdala". Nat Neurosci. 12 (10): 1224–12225. doi:10.1038/nn.2380. PMC 2756300. PMID 19718036.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vyas; Kim, SK; Giacomini, N; Boothroyd, JC; Sapolsky, RM; et al. (2007). "Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors". Proc Natl Acad Sci U S A. 104 (15): 6442–7. doi:10.1073/pnas.0608310104. PMC 1851063. PMID 17404235.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b http://www.pnas.org/content/105/30/10273.full
- ^ Bickart, Kevin C., Wright, Christopher I., Dautoff, Rebecca J., Dickerson, Bradford C., Barrett, Lisa Feldman (2010). "Amygdala volume and social network size in humans". Nature neuroscience. 14 (2): 163–164. doi:10.1038/nn.2724. PMC 3079404. PMID 21186358. Retrieved 2010-12- 27.
{{cite journal}}: Check date values in:|accessdate=(help); Cite has empty unknown parameters:|laydate=,|laysummary=, and|laysource=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Szalavitz, Maia (December 28, 2010). "How to Win Friends: Have a Big Amygdala?". Time Healthland. Time.com. Retrieved December 30, 2010.
- ^ Bzdok, D., Langner, R., Caspers, S., Kurth, F., Habel, U., Zilles, K., Laird, A., Eickhoff, S.B., 2011. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Struct Funct 215, 209-223.
- ^ Buchanan, T.W., Tranel, D. & Adolphs, R. in The Human Amygdala (eds. Whalen, P.J. & Phelps, E.A.) 289–318 (Guilford, New York, 2009).
- ^ a b Kennedy DP, Gläscher J, Tyszka JM, Adolphs R (2009). "Personal space regulation by the human amygdala". Nat Neurosci. 12 (10): 1226–1227. doi:10.1038/nn.2381. PMC 2753689. PMID 19718035.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://rstb.royalsocietypublishing.org/content/363/1507/3169.long
- ^ Marinkovic K (2009). "Alcoholism and dampened temporal limbic activation to emotional faces". Alcohol Clin Exp Res. 33 (11): 1880–92. doi:10.1111/j.1530-0277.2009.01026.x. PMID 19673745.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WP9-4NKXWJX-4&_user=2251208&_coverDate=06%2F30%2F2007&_rdoc=1&_fmt=high&_orig=gateway&_origin=gateway&_sort=d&_docanchor=&view=c&_acct=C000056758&_version=1&_urlVersion=0&_userid=2251208&md5=c865afb9018b406bda26bb7c7ef964da&searchtype=a
- ^ Lesscher et al, Amygdala protein kinase C epsilon controls alcohol consumption http://www3.interscience.wiley.com/journal/122210376/abstract?CRETRY=1&SRETRY=0
- ^ Gazzaniga, M.S., Ivry, R.B., & Mangun, G.R. (2009). Cognitive neuroscience: the biology of the mind. NY: W.W.Norton&Company.
External links
 Media related to amygdala at Wikimedia Commons
Media related to amygdala at Wikimedia Commons- Stained brain slice images which include the "amygdala " at the BrainMaps project
- Scholarpedia article by Dr. Joseph LeDoux
- NIF Search - Amygdala via the Neuroscience Information Framework
