Management of multiple sclerosis: Difference between revisions
WhatamIdoing (talk | contribs) Section heading |
→Therapies under investigation: Copied from multiple sclerosis article |
||
| Line 143: | Line 143: | ||
{{Main|Therapies under investigation for multiple sclerosis}} |
{{Main|Therapies under investigation for multiple sclerosis}} |
||
[[Image:Alemtuzumab Fab 1CE1.png|thumb|Chemical structure of [[alemtuzumab]]]] |
|||
Scientists continue their extensive efforts to create new and better therapies for MS. There are a number of treatments under investigation that may curtail attacks or improve function. Some of these treatments involve the combination of drugs that are already in use for multiple sclerosis, such as the joint administration of [[mitoxantrone]] and [[glatiramer acetate]] (''Copaxone'').<ref>[http://www.mxga-mstrial.co.uk/ United Kingdom early Mitoxantrone Copaxone trial.] Onyx Healthcare (2006-01-01). Retrieved on 2007-09-02.</ref> |
|||
Research directions on MS treatments include investigations of MS pathogenesis and heterogeneity; research of more effective, convenient, or tolerable new treatments for RRMS; creation of therapies for the progressive subtypes; neuroprotection strategies; and the search for effective symptomatic treatments.<ref name="pmid19597083">{{cite journal |author=Cohen JA |title=Emerging therapies for relapsing multiple sclerosis |journal=Arch. Neurol. |volume=66 |issue=7 |pages=821–8 |year=2009 |month=July |pmid=19597083 |doi=10.1001/archneurol.2009.104 |url=}}</ref> |
|||
Advances during the last decades has led to the recent approval of several oral drugs. These drugs are expected to gain in popularity and frequency of use at the expense of previously existing therapies.<ref name="pmid21425270"/> Further oral drugs are still under investigation, the most notable example being [[laquinimod]], which was announced in August 2012 to be the focus of a third phase III trial after mixed results in the previous ones.<ref>{{cite news|last=Jeffrey|first=susan|title=CONCERTO: A Third Phase 3 Trial for Laquinimod in MS|url=http://www.medscape.com/viewarticle/768902|accessdate=21 May 2013|newspaper=Medscape Medical News|date=09 Aug 2012}}</ref> Similarly, Other studies are aimed to improve efficacy and ease of use of already existing therapies through the use of novel preparations. Such is the case the [[PEGylation|PEGylated]] version of interferon-β-1a, that has a longer life than normal interferon and therefore it is being studied if given at less frequent doses has a similar efficacy than the existing product.<ref name="pmid22201341">{{cite journal |author=Kieseier BC, Calabresi PA |title=PEGylation of interferon-β-1a: a promising strategy in multiple sclerosis |journal=CNS Drugs |volume=26 |issue=3 |pages=205–14 |year=2012 |month=March |pmid=22201341 |doi=10.2165/11596970-000000000-00000 |url=}}</ref><ref name=PEG>{{cite news|last=Biogen Idec|title=Biogen Idec Announces Positive Top-Line Results from Phase 3 Study of Peginterferon Beta-1a in Multiple Sclerosis-Press release|url=http://www.biogenidec.com/press_release_details.aspx?ID=5981&ReqId=1777510|accessdate=21 May 2013|date=24 Jan 2013}}</ref> Request for approval of ''peginterferon beta-1a'' is expected during 2013.<ref name=PEG/> |
|||
However most treatments already in clinical trials involve drugs that are used in other diseases. These are the cases of [[alemtuzumab]] (''Campath''),<ref>[http://www.genzyme.com/corp/media/GENZ%20PR-050207.asp Genzyme and Bayer HealthCare Announce Detailed Interim Two-Year Alemtuzumab in Multiple Sclerosis Data Presented at AAN.]{{dead link|date=August 2012}} Genzyme (2007-02-01). Retrieved on 2007-09-02.</ref> [[daclizumab]] (''Zenapax''),<ref>[http://www.pdl.com/index.cfm?navId=49 Daclizumab.] PDL Biopharma (2006-01-01). Retrieved on 2007-09-02. {{dead link| date=May 2010 | bot=DASHBot}} {{Wayback|url=http://www.pdl.com/index.cfm?navId=49|date =20070915190712|bot=DASHBot}}</ref> or [[inosine]],<ref>[http://www.clinicaltrials.gov/ct/show/NCT00067327 Treatment of Multiple Sclerosis Using Over the Counter Inosine.] ClinicalTrials.gov (2006-03-16). Retrieved on 2007-09-02.</ref> Alemtuzumab performed better than interferon beta-1a in relapsing-remitting MS reducing disability, imaging abnormalities and frequence of relapses, at the cost of increased [[autoimmunity]] problems. These included three cases of [[immune thrombocytopenic purpura|thrombocytopenic purpura]] which led to the suspension of the therapy.<ref name="pmid1234567">{{cite journal |author=The CAMMS223 Trial Investigators |title=Alemtuzumab vs. Interferon Beta-1a in Early Multiple Sclerosis |journal=N Engl J Med |volume=359 |issue=17 |pages=1786–1801 |year=2008 |pmid=18946064 |doi= 10.1056/NEJMoa0802670|url=}}</ref> Other drugs in clinical trials have been designed specifically for MS, such as [[fingolimod]],<ref>[http://www.clinicaltrials.gov/ct/show/NCT00289978 Efficacy and Safety of Fingolimod in Patients With Relapsing-Remitting Multiple Sclerosis.] ClinicalTrials.gov (2006-02-09). Retrieved on 2007-09-02.</ref> [[laquinimod]],<ref name="pmid15781813">{{cite journal |author=Polman C, Barkhof F, Sandberg-Wollheim M, Linde A, Nordle O, Nederman T |title=Treatment with laquinimod reduces development of active MRI lesions in relapsing MS |journal=Neurology |volume=64 |issue=6 |pages=987–91 |year=2005 |pmid=15781813 |doi=10.1212/01.WNL.0000154520.48391.69}}</ref> or ''[[Neurovax]]''.<ref>{{cite journal |author=Darlington CL |title=Technology evaluation: NeuroVax, Immune Response Corp |journal=Curr. Opin. Mol. Ther. |volume=7 |issue=6 |pages=598–603 |year=2005 |pmid=16370383 |doi=}}</ref> |
|||
Monoclonal antibodies, which are drugs of the same family as natalizumab, have also raised high levels of interest and research. [[Alemtuzumab]], [[daclizumab]] and [[CD20]] monoclonal antibodies such as [[rituximab]], [[ocrelizumab]] and [[ofatumumab]] have all shown some benefit and are under study as potential treatments for MS.<ref name="pmid22224673">{{cite journal |author=Saidha S, Eckstein C, Calabresi PA |title=New and emerging disease modifying therapies for multiple sclerosis |journal=Ann. N. Y. Acad. Sci. |volume=1247 |issue= |pages=117–37 |year=2012 |month=January |pmid=22224673 |doi=10.1111/j.1749-6632.2011.06272.x |url=}}</ref> Nevertheless their use has also been accompanied by the appearance of potentially dangerous adverse effects, most importantly opportunistic infections.<ref name="pmid21425270"/> Related to these investigations is the recent development of a test against [[JC virus]] antibodies which might help to predict what patients are at a greater risk of developing progressive multifocal leukoencephalopathy when taking natalizumab.<ref name="pmid21425270"/> While monoclonal antibodies are probably going to have some role in the treatment of the disease in the future, it is believed that it will be small due to the risks associated to them.<ref name="pmid21425270"/> |
|||
In humans, [[BCG vaccine]], the common, live, attenuated vaccine against [[tuberculosis]], has substantially reduced recurrence of symptoms in multiple sclerosis patients.<ref name="Ristori_1999"> |
|||
{{cite journal | journal=Neurology | year=1999 | month=Oct |volume=53|issue=7|pages=1588–1589 | |
|||
title=Use of Bacille Calmette-Guèrin (BCG) in multiple sclerosis | |
|||
last1=Ristori|first1=G |coauthors= Buzzi MG, Sabatini U, Giugni E, Bastianello S, Viselli F, Buttinelli C, Ruggieri S, Colonnese C, Pozzilli C, Salvetti M | pmid=10534275 |
|||
}}</ref> |
|||
The frequency of new enhancing lesions as detected by Gd-enhanced MRI was reduced by more than half in 12 patients, comparing the six-month run-in phase to the six-month post BCG phase of the experiment. Persistence at subsequent MR scan was reduced from 18 to 1 lesion, and evolution to black holes was reduced from 28 to 6 lesions.<ref>{{cite journal | doi=10.1007/s00415-003-0967-6 | last1=Paolillo|first1=A | coauthors= Buzzi MG, Giugni E, Sabatini U, Bastianello S, Pozzilli C, Salvetti M, Ristori G. | title=The effect of Bacille Calmette-Guérin on the evolution of new enhancing lesions to hypointense T1 lesions in relapsing remitting MS | journal=J Neurol | year=2003 | month=February|volume=250|issue=2|pages=247–248 | pmid=12622098 |
|||
}}</ref> |
|||
The conventional explanation of such protection is that parasites (including bacteria) modulate the sensitivity of the immune system. BCG appears safe as a treatment for multiple sclerosis.<ref name="Ristori_1999"/><ref> |
|||
{{cite journal | journal=Neurology | year=2002|month=Dec|volume=59|issue=12|pages=1837–1843 | |
|||
title=Immunization and MS: a summary of published evidence and recommendations | |
|||
last1=Rutschmann|first1=OT | last2=McCrory|first2=DC | last3=Matchar|first3=DB |pmid=12499473 | author4=Immunization Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines |
|||
}}</ref> |
|||
Another research strategy is to evaluate the [[combination therapy|combined effectiveness]] of two or more drugs.<ref name="pmid21111490">{{cite journal |author=Milo R, Panitch H |title=Combination therapy in multiple sclerosis |journal=J. Neuroimmunol. |volume=231 |issue=1-2 |pages=23–31 |year=2011 |month=February |pmid=21111490 |doi=10.1016/j.jneuroim.2010.10.021 |url=}}</ref> The main rationale for polytherapy in MS is that the involved treatments target different mechanisms of the disease and therefore their use is not neccesarily exclusive.<ref name="pmid21111490"/> Moreover [[Synergy#Drug_synergy|synergies]], in which a drug potentiates the effect of another are also possible. Nevertheless there can also appear important drawbacks such as antagonizing mechanisms of action or potentiation of deleterious secondary effects.<ref name="pmid21111490"/> While there have been several clinical trials of combined therapy none has shown positive enough effects to merit the consideration as a viable treatment for MS.<ref name="pmid21111490"/> |
|||
Many anecdotes are found on the Internet about the effectiveness of [[low dose naltrexone]] for MS, but no published scientific studies or case reports address its effectiveness.<ref>{{cite journal |author= Patel PN |title= Low-dose naltrexone for treatment of multiple sclerosis: clinical trials are needed |journal= Ann Pharmacother |volume=41 |issue=9 |pages=1549–50 |year=2007 |pmid=17623758 |doi=10.1345/aph.1H083}}</ref> Statins administered in combination with interferons have not shown an improved efficacy over interferons only.<ref name='"pmid22899744"'>{{cite journal|last=Bhardwaj|first=S.|coauthors=Coleman, C. I.; Sobieraj, D. M.|title=Efficacy of statins in combination with interferon therapy in multiple sclerosis: A meta-analysis|journal=American Journal of Health-System Pharmacy|date=16 August 2012|volume=69|issue=17|pages=1494–1499|doi=10.2146/ajhp110675|pmid=22899744}}</ref> Finally, there are also many early-stage investigations that in the future may emerge as new treatments. Examples of these are the studies trying to understand the influence of ''[[Chlamydophila pneumoniae]]'' or [[vitamin D]] in the origin of the disease,<ref>{{cite journal |author=Sriram S, Yao SY, Stratton C, Moses H, Narayana PA, Wolinsky JS |title=Pilot study to examine the effect of antibiotic therapy on MRI outcomes in RRMS |journal=J. Neurol. Sci. |volume=234 |issue=1–2 |pages=87–91 |year=2005 |pmid=15935383 |doi=10.1016/j.jns.2005.03.042}}</ref><ref>{{cite journal |author=Munger KL, Zhang SM, O'Reilly E, ''et al.'' |title=Vitamin D intake and incidence of multiple sclerosis |journal=Neurology |volume=62 |issue=1 |pages=60–5 |year=2004 |pmid=14718698 |doi=}}</ref> or preliminary investigations on the use of [[helminthic therapy]],<ref>{{cite journal |author= Elliott DE, Summers RW, Weinstock JV |title= Helminths as governors of immune-mediated inflammation |journal= Int J Parasitol |volume=37 |issue=5 |pages=457–64 |year=2007 |pmid=17313951 |doi=10.1016/j.ijpara.2006.12.009}}</ref> or [[angioplasty]] and [[stent|venous stents]] based on the theory that an [[Chronic cerebrospinal venous insufficiency|incorrect blood drainage system]] weakens the blood–brain barrier.<ref name="pmid21106999">{{cite journal |author=Zamboni P, Galeotti R |title=The chronic cerebrospinal venous insufficiency syndrome |journal=Phlebology |volume=25 |issue=6 |pages=269–79 |year=2010 |pmid=21106999 |doi=10.1258/phleb.2010.009083 |url=}}</ref> [[Parenchyma]]l [[stem cell]]s have a great potential as a therapy since they could theoretically help to modulate the immune system to stop axonal damage and even repair the central nervous system. Nevertheless [[Stem-cell therapy|such therapies]] are still at the initial stages of research.<ref name='"pmid22642335"'>{{cite journal|last=Auletta|first=Jeffery J|coauthors=Bartholomew, Amelia M; Maziarz, Richard T; Deans, Robert J; Miller, Robert H; Lazarus, Hillard M; Cohen, Jeffrey A|title=The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis|journal=Immunotherapy|date=1 May 2012|volume=4|issue=5|pages=529–547|doi=10.2217/imt.12.41|pmid=22642335}}</ref> |
|||
Finally, regarding neuroprotective and specially regenerative treatments, such as [[stem cell therapy]], while their research is considered of high importance at the moment they are only a promise of future therapeutic approaches.<ref name="pmid23039386">{{cite journal |author=Luessi F, Siffrin V, Zipp F |title=Neurodegeneration in multiple sclerosis: novel treatment strategies |journal=Expert Rev Neurother |volume=12 |issue=9 |pages=1061–76; quiz 1077 |year=2012 |month=September |pmid=23039386 |doi=10.1586/ern.12.59 |url=http://www.expert-reviews.com/doi/full/10.1586/ern.12.59}}</ref> Likewise, there are not any effective treatments for the progressive variants of the disease. Many of the newest drugs as well as those under development are probably going to be evaluated as therapies for PPMS or SPMS, and their improved effectiveness when compared with previously existing drugs may eventually lead to a positive result in these groups of patients.<ref name="pmid21425270"/> |
|||
The Percutaneous Transluminal Angioplasty (PTA) that was proposed by Paolo Zamboni in 2009 as a treatment for chronic cerebrospinal venous insufficiency (CCSVI), is a controversial condition characterized anomalies of cerebrospinal veins that interfere with venous drainage from the brain.<ref name="CCSVI">{{cite journal|authors=Awad, A. M., Marder, E., Milo, R., & Stüve, O|title=Multiple sclerosis and chronic cerebrospinal venous insufficiency: A critical review|year=2011|journal=Therapeutic Advances in Neurological Disorders|volume=4|issue=4|231-235}}</ref> However, there is limited data supporting these endovascular interventions for CCSVI also known as “liberation procedures” because researchers are not able to reproduce Zamboni's results. The first report from Zamboni was an open-label study of 65 patients with MS who met criteria for CCSVI where they found that PTA was associated with significant improvement in some MS outcome measures, particularly for 35 patients with relapsing-remitting MS.<ref>{{cite journal|authours=Zamboni, P., Galeotti, R., Menegatti, E., Malagoni, A. M., Gianesini, S., Bartolomei, et.al.|title=A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency|journal=Journal of Vascular Surgery|volume=50|issue=6|pages=1348–1358}}</ref> A subsequent study of 31 patients with MS and CCSVI reported that treatment with balloon dilatation of extracranial veins was associated with improvement in fatigue scores.<ref>{{cite journal|authors=Malagoni AM, Galeotti R, Menegatti E, Manfredini F, Basaglia N, Salvi F, Zamboni P|title=Is chronic fatigue the symptom of venous insufficiency associated with multiple sclerosis? A longitudinal pilot study|year=2010|volume=29|issue=2|pages=176|journal=Int Angiol}}</ref> Criticisms of Zamboni's approach include the lack of control groups, the small number of patients, the study was not blinded and the results observed could be due to the placebo effect as all of the patients who were treated were previously on disease-modifying treatments.<ref name="CCSVI" /> Several complications have occurred due to the lack of follow-up of treatments including deaths due to the migration of stents into the right ventricle and deaths due to brain haemorrhage while on anticoagulant warfarin.<ref name="CCSVI" /> Currently, a $6 million study led by Dr Anthony Traboulsee is being conducted in Vancouver and Montreal where a total of 100 MS patients is randomly selected to have either the venoplasty or the placebo venoplasty and after one year the groups will be switched so everyone will receive treatment and be assessed without the patients' knowledge to eliminate the placebo effect and bias.<ref>Multiple sclerosis: Canada launching clinical trial of controversial treatment developed by Zamboni http://www.thestar.com/news/2012/09/28/multiple_sclerosis_canada_launching_clinical_trial_of_controversial_treatment_developed_by_zamboni.html</ref> |
|||
===Chronic cerebrospinal venous insufficiency=== |
|||
{{Main|Chronic cerebrospinal venous insufficiency}} |
|||
In 2008, vascular surgeon [[Paolo Zamboni]] suggested that MS involves a [[Circulatory system|vascular]] process he referred to as [[chronic cerebrospinal venous insufficiency]] (CCSVI), in which veins from the brain are constricted. He found CCSVI in all patients with MS in his study, performed a surgical procedure, later called in the media "liberation procedure" to correct it and claimed that 73% of participants improved.<ref name="pmid19060024">{{Cite journal |author=Zamboni P, Galeotti R, Menegatti E, ''et al.'' |title=Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis |journal=J. Neurol. Neurosurg. Psychiatr. |volume=80 |issue=4 |pages=392–9 |date=April 2009|pmid=19060024 |pmc=2647682 |doi=10.1136/jnnp.2008.157164 |url=http://jnnp.bmj.com/cgi/content/full/80/4/392}}</ref> This theory received important attention in the media and among MS patients, specially in Canada.<ref name="pmid23402260">{{cite journal |author=Pullman D, Zarzeczny A, Picard A |title="Media, politics and science policy: MS and evidence from the CCSVI Trenches" |journal=BMC Med Ethics |volume=14 |issue= |pages=6 |year=2013 |pmid=23402260 |pmc=3575396 |doi=10.1186/1472-6939-14-6 |url=}}</ref> Concern has been raised with Zamboni's research as it was neither blinded nor controlled, and additionally its assumptions about the pathophisiology of the disease may not be backed by known data.<ref name="pmid20398855">{{cite journal |author=Qiu J |title=Venous abnormalities and multiple sclerosis: another breakthrough claim? |journal=Lancet Neurol |volume=9 |issue=5 |pages=464–5 |year=2010 |month=May |pmid=20398855 |doi=10.1016/S1474-4422(10)70098-3 }}</ref> Also further studies have either not found a relationship or found a much less strong one.<ref name="pmid21161309">{{cite journal |author=Ghezzi A, Comi G, Federico A |title=Chronic cerebro-spinal venous insufficiency (CCSVI) and multiple sclerosis |journal=Neurol. Sci. |volume=32 |issue=1 |pages=17–21 |year=2011 |month=February |pmid=21161309 |doi=10.1007/s10072-010-0458-3 }}</ref> This has raised serious objections to the hypothesis of CCSVI originating MS.<ref name="Dorne">{{Cite journal|author=Dorne H, Zaidat OO, Fiorella D, Hirsch J, Prestigiacomo C, Albuquerque F, Tarr RW.|title=Chronic cerebrospinal venous insufficiency and the doubtful promise of an endovascular treatment for multiple sclerosis|journal=J NeuroIntervent Surg |volume=2|issue=4|pages=309–311|date=October 2010|pmid=21990639|doi=10.1136/jnis.2010.003947 }}</ref> The "liberation procedure" has been criticized for possibly resulting in serious complications and deaths while its benefits have not been proven.<ref name="pmid20398855"/> Currently it is recommended not to use the proposed treatment unless its effectiveness is confirmed by controlled studies.<ref name="Annals of Neurology, Gep 2010">{{cite journal |title=Chronic cerebrospinal venous insufficiency and multiple sclerosis |author=Khan O, Filippi M, Freedman MS, Barkhof F, Dore-Duffy P, Lassmann H, Trapp B, Bar-Or A, Zak I, Siegel MJ, Lisak R |date=2010-02-12 |journal=Annals of neurology|publisher=Annals of Neurology |volume=67 |issue=3 |pages=286–90 |pmid=20373339 |doi=10.1002/ana.22001 }}</ref>Research on CCSVI has been [[FDA Fast Track Development Program|fast tracked]] but researchers have been unable to confirm whether CCSVI has a role in causing MS.<ref name="pmid21161309"/> |
|||
== Alternative treatments == |
== Alternative treatments == |
||
Revision as of 21:03, 24 May 2013
Several therapies for multiple sclerosis (MS) exist, although there is no known cure. Multiple sclerosis is a chronic inflammatory demyelinating disease that affects the central nervous system (CNS).
The most common initial course of the disease is the relapsing-remitting subtype, which is characterized by unpredictable attacks (relapses) followed by periods of relative remission with no new signs of disease activity. After some years, many of the people who have this subtype begin to experience neurologic decline without acute relapses. When this happens it is called secondary progressive multiple sclerosis. Other, less common, courses of the disease are the primary progressive (decline from the beginning without attacks) and the progressive-relapsing (steady neurologic decline and superimposed attacks). Different therapies are used for patients experiencing acute attacks, for patients who have the relapsing-remitting subtype, for patients who have the progressive subtypes, for patients without a diagnosis of MS who have a demyelinating event, and for managing the various consequences of MS.
The primary aims of therapy are returning function after an attack, preventing new attacks, and preventing disability. As with any medical treatment, medications used in the management of MS may have several adverse effects, and many possible therapies are still under investigation. At the same time different alternative treatments are pursued by many patients, despite the paucity of supporting, comparable, replicated scientific study.
This article focuses on therapies for standard MS; borderline forms of MS have particular treatments that are excluded.
Management of acute attacks

Administration of high doses of intravenous corticosteroids, such as methylprednisolone, is the routine therapy for acute relapses. This is administered over a period of three to five days, and has a well-established efficacy in promoting a faster recovery from disability after an attack.[1][2] There is however insufficient evidence to indicate any significant impact on long-term disability of corticosteroid treatments.[3] Steroids administered orally have a similar effectiveness and safety profile at treating MS symptoms as intravenous treatment.[4] Consequences of severe attacks which do not respond to corticosteroids might be treated by plasmapheresis.[5][6]
Disease-modifying treatments
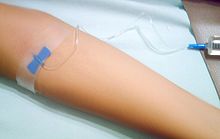
As of April 2013, eight disease-modifying treatments have been approved by regulatory agencies of different countries, including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMEA) and the Japanese PMDA.
The approved drugs with their trademarks are interferon beta-1a (Avonex, Rebif, CinnoVex, ReciGen), interferon beta-1b (Betaseron), glatiramer acetate (Copaxone), mitoxantrone (Novantrone), natalizumab (Tysabri), fingolimod (Gilenya), teriflunomide (Aubagio)[5][7][8] and dimethyl fumarate (BG12, Tecfidera).[9][10]
Description of the drugs
In 1993 interferon beta-1b was the first drug to ever be approved for MS, being soon followed by interferon beta-1a and glatiramer acetate.[11] Interferon beta-1a is injected either weekly (intramuscular injection) or three times a week (subcutaneous injection) depending on commercial formulations,[12][13] while interferon beta-1b is injected subcutaneously every second day.[14] Interferon beta balances the expression of pro- and anti-inflammatory agents in the brain, and reduces the number of inflammatory cells that cross the blood brain barrier.[15] Overall, therapy with interferon beta leads to a reduction of neuron inflammation.[15] Moreover, it is also thought to increase the production of nerve growth factor and consequently improve neuronal survival.[15]
Glatiramer acetate is a mixture of random polymers of four amino acids which is antigenically similar to the myelin basic protein, a component of the myelin sheath of nerves with which it competes for presentation to T cells . It is injected subcutaneously on a daily basis.[16][17][18]
Mitoxantrone is an immunosuppressant also used in cancer chemotherapy which was approved for MS in the year 2000;[19] whereas natalizumab is a monoclonal antibody that was initially approved in 2004.[20] Both are given by intravenous infusion at monthly intervals in the case of natalizumab and every three months in the case of mitoxantrone.[19][21][20]
In 2010 fingolimod, a sphingosine-1-phosphate receptor modulator, became the first oral drug approved by the FDA, being followed in 2012 by teriflunomide, a drug that inhibits the synthesis of pyrimidine and disrupts the interaction of T cells with antigen presenting cell.[7][8][22][23] Fingolimod and teriflunomide are taken through a daily single dose.[8][24] In 2013 one further oral drug, dimethyl fumarate, was approved by the FDA. Dimethyl fumarate is taken twice daily.[10]
Another oral drug, cladribine, was approved in Russia and Australia in 2010. Its application was rejected by the FDA and EMEA in 2011 due to safety concerns in spite of the promising efficacy of the drug. This led the pharmaceutical to discontinue commercialization and withdraw all marketing applications.[25]
Most of these drugs are approved only for Relapsing-Remitting multiple sclerosis (RRMS).
Side effects
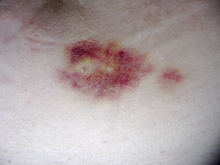

Both the interferons and glatiramer acetate are available only in injectable forms, and both can cause skin reactions at the injection site, specially with subcutaneous administration.[26][27] Skin reactions vary greatly in their clinical presentation and may include bruising, erythema, pain, pruritus, irritation, swelling and in the most extreme cases cutaneous necrosis.[26][27] They usually appear within the first month of treatment albeit their frequence and importance diminish after six months of use.[26] Mild skin reactions usually do not impede treatment whereas necroses appear in around 5% of patients and lead to the discontinuation of the therapy.[26][26] Also over time, a visible dent at the injection site due to the local destruction of fat tissue, known as lipoatrophy, may develop.[26][26]
Interferons, a subclass of cytokines, are produced in the body during illnesses such as influenza in order to help fight the infection. They are responsible of many of the symptoms of influenza infections, including fever, muscle aches, fatigue, and headaches.[28] Many patients report influenza-like symptoms hours after taking interferon-beta that usually improve within 24 hours, being such symptoms related to the temporary increase of cytokines.[5][26] This reaction tends to disappear after 3 months of treatment and its symptoms can be treated with over-the-counter nonsteroidal anti-inflammatory drugs, such as ibuprofen, that reduce fever and pain.[26] Another common transient secondary effect with interferon-beta is a functional deterioration of already existing symptoms of the disease.[26] Such deterioration is similar to the one produced in MS patients due to heat, fever or stress (Uhthoff's phenomenon), usually appears within 24 hours of treatment, is more common in the initial months of treatment, and may last several days.[26] A symptom specially sensitive to worsening is spasticity.[26] Interferon-beta can also reduce numbers of white blood cells (leukopenia), lymphocytes (lymphopenia) and neutrophils (neutropenia), as well as affect liver function.[26] In most cases these effects are non-dangerous and reversible after cessation or reduction of treatment.[26] Nevertheless, recommendation is that all patients should be monitored through laboratory blood analyses, including liver function tests, to ensure safe use of interferons.[26]
Glatiramer acetate is generally well tolerated.[27] The most common secondary effect with glatiramer acetate after skin problem is a post-injection reaction manifested by flushing, chest tightness, heart palpitations, breathlessness, and anxiety, which usually lasts less than thirty minutes and does not require of additional treatment.[27][29]
Mitoxantrone therapy may be associated with immunosuppressive effects and liver damage; however its most dangerous side effect is its dose-related cardiac toxicity. Careful adherence to the administration and monitoring guidelines is therefore essential; this includes obtaining an echocardiogram and a complete blood count before treatment to decide whether the therapy is suitable for the patient or the risks are too great. It is recommended that mitoxantrone be discontinued at the first signs of heart damage, infection or liver dysfunction during therapy.[30] Heart problems (mainly systolic dysfunction) appear in over 10% of patients, while leukemia prevalence is 0.8%.[19]
Soon after its approval natalizumab was withdrawn from the market by its manufacturer after it was linked with three cases of the rare but hazardous neurological condition called progressive multifocal leukoencephalopathy (PML).[20] PML is an opportunistic infection with neurological progressive symptoms caused by the replication of the JC virus in the glial cells of the brain.[20] All 3 initial cases were taking natalizumab in combination with interferon beta-1a. After a safety review the drug was returned to the market in 2006 as a monotherapy for MS under a special prescription program.[20] As of May 2011, over 130 cases of PML had been reported, all in patients who had taken natalizumab for more than a year.[20] While none of them had taken the drug in combination with other disease-modifying treatments, previous use of MS treatments increases the risk of PML between 3 and 4-fold.[20] The estimated prevalence of PML is 1.5 cases per thousand natalizumab users.[20] Around 20% of MS patients with PML die, while most of the remaining are importantly disabled.[20]
During clinical trials fingolimod gave rise to side effects such as hypertension and bradycardia, macular edema, elevated liver enzymes or reduction in lymphocite levels.[23] Teriflunomide is considered a very safe drug. Nevertheless there have been reports of liver failure, and PML.[23] Teriflunomide is also known to be dangerous for fetal development.[23] Most common secondary effects of dimethyl fumarate during clinical trials were flushing and gastrointestinal problems.[9][10][23] These problems were generally mild and occurred more frequently during the first month of treatment.[9][10][23] While dimethyl fumarate leads to a reduction in white blood cell count and levels should be monitored in patients, there were no reported cases of opportunistic infections during the clinical trials.[9][10] Moreover, dymethil fumarate is also used to treat psoriasis, another autoinmune disorder, so there is long term safety data from over 14 years of use without any indication of further dangerous secondary effects.[23]
Clinically isolated syndrome
The earliest clinical presentation of RRMS is the clinically isolated syndrome (CIS), that is, a single attack of a single symptom. During a CIS, there is a subacute attack suggestive of demyelination but the patient does not fulfill the criteria for diagnosis of multiple sclerosis.[31] Treatment with interferons or glatiramer acetate after an initial attack decreases the risk of developing clinical definite MS.[5][32]
Relapsing-remitting MS
Medications are modestly effective at decreasing the number of attacks in RRMS and in reducing the accumulation of brain lesions, which is measured using gadolinium-enhanced magnetic resonance imaging (MRI).[5] Interferons and glatiramer acetate are roughly equivalent, reducing relapses by approximately 30% and their safe profile make them the first-line treatments.[5] Nevertheless, not all the patients are responsive to these therapies. It is known that 30% of MS patients are non-responsive to Beta interferon.[33] One of the factors related to non-respondance is the presence of high levels of interferon beta neutralizing antibodies. Interferon therapy, and specially interferon beta-1b, induces the production of neutralizing antibodies, usually in the second 6 months of treatment, in 5 to 30% of treated patients.[5][34] Moreover, a subset of RRMS patients with specially active MS, sometimes called "rapidly worsening MS" are normally non-responders to immunomodulators and are treated with either mitoxantrone or natalizumab.[35]
Natalizumab and mitoxantrone are considered highly effective both in terms of relapse rate reduction and halting disability progression, however, they are related to dangerous side-effects that have led them to be considered second-line treatments. Natalizumab halfs the risk of suffering relapses when compared to interferons, having an overall efficacy of over 70%.[20] Moreover, mitoxantrone is also highly useful to reduce attacks and disability, but it is generally not considered as a long-term therapy due to its severe cardiac toxicity.[5][36]
There are no official guidelines yet on the use of disease-modifying oral treatments due to their recent development.[23] While some believe that they will probably reduce the usage of first-line treatments the long-term safety of interferons and glatiramer acetate will probably slow this trend.[23] It has been recommended that at the moment oral treatments should be mainly offered in those cases where patients do not use existing treatments due to needle phobia or other reasons such as perceived inefficacy of interferons and glatiramer acetate.[23] They could also be used in patients taking natalizumab who have developed JC virus antibodies and are therefore at an increased risk of PML.[23] Dymethil fumarate is potentially one of the most interesting oral drugs due to the long term data from use in psoriais which points towards a very good safety profile.[23]
While more studies of the long-term effects of the drugs are needed,[5][36][37] specially for the newest treatments,[38][20] existing data on the effects of interferons and glatiramer acetate indicate that early-initiated long-term therapy is safe and it is related to better outcomes.[37]
Even with appropriate use of medication, to varying degrees most patients with relapsing-remitting MS still suffer from some attacks and many suffer subsequent disability.
Secondary progressive MS and progressive relapsing MS
Treatment of advanced forms of MS is more difficult than relapsing-remitting MS. A wide range of medications have been used to try to slow the progression of the disease, with results that have been at best fair.
Mitoxantrone has shown positive effects in patients with a secondary progressive and progressive relapsing courses. It is moderately effective in reducing the progression of the disease and the frequency of relapses in patients in short-term follow-up.[39] In 2007 it was the only medication approved in the USA for both secondary progressive and progressive relapsing multiple sclerosis; however, it causes dose-dependent cardiac toxicity which limits its long-term use. It is also not approved in Europe. Natalizumab has shown efficacy and has been approved for secondary progressive MS with relapses. Studies on the use of Interferon-beta-1b in secondary progressive and progressive relapsing MS do not support that it slows progression of the disease, although it is effective in reducing the number of relapses.[40]
Primary progressive MS
Treatment of primary progressive multiple sclerosis (PPMS) is problematic as many patients do not respond to any available therapy, and no treatment has been approved specifically for use in this form of the disease. There have been several trials investigating the efficacy of different drugs for PPMS without positive results. Drugs tested include interferon beta, mitoxantrone, glatiramer acetate or riluzole.[41] PPMS patients have also been included in trials of azathioprine, methotrexate, intravenous immunoglobulin, cyclophosphamide and hematopoietic stem cell transplantation.[42]
Managing the effects of MS
Disease-modifying treatments only reduce the progression rate of the disease but do not stop it. As multiple sclerosis progresses, the symptoms tend to increase. The disease is associated with a variety of symptoms and functional deficits that result in a range of progressive impairments and handicap. Management of these deficits is therefore very important.
Both drug therapy and neurorehabilitation have shown to ease the burden of some symptoms, even though neither influence disease progression. For other symptoms the efficacy of treatments is still very limited.[43]
Neurorehabilitation

Although there are relatively few studies of rehabilitation in MS,[44][45] its general effectiveness, when conducted by a team of specialists, has been clearly demonstrated in other pathologies such as stroke[46] or head trauma.[47] As for any patient with neurologic deficits, a multidisciplinary approach is key to limiting and overcoming disability; however there are particular difficulties in specifying a 'core team' because people with MS may need help from almost any health profession or service at some point.[48] Neurologists are mainly involved in the diagnosis and ongoing management of multiple sclerosis, and any exacerbations. The comprehensive rehabilitation process for patients with multiple sclerosis is generally managed by physiatrists. Allied treatments such as physiotherapy,[49][50] speech and language therapy[51] or occupational therapy[52] can also help to manage some symptoms and maintain quality of life. Treatment of neuropsychiatric symptoms such as emotional distress and clinical depression should involve mental health professionals such as therapists, psychologists, and psychiatrists,[53] while neuropsychologists can help to evaluate and manage cognitive deficits.[54] Multidisciplinary approaches have been shown to be effective in increasing activity levels and participation in multiple sclerosis.[55][56] Due to the paucity of randomized controlled studies, there is limited evidence of the overall efficacy of individual therapy disciplines,[57][58] though there is good evidence that specific approaches, such as exercise,[59][60] psychology therapies, particularly cognitive behavioral approaches[61] and energy conservation instruction[62] are effective. More specifically psychological interventions seem useful in the treatment of depression, while evidence on effectiveness for other uses such as the treatment of cognitive impairments or vocational counseling is less strong.[63][64] It is difficult to be specific about what types of rehabilitation will be most beneficial because therapies are tailored to meet the individual's specific needs [65]
In regards to well-being, physical therapy focused on gait training can be vital to maximizing MS patient participation via reduction of fatigue during walking and activities of daily living (ADLs).[66] Most gait training is performed over-ground (i.e., in a gym room or outside on uneven ground), on treadmills or, less commonly, using robotic-assisted devices. Robotic-assisted body weight-supported treadmill training may be an effective therapeutic option in MS patients with severe walking impairments.[67] In contrast, over-ground gait training may be most effective in improving gait speed in MS patients with less severe impairments.[67] Equine-assisted therapies such as therapeutic horseback riding and hippotherapy are additional treatments that can positively influence gait,[68] balance and quality of life in people with MS.[69]
Historically, individuals with MS were advised against participation in physical activity due to worsening symptoms.[70] However, under the direction of an expert, participation in physical activity can be safe and has been proven beneficial for persons with MS.[71] Research has supported the rehabilitative role of physical activity in improving muscle power,[72] mobility,[72] mood,[73] bowel health,[74] general conditioning and quality of life.[72] Depending on the person, activities may include resistance training,[75] walking, swimming, yoga, tai chi, and others.[74] Determining an appropriate and safe exercise program is challenging and must be carefully individualized to each person being sure to account for all contraindications and precautions.[71]
An elevated core temperature, leading to increased symptom presentation has been noted during exercise, due to variations in circadian body temperature throughout the day, and due to heat exposure including warm temperatures, warm showers, sun bathing, etc. Care should be taken not to overheat a person with MS during the course of exercise. There is some evidence that cooling measures are effective in allowing a greater degree of exercise: cold showers, cold water limb immersion, applying ice packs, and drinking cold beverages. These strategies are effective when attempting to decrease core temperature post-exercise, and as a method of pre-cooling prior to physical activity or heat exposure.[76] The interaction between an elevated core temperature and the pathological demyelination can cause a transient nerve conduction block that leads to temporarily impaired physical and cognitive function. These effects translate to reduced patient safety and performance of ADLs, however there are viable prevention strategies. Behavioral strategies to minimize heat exposure include performing outdoor physical activity when temperatures are cooler, or installing an air conditioner.
Medical treatments for symptoms
Multiple sclerosis can cause a variety of symptoms including changes in sensation (hypoesthesia), muscle weakness, abnormal muscle spasms, impaired movement, difficulties with coordination and balance, problems in speech (known as dysarthria) or swallowing (dysphagia), visual problems (nystagmus, optic neuritis, or diplopia), fatigue and acute or chronic pain syndromes, bladder and bowel difficulties, cognitive impairment, or emotional symptoms (mainly depression). At the same time for each symptom there are different treatment options. Treatments should therefore be individualized depending both on the patient and the physician.
- Bladder: Symptomatology of the urinary tract is common in MS.[77] Treatments for bladder problems vary depending on the origin or type of dysfunction but can mainly divided into treatment of bladder control and incontinence, and of urinary tract infections.[77] Regarding bladder control, some examples of medications used are desmopressin for nocturia and anticholinergic drugs such as oxybutynin and tolterodine for urinary urgency.[77][78] Non-pharmacological management includes pelvic floor muscle training, stimulation, pessaries, bladder retraining, changes to daily life habits such as clothing, use of external urine collection devices for men and incontinence pads for women; and sometimes intermittent urinary catheterization.[79][77] Regarding long term catheterization, it is associated to urinary tract infections and should be avoided whenever possible.[77] Some of these recommendations do not come from specific studies in MS but are general recommendations for those who have neurogenic bladder.[77]
- Bowel: bowel problems affect around 70% of the patients, with around 50% of the patients suffering from constipation and up to 30% from fecal incontinence.[77] Cause of bowel impairments in MS patients is usually either a reduced gut motility or an impairment in neurological control of defecation. The former is commonly related to inmobility or secondary effects from drugs used in the treatment of the disease.[77] Pain or problems with defecation can be helped with a diet change which includes among other changes an increased fluid intake, oral laxatives or suppositories and enemas when habit changes and oral measures are not enough to control the problems.[77][80]
- Cognitive and emotional: neuropsychiatric symptomatology is common in the course of the disease. Depression and anxiety appear in up to 80% of patients,[81]. Emotional lability leading to uncontrollable crying is also common.[77] These symptoms can be treated with antidepressants and cognitive behavioral therapy[77]; however, high quality studies on efficacy are lacking.[77] For example, in the specific case of antidepressants and depression, only two studies were considered worth considering as of 2011 by the Cochrane collaboration and they only showed a trend towards efficacy.[82] Other neuropsychiatric symptoms are euphoria and disinhibition. Cognitive impairment is a frequent complication of MS even after the introduction of disease-modifying treatments in the last 20 years.[83] Although the disease is usually the primary cause of cognitive problems, other factors such as medications, relapses or depression may be enhancing them so a correct evaluation of the deficits and factors exacerbating them is important.[77] Regarding primary deficits, data point towards administration of L-amphetamine and methylphenidate being useful, whereas memantine and anticholinesterase drugs such as donepezil[84]—commonly used in Alzheimer disease— are not considered effective in improving cognitive functions.[83][64][85][86] Effectiveness of cognitive rehabilitation therapy is more controverted.[87][86][83]
- Dysphagia and dysarthria: dysphagia is a difficulty with eating and swallowing which may cause choking and aspiration of food or liquid into the lungs, while dysarthria is a neurological motor speech disorder characterized by poor control over the subsystems and muscles responsible for speech ("articulation"). A speech and language therapist may give advice on specific swallowing techniques, on adapting food consistencies and dietary intake, on techniques to improve and maintain speech production and clarity, and on alternative communication approaches.[48][51] In the case of advanced dysphagia, food can be supplied by a nasogastric tube, which is a tube that goes through the nose directly to the stomach; or a percutaneous endoscopic gastrostomy (PEG), which is a procedure for placing a tube into the stomach and therefore administering food directly to it. This second system, although more invasive, has better results in the long term than nasogastric intake.[88]
- Erectile dysfunction: erectile dysfunction is common in male patients with MS. There is some evidence indicating that sildenafil citrate may be a useful treatment.[89]
- Fatigue: fatigue is very common and disabling in MS, and at the same time it has a close relationship with depressive symptomatology.[90] When depression is reduced fatigue also tends to improve, so patients should be evaluated for depression before other therapeutic approaches are used.[91] In a similar way, other factors such as disturbed sleep, chronic pain, poor nutrition, or even some medications can contribute to fatigue; medical professionals are therefore encouraged to identify and modify them.[48] A few medications have been studied to treat MS-related fatigue, such as amantadine[92][93] pemoline (which is a psychostimulant also used for attention-deficit hyperactivity disorder and narcolepsy),[94][95] or
modafinil,[96][97] as well as psychological interventions of energy conservation,[98][99] but the effects of all of them are small. Fatigue is therefore a very difficult symptom to manage for which no drugs are recommended.[92]
- Pain: acute pain is mainly due to optic neuritis (with corticosteroids being the best treatment available), as well as trigeminal neuralgia, Lhermitte's sign, or dysesthesias.[100] Subacute pain is usually secondary to the disease and can be a consequence of spending too long in the same position, urinary retention, and infected skin ulcers, amongst others. Treatment will depend on cause. Chronic pain is very common and harder to treat as its most common cause is dysesthesias. Acute pain due to trigeminal neuralgia is usually successfully treated with anticonvulsants such as carbamazepine[101] or phenytoin.[102][103][104] Both Lhermitte's sign and painful dysesthesias usually respond to treatment with carbamazepine, clonazepam,[105] or amitriptyline.[106][107] Sativex is approved for treatment of pain in MS in different countries, but due to its derivation from cannabis, it is currently not available in others, such as the USA.[108] This medication is also being investigated for the management of other MS symptoms, such as spasticity,[109] and has shown long-term safety and efficacy.[110]
- Spasticity: spasticity is characterized by increased stiffness and slowness in limb movement, the development of certain postures, an association with weakness of voluntary muscle power, and with involuntary and sometimes painful spasms of limbs.[48] A physiotherapist can help to reduce spasticity and avoid the development of contractures with techniques such as passive stretching.[111] Trials with Sativex have shown encouraging improvements of spasticicy.[112] There is evidence, albeit limited, of the clinical effectiveness of baclofen,[113] dantrolene,[114] diazepam,[115] and tizanidine.[116][117][118] In the most complicated cases intrathecal injections of baclofen can be used.[119] There are also palliative measures like castings, splints or customized seatings.[48]
- Vision: different drugs as well as optic compensatory systems and prisms can be used to improve the symptoms of nystagmus or diplopia (double vision).[120][121][122] Surgery can also be used in some cases.[123]
- Walking : dalfampridine (ampyra) is a broad-spectrum potassium channel blocker. It is approved by the FDA to treat walking difficulties in MS. It has been shown to increase walking speed, although its high cost (over 1000 dollars a month) limits its usage.[124]
Unfortunately, other symptoms, such as ataxia, tremor or sensory losses, do not have proven treatments.[48]
Therapies under investigation

Research directions on MS treatments include investigations of MS pathogenesis and heterogeneity; research of more effective, convenient, or tolerable new treatments for RRMS; creation of therapies for the progressive subtypes; neuroprotection strategies; and the search for effective symptomatic treatments.[125]
Advances during the last decades has led to the recent approval of several oral drugs. These drugs are expected to gain in popularity and frequency of use at the expense of previously existing therapies.[126] Further oral drugs are still under investigation, the most notable example being laquinimod, which was announced in August 2012 to be the focus of a third phase III trial after mixed results in the previous ones.[127] Similarly, Other studies are aimed to improve efficacy and ease of use of already existing therapies through the use of novel preparations. Such is the case the PEGylated version of interferon-β-1a, that has a longer life than normal interferon and therefore it is being studied if given at less frequent doses has a similar efficacy than the existing product.[128][129] Request for approval of peginterferon beta-1a is expected during 2013.[129]
Monoclonal antibodies, which are drugs of the same family as natalizumab, have also raised high levels of interest and research. Alemtuzumab, daclizumab and CD20 monoclonal antibodies such as rituximab, ocrelizumab and ofatumumab have all shown some benefit and are under study as potential treatments for MS.[130] Nevertheless their use has also been accompanied by the appearance of potentially dangerous adverse effects, most importantly opportunistic infections.[126] Related to these investigations is the recent development of a test against JC virus antibodies which might help to predict what patients are at a greater risk of developing progressive multifocal leukoencephalopathy when taking natalizumab.[126] While monoclonal antibodies are probably going to have some role in the treatment of the disease in the future, it is believed that it will be small due to the risks associated to them.[126]
Another research strategy is to evaluate the combined effectiveness of two or more drugs.[131] The main rationale for polytherapy in MS is that the involved treatments target different mechanisms of the disease and therefore their use is not neccesarily exclusive.[131] Moreover synergies, in which a drug potentiates the effect of another are also possible. Nevertheless there can also appear important drawbacks such as antagonizing mechanisms of action or potentiation of deleterious secondary effects.[131] While there have been several clinical trials of combined therapy none has shown positive enough effects to merit the consideration as a viable treatment for MS.[131]
Finally, regarding neuroprotective and specially regenerative treatments, such as stem cell therapy, while their research is considered of high importance at the moment they are only a promise of future therapeutic approaches.[132] Likewise, there are not any effective treatments for the progressive variants of the disease. Many of the newest drugs as well as those under development are probably going to be evaluated as therapies for PPMS or SPMS, and their improved effectiveness when compared with previously existing drugs may eventually lead to a positive result in these groups of patients.[126]
Chronic cerebrospinal venous insufficiency
In 2008, vascular surgeon Paolo Zamboni suggested that MS involves a vascular process he referred to as chronic cerebrospinal venous insufficiency (CCSVI), in which veins from the brain are constricted. He found CCSVI in all patients with MS in his study, performed a surgical procedure, later called in the media "liberation procedure" to correct it and claimed that 73% of participants improved.[133] This theory received important attention in the media and among MS patients, specially in Canada.[134] Concern has been raised with Zamboni's research as it was neither blinded nor controlled, and additionally its assumptions about the pathophisiology of the disease may not be backed by known data.[135] Also further studies have either not found a relationship or found a much less strong one.[136] This has raised serious objections to the hypothesis of CCSVI originating MS.[137] The "liberation procedure" has been criticized for possibly resulting in serious complications and deaths while its benefits have not been proven.[135] Currently it is recommended not to use the proposed treatment unless its effectiveness is confirmed by controlled studies.[138]Research on CCSVI has been fast tracked but researchers have been unable to confirm whether CCSVI has a role in causing MS.[136]
Alternative treatments
A recent study found that over 60% of MS patients use complementary and alternative medicine, possibly because conventional treatments lack effectiveness. Except for vitamin D, evidence is lacking for these treatments and there are no clear guidelines for their use.[139]
The effect of diet on MS is unclear, with studies unable to show whether diet contributes to MS or can alter its course. Ecologic studies have found a link between high consumptions of polyunsaturated fats and low MS prevalence. The most promising evidence comes from daily supplementation with vitamin D. Evidence is lacking for several other widely promoted non-herbal supplements, including vitamin B12, alpha-lipoic acid, luteolin, and evening primrose oil.[139] Many diets have been proposed for treating the symptoms of the disease. Patients have reported a decrease in symptoms after long-term application of changes in diet; however, no controlled trials have been able to prove their efficacy.[140] Even if these diets are genuinely beneficial for people with MS, it is not known whether this is due to any special traits for MS, as opposed to their known benefits for whole body health.[139] Two examples of such diets are the low saturated fat Swank Multiple Sclerosis Diet.[141][142]
Herbal medicine is another source of alternative treatments. Many patients use medical marijuana to help alleviate symptoms; however, the results of experimental studies are scarce and further clinical trials are required.[143][144] Derivatives from the herb common rue (Ruta graveolens); which contain compounds that block Kv1.3 channels in T cells; have also been suggested to ameliorate MS symptoms.[145][146] Kv1.3 channel-blockers are in development for the treatment of the disease.[147][148]
Bee venom therapy has been promoted as a possible treatment for MS, as it is thought to be an anti-inflammatory and inflammation is a component of MS. Research results have been variable and its benefit has not been demonstrated. Its greatest safety issue is the risk of anaphylactic shock, as about 15% of the population is allergic to bee stings.[139]
Hyperbaric oxygenation has been the subject of several small studies with heterogeneous results which, overall, do not support its use.[149]
Relaxation disciplines such as yoga, and general exercise, seem to mitigate fatigue and improve quality of life.[150] Some studies also show benefits on physical variables, such as balance and strength or cardiovascular and respiratory function, but more investigation is needed as the studies are usually of low quality.[151]
Further reading
Clinical guidelines: clinical guidelines are documents with the aim of guiding decisions and criteria in specific areas of healthcare, as defined by an authoritative examination of current evidence (evidence-based medicine).
- The Royal College of Physicians (2004). Multiple Sclerosis. National clinical guideline for diagnosis and management in primary and secondary care. Salisbury, Wiltshire: Sarum ColourView Group. ISBN 1-86016-182-0.Free full text[dead link] (2004-08-13). Retrieved on 2007-10-01.
- Multiple sclerosis. Understanding NICE guidance. Information for people with multiple sclerosis, their families and carers, and the public. London: National Institute of Clinical Excellence. 2003. ISBN 1-84257-445-0. Free full text (2003-11-26). Retrieved on 2007-10-25.
Notes and references
- ^ Sellebjerg F, Barnes D, Filippini G; et al. (2005). "EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses". Eur. J. Neurol. 12 (12): 939–46. doi:10.1111/j.1468-1331.2005.01352.x. PMID 16324087.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Goodin DS, Frohman EM, Garmany GP; et al. (2002). "Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines". Neurology. 58 (2): 169–78. PMID 11805241.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Template:Cite cochrane
- ^ Template:Cite cochrane
- ^ a b c d e f g h i Compston A, Coles A (2008). "Multiple sclerosis". Lancet. 372 (9648): 1502–17. doi:10.1016/S0140-6736(08)61620-7. PMID 18970977.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Koziolek, MJ (2013 Jan). "Immunoadsorption in steroid-refractory multiple sclerosis". Atherosclerosis. Supplements. 14 (1): 175–8. doi:10.1016/j.atherosclerosissup.2012.10.026.. PMID 23357161.
{{cite journal}}: Check|doi=value (help); Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b "FDA approves first oral drug to reduce MS relapses" (Press release). US FDA. 2010-09-22. Retrieved 2013-01-21.
- ^ a b c "FDA approves new multiple sclerosis treatment Aubagio" (Press release). US FDA. 2012-09-12. Retrieved 2013-01-21.
- ^ a b c d "Biogen Idec's TECFIDERA™ (Dimethyl Fumarate) Approved in US as a First-Line Oral Treatment for Multiple Sclerosis". Biogen Idec Press Release. 2013-03-27.
- ^ a b c d e "NDA 204063 - FDA Approved Labeling Text" (PDF). US Food and Drug Agency. 27 March 2013. Retrieved 5 April 2013.
"NDA Approval" (PDF). US Food and Drug Agency. 27 March 2013. Retrieved 5 April 2013. - ^ Lublin, F (2005 Sep). "History of modern multiple sclerosis therapy". Journal of neurology. 252 Suppl 3: iii3–iii9. PMID 16170498.
{{cite journal}}: Check date values in:|date=(help) - ^ Interferon beta-1a Intramuscular Injection. US National Library of Medicine (Medline) (2006-04-01). Retrieved on 2007-09-02.
- ^ Interferon beta-1a Subcutaneous Injection. US National Library of Medicine (Medline) (2004-04-01). Retrieved on 2007-09-02.
- ^ Interferon Beta-1b Injection. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-09-02
- ^ a b c Kieseier, Bernd C. (1 June 2011). "The Mechanism of Action of Interferon-β in Relapsing Multiple Sclerosis". CNS Drugs. 25 (6): 491–502. doi:10.2165/11591110-000000000-00000. PMID 21649449.
- ^ "Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications". Proceedings of the National Academy of Sciences of the United States of America. 101: 14593–14598. 2004.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Glatiramer injection. US National Library of Medicine (Medline) (2003-07-01). Retrieved on 2007-09-02.
- ^ Lalive, Patrice H. (1 May 2011). "Glatiramer Acetate in the Treatment of Multiple Sclerosis". CNS Drugs. 25 (5): 401–414. doi:10.2165/11588120-000000000-00000. PMID 21476611.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Marriott, J. J. (3 May 2010). "Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology". Neurology. 74 (18): 1463–1470. doi:10.1212/WNL.0b013e3181dc1ae0. PMID 20439849.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i j k Kappos, L (2011 Aug). "Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring". Lancet neurology. 10 (8): 745–58. doi:10.1016/S1474-4422(11)70149-1. PMID 21777829.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Natalizumab Injection. US National Library of Medicine (Medline) (2006-10-01). Retrieved on 2007-09-02.
- ^ "Disruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formation". Arthritis Rheum. 52 (9): 2730. 2005.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b c d e f g h i j k l Killestein, Joep (1 November 2011). "Oral treatment for multiple sclerosis". The Lancet Neurology. 10 (11): 1026–1034. doi:10.1016/S1474-4422(11)70228-9. PMID 22014437.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gylenya medication guide (pdf). Novartis Pharmaceuticals Corporation. 2012. p. 2. Retrieved 2013-01-21.
{{cite book}}: Unknown parameter|month=ignored (help) - ^ Comi, G (2013 Jan). "Cladribine tablets for the treatment of relapsing-remitting multiple sclerosis". Expert opinion on pharmacotherapy. 14 (1): 123–36. PMID 23256518.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i j k l m n o Walther, EU (1999 Nov 10). "Multiple sclerosis: side effects of interferon beta therapy and their management". Neurology. 53 (8): 1622–7. PMID 10563602.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d Carter, Natalie J. (1 August 2010). "Glatiramer Acetate". Drugs. 70 (12): 1545–1577. doi:10.2165/11204560-000000000-00000. PMID 20687620.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Eccles, R (2005 Nov). "Understanding the symptoms of the common cold and influenza". The Lancet infectious diseases. 5 (11): 718–25. PMID 16253889.
{{cite journal}}: Check date values in:|date=(help) - ^ Munari L, Lovati R, Boiko A (2004). Munari, Luca M. (ed.). "Therapy with glatiramer acetate for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD004678. doi:10.1002/14651858.CD004678. PMID 14974077.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fox EJ (2006). "Management of worsening multiple sclerosis with mitoxantrone: a review". Clinical therapeutics. 28 (4): 461–74. doi:10.1016/j.clinthera.2006.04.013. PMID 16750460.
- ^ Miller D, Barkhof F, Montalban X, Thompson A, Filippi M (2005). "Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis". Lancet neurology. 4 (5): 281–8. doi:10.1016/S1474-4422(05)70071-5. PMID 15847841.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bates, D (2011 Jan 4). "Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials". Neurology. 76 (1 Suppl 1): S14-25. PMID 21205678.
{{cite journal}}: Check date values in:|date=(help) - ^ Bertolotto A, Gilli F (2008). "Interferon-beta responders and non-responders. A biological approach". Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 29 Suppl 2: S216–7. doi:10.1007/s10072-008-0941-2. PMID 18690496.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Plosker, Greg L. (1 January 2011). "Interferon-β-1b". CNS Drugs. 25 (1): 67–88. doi:10.2165/11206430-000000000-00000. PMID 21128695.
- ^ Boster A, Edan G, Frohman E, Javed A, Stuve O, Tselis A, Weiner H, Weinstock-Guttman B, Khan O (2008). "Intense immunosuppression in patients with rapidly worsening multiple sclerosis: treatment guidelines for the clinician". Lancet neurology. 7 (2): 173–83. doi:10.1016/S1474-4422(08)70020-6. PMID 18207115.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Comi G (2009). "Treatment of multiple sclerosis: role of natalizumab". Neurol. Sci. 30. Suppl 2 (S2): S155–8. doi:10.1007/s10072-009-0147-2. PMID 19882365.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Freedman, M. S. (27 December 2010). "Long-term follow-up of clinical trials of multiple sclerosis therapies". Neurology. 76 (1, Supplement 1): S26–S34. doi:10.1212/WNL.0b013e318205051d. PMID 21205679.
- ^ He, D (2012 Dec 12). "Teriflunomide for multiple sclerosis". Cochrane database of systematic reviews (Online). 12: CD009882. PMID 23235682.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Martinelli Boneschi F, Rovaris M, Capra R, Comi G (2005). Martinelli Boneschi, Filippo (ed.). "Mitoxantrone for multiple sclerosis". Cochrane database of systematic reviews (Online) (4): CD002127. doi:10.1002/14651858.CD002127.pub2. PMID 16235298.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Template:Cite cochrane
- ^ Riluzole. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.
- ^ Leary SM, Thompson AJ (2005). "Primary progressive multiple sclerosis: current and future treatment options". CNS Drugs. 19 (5): 369–76. doi:10.2165/00023210-200519050-00001. PMID 15907149.
- ^ Kesselring J, Beer S (2005). "Symptomatic therapy and neurorehabilitation in multiple sclerosis". Lancet neurology. 4 (10): 643–52. doi:10.1016/S1474-4422(05)70193-9. PMID 16168933.
- ^ Di Fabio RP, Soderberg J, Choi T, Hansen CR, Schapiro RT (1998). "Extended outpatient rehabilitation: its influence on symptom frequency, fatigue, and functional status for persons with progressive multiple sclerosis". Archives of physical medicine and rehabilitation. 79 (2): 141–6. doi:10.1016/S0003-9993(98)90290-8. PMID 9473994.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Solari A, Filippini G, Gasco P; et al. (1999). "Physical rehabilitation has a positive effect on disability in multiple sclerosis patients". Neurology. 52 (1): 57–62. PMID 9921849.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Langhorne, Peter (2000). Langhorne, Peter (ed.). "Organised inpatient (stroke unit) care for stroke. Stroke Unit Trialists' Collaboration". Cochrane database of systematic reviews (Online) (2): CD000197. doi:10.1002/14651858.CD000197. PMID 10796318.
- ^ Turner-Stokes L, Disler PB, Nair A, Wade DT (2005). Turner-Stokes, Lynne (ed.). "Multi-disciplinary rehabilitation for acquired brain injury in adults of working age". Cochrane database of systematic reviews (Online) (3): CD004170. doi:10.1002/14651858.CD004170.pub2. PMID 16034923.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Multiple sclerosis : national clinical guideline for diagnosis and management in primary and secondary care (pdf). London: Royal College of Physicians. 2004. ISBN 1-86016-182-0. PMID 21290636. Retrieved 6 February 2013.
{{cite book}}:|first=missing|last=(help) - ^ Heesen C, Romberg A, Gold S, Schulz KH (2006). "Physical exercise in multiple sclerosis: supportive care or a putative disease-modifying treatment". Expert Review of Neurotherapeutics. 6 (3): 347–55. doi:10.1586/14737175.6.3.347. PMID 16533139.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rietberg MB, Brooks D, Uitdehaag BM, Kwakkel G (2005). Kwakkel, Gert (ed.). "Exercise therapy for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD003980. doi:10.1002/14651858.CD003980.pub2. PMID 15674920.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Merson RM, Rolnick MI (1998). "Speech-language pathology and dysphagia in multiple sclerosis". Physical medicine and rehabilitation clinics of North America. 9 (3): 631–41. PMID 9894114.
- ^ Baker NA, Tickle-Degnen L (2001). "The effectiveness of physical, psychological, and functional interventions in treating clients with multiple sclerosis: a meta-analysis". The American Journal of Occupational Therapy. 55 (3): 324–31. doi:10.5014/ajot.55.3.324. PMID 11723974.
- ^ Ghaffar O, Feinstein A (2007). "The neuropsychiatry of multiple sclerosis: a review of recent developments". Curr Opin Psychiatry. 20 (3): 278–85. doi:10.1097/YCO.0b013e3280eb10d7. PMID 17415083.
- ^ Benedict RH, Bobholz JH (2007). "Multiple sclerosis". Seminars in neurology. 27 (1): 78–85. doi:10.1055/s-2006-956758. PMID 17226744.
- ^ Khan F, Turner-Stokes L, Ng L, Kilpatrick T (2008). "Multidisciplinary rehabilitation for adults with multiple sclerosis". J. Neurol. Neurosurg. Psychiatr. 79 (2): 114. doi:10.1136/jnnp.2007.127563. PMID 18202203.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khan F, Turner-Stokes L, Ng L, Kilpatrick T (2007). Khan, Fary (ed.). "Multidisciplinary rehabilitation for adults with multiple sclerosis". Cochrane Database of Systematic Reviews (2): CD006036. doi:10.1002/14651858.CD006036.pub2. PMID 17443610.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Steultjens EM, Dekker J, Bouter LM, Leemrijse CJ, van den Ende CH (2005). "Evidence of the efficacy of occupational therapy in different conditions: an overview of systematic reviews". Clinical rehabilitation. 19 (3): 247–54. doi:10.1191/0269215505cr870oa. PMID 15859525.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Steultjens EM, Dekker J, Bouter LM, Cardol M, Van de Nes JC, Van den Ende CH (2003). Steultjens, Esther EMJ (ed.). "Occupational therapy for multiple sclerosis". Cochrane database of systematic reviews (Online) (3): CD003608. doi:10.1002/14651858.CD003608. PMID 12917976.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gallien P, Nicolas B, Robineau S, Pétrilli S, Houedakor J, Durufle A (2007). "Physical training and multiple sclerosis". Ann Readapt Med Phys. 50 (6): 373–6, 369–72. doi:10.1016/j.annrmp.2007.04.004. PMID 17482708.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G (2005). Kwakkel, Gert (ed.). "Exercise therapy for multiple sclerosis". Cochrane Database of Systematic Reviews (1): CD003980. doi:10.1002/14651858.CD003980.pub2. PMID 15674920.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thomas PW, Thomas S, Hillier C, Galvin K, Baker R (2006). Thomas, Peter W (ed.). "Psychological interventions for multiple sclerosis". Cochrane Database of Systematic Reviews (1): CD004431. doi:10.1002/14651858.CD004431.pub2. PMID 16437487.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mathiowetz V, Matuska KM, Murphy ME (2001). "Efficacy of an energy conservation course for persons with multiple sclerosis". Arch Phys Med Rehabil. 82 (4): 449–56. doi:10.1053/apmr.2001.22192. PMID 11295003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khan F, Ng L, Turner-Stokes L (2009). Khan, Fary (ed.). "Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis". Cochrane Database Syst Rev (1): CD007256. doi:10.1002/14651858.CD007256.pub2. PMID 19160331.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Thomas PW, Thomas S, Hillier C, Galvin K, Baker R (2006). Thomas, Peter W (ed.). "Psychological interventions for multiple sclerosis". Cochrane Database Syst Rev (1): CD004431. doi:10.1002/14651858.CD004431.pub2. PMID 16437487.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid16437487" was defined multiple times with different content (see the help page). - ^ "Multidisciplinary Rehabilitation for MS". Making Sense of MS Research. Retrieved 8 November 2012.
{{cite web}}:|first=missing|last=(help) - ^ Sacco, R., Bussman, R., Oesch, P., Kesselring, J., & Beer, S. (2011). Assessment of gait parameters and fatigue in MS patients during inpatient rehabilitation: a pilot trial. Journal of Neurology, 258: 889-894.
- ^ a b Vaney, C., Gattlen, B., Lugon-Moulin, V., Meichtry, A., Hausammann, R., Foinant, D., Anchisi-Bellwald, A.M., Palaci, C., & Hilfiker, R. (2012). Robotic-assisted step training (lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabiliation and Neural Repair, 26(3): 212-21.
- ^ Muñoz-Lasa, S., Ferriero, G., Valero, R., Gomez-Muñiz, F., Rabini, A., & Varela, E. (2011.) Effect of therapeutic horseback riding on balance and gait of people with multiple sclerosis. G Ital Med Lav Ergon. 33(4): 462-7.
- ^ Bronson, C., Brewerton, K., Ong, J., Palanca, C., & Sullivan, S.J. (2010). Does hippotherapy improve balance in persons with multiple sclerosis: a systematic review. European Journal of Physical and Rehabilitation Medicine. 46(3): 347-53.
- ^ Smith, Cath (2009). "How does exercise influence fatigue in people with multiple sclerosis?". Disability and Rehabilitation. 31 (9): 685–692. doi:10.1080/09638280802273473. PMID 18841515.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b O'Sullivan, Susan (2007). Physical Rehabilitation Fifth Edition. Philadelphia: F.A. Davis Company. pp. 136–146. ISBN 978- 0-8036-1247-1.
- ^ a b c Stroud NM, Minahan CL (2009). "The impact of regular physical activity on fatigue, depression and quality of life in persons with multiple sclerosis". Health and Quality of Life Outcomes. 7: 68. doi:10.1186/1477-7525-7-68. PMC 2717927. PMID 19619337.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G (2005). Kwakkel, Gert (ed.). "Exercise therapy for multiple sclerosis". Cochrane Database of Systematic Reviews. CD003980 (1): CD003980. doi:10.1002/14651858.CD003980.pub2. PMID 15674920.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Multiple sclerosis: its effects on you and those you love" (pdf). Multiple Sclerosis Society of Canada. 2008. Retrieved 2011-05-11.
- ^ Dalgas U, Stenager E, Jakobsen J; et al. (2009). "Resistance training improves muscle strength and functional capacity in multiple sclerosis". Neurology. 73 (18): 1478–1484. doi:10.1212/WNL.0b013e3181bf98b4. PMID 19884575.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Davis, SL (2010 Nov). "Thermoregulation in multiple sclerosis". Journal of applied physiology (Bethesda, Md. : 1985). 109 (5): 1531–7. doi:10.1152/japplphysiol.00460.2010. PMC 2980380. PMID 20671034.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i j k l m The National Collaborating Centre for Chronic Conditions (UK) (2004). "Diagnosis and treatment of specific impairments". [http://www.ncbi.nlm.nih.gov/books/NBK48919/pdf/TOC.pdf Multiple sclerosis: national clinical guideline for diagnosis and management in primary and secondary care]. NICE Clinical Guidelines. Vol. 8. London: Royal College of Physicians (UK). pp. 87–132. ISBN 1-86016-182-0. PMID 21290636. Retrieved 6 February 2013.
{{cite book}}: External link in|title= - ^ Bosma R, Wynia K, Havlíková E, De Keyser J, Middel B (2005). "Efficacy of desmopressin in patients with multiple sclerosis suffering from bladder dysfunction: a meta-analysis". Acta Neurol. Scand. 112 (1): 1–5. doi:10.1111/j.1600-0404.2005.00431.x. PMID 15932348.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Frances M Dyro (2012-02-01). Urological Management in Neurological Disease "Urologic Management in Neurologic Disease". WebMD LLC. Retrieved 2013-04-24.
{{cite web}}: Check|url=value (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ DasGupta R, Fowler CJ (2003). "Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies". Drugs. 63 (2): 153–66. doi:10.2165/00003495-200363020-00003. PMID 12515563.
- ^ Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C (1999). "Neuropsychiatric manifestations of multiple sclerosis". The Journal of neuropsychiatry and clinical neurosciences. 11 (1): 51–7. PMID 9990556.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Template:Cite cochrane
- ^ a b c Lovera J, Kovner B (2012). "Cognitive impairment in multiple sclerosis". Curr Neurol Neurosci Rep. 12 (5): 618–27. doi:10.1007/s11910-012-0294-3. PMID 22791241.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Donepezil. US National Library of Medicine (Medline) (2007-04-01). Retrieved on 2007-09-02.
- ^ Template:Cite cochrane
- ^ a b Patti F (2012). "Treatment of cognitive impairment in patients with multiple sclerosis". Expert Opin Investig Drugs. 21 (11): 1679–99. doi:10.1517/13543784.2012.716036. PMID 22876911.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ das Nair R, Ferguson H, Stark DL, Lincoln NB (2012). "Memory Rehabilitation for people with multiple sclerosis". Cochrane Database Syst Rev. 3: CD008754. doi:10.1002/14651858.CD008754.pub2. PMID 22419337.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bath PM, Bath FJ, Smithard DG (2000). Bath, Philip MW (ed.). "Interventions for dysphagia in acute stroke". Cochrane database of systematic reviews (Online) (2): CD000323. doi:10.1002/14651858.CD000323. PMID 10796343.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Xiao, Y (2012 Apr 18). "Sildenafil citrate for erectile dysfunction in patients with multiple sclerosis". Cochrane database of systematic reviews (Online). 4: CD009427. PMID 22513975.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bakshi R (2003). "Fatigue associated with multiple sclerosis: diagnosis, impact and management". Mult. Scler. 9 (3): 219–27. doi:10.1191/1352458503ms904oa. PMID 12814166.
- ^ Mohr DC, Hart SL, Goldberg A (2003). "Effects of treatment for depression on fatigue in multiple sclerosis". Psychosomatic Medicine. 65 (4): 542–7. doi:10.1097/01.PSY.0000074757.11682.96. PMID 12883103.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Pucci E, Branãs P, D'Amico R, Giuliani G, Solari A, Taus C (2007). Pucci, Eugenio (ed.). "Amantadine for fatigue in multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD002818. doi:10.1002/14651858.CD002818.pub2. PMID 17253480.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Amantadine. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-07.
- ^ Weinshenker BG, Penman M, Bass B, Ebers GC, Rice GP (1992). "A double-blind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis". Neurology. 42 (8): 1468–71. PMID 1641137.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C (2000). "Treatments for fatigue in multiple sclerosis: a rapid and systematic review". Health Technol Assess. 4 (27): 1–61. PMID 11074395.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.guardian.co.uk/lifeandstyle/2013/may/03/brain-enhancing-drugs-mj-hyland
- ^ Rammohan KW, Rosenberg JH, Lynn DJ, Blumenfeld AM, Pollak CP, Nagaraja HN (2002). "Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study". J. Neurol. Neurosurg. Psychiatr. 72 (2): 179–83. doi:10.1136/jnnp.72.2.179. PMC 1737733. PMID 11796766.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P (2005). "Randomized controlled trial of an energy conservation course for persons with multiple sclerosis". Mult Scler. 11 (5): 592–601. doi:10.1191/1352458505ms1198oa. PMID 16193899.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Matuska K, Mathiowetz V, Finlayson M (2007). "Use and perceived effectiveness of energy conservation strategies for managing multiple sclerosis fatigue". The American Journal of Occupational Therapy. 61 (1): 62–9. doi:10.5014/ajot.61.1.62. PMID 17302106.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kerns RD, Kassirer M, Otis J (2002). "Pain in multiple sclerosis: a biopsychosocial perspective". Journal of rehabilitation research and development. 39 (2): 225–32. PMID 12051466.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carbamazepine. US National Library of Medicine (Medline) (2007-05-01). Retrieved on 2007-09-02.
- ^ Phenytoin. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.
- ^ Brisman R (1987). "Trigeminal neuralgia and multiple sclerosis". Arch. Neurol. 44 (4): 379–81. PMID 3493757.
- ^ Bayer DB, Stenger TG (1979). "Trigeminal neuralgia: an overview". Oral Surg. Oral Med. Oral Pathol. 48 (5): 393–9. doi:10.1016/0030-4220(79)90064-1. PMID 226915.
- ^ Clonazepam. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.
- ^ Amitriptyline. US National Library of Medicine (Medline) (2007-08-01). Retrieved on 2007-09-02.
- ^ Moulin DE, Foley KM, Ebers GC (1988). "Pain syndromes in multiple sclerosis". Neurology. 38 (12): 1830–4. PMID 2973568.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR (2007). "Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain". Current medical research and opinion. 23 (1): 17–24. doi:10.1185/030079906X158066. PMID 17257464.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Perras C (2005). "Sativex for the management of multiple sclerosis symptoms". Issues in emerging health technologies (72): 1–4. PMID 16317825.
- ^ Wade DT, Makela PM, House H, Bateman C, Robson P (2006). "Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis". Mult. Scler. 12 (5): 639–45. doi:10.1177/1352458505070618. PMID 17086911.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cardini RG, Crippa AC, Cattaneo D (2000). "Update on multiple sclerosis rehabilitation". J. Neurovirol. 6 Suppl 2: S179–85. PMID 10871810.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Oreja-Guevara, C (2012 Apr). "Clinical efficacy and effectiveness of Sativex, a combined cannabinoid medicine, in multiple sclerosis-related spasticity". Expert review of neurotherapeutics. 12 (4 Suppl): 3–8. PMID 22509985.
{{cite journal}}: Check date values in:|date=(help) - ^ Baclofen oral. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-17.
- ^ Dantrolene oral. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-17.
- ^ Diazepam. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-10-17.
- ^ Tizanidine. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-10-17.
- ^ Beard S, Hunn A, Wight J (2003). "Treatments for spasticity and pain in multiple sclerosis: a systematic review". Health technology assessment (Winchester, England). 7 (40): iii, ix–x, 1–111. PMID 14636486.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Paisley S, Beard S, Hunn A, Wight J (2002). "Clinical effectiveness of oral treatments for spasticity in multiple sclerosis: a systematic review". Mult. Scler. 8 (4): 319–29. doi:10.1191/1352458502ms795rr. PMID 12166503.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Becker WJ, Harris CJ, Long ML, Ablett DP, Klein GM, DeForge DA (1995). "Long-term intrathecal baclofen therapy in patients with intractable spasticity". The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 22 (3): 208–17. PMID 8529173.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leigh RJ, Averbuch-Heller L, Tomsak RL, Remler BF, Yaniglos SS, Dell'Osso LF (1994). "Treatment of abnormal eye movements that impair vision: strategies based on current concepts of physiology and pharmacology". Ann. Neurol. 36 (2): 129–41. doi:10.1002/ana.410360204. PMID 8053648.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Starck M, Albrecht H, Pöllmann W, Straube A, Dieterich M (1997). "Drug therapy for acquired pendular nystagmus in multiple sclerosis". J. Neurol. 244 (1): 9–16. doi:10.1007/PL00007728. PMID 9007739.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Menon GJ, Thaller VT (2002). "Therapeutic external ophthalmoplegia with bilateral retrobulbar botulinum toxin- an effective treatment for acquired nystagmus with oscillopsia". Eye (London, England). 16 (6): 804–6. doi:10.1038/sj.eye.6700167. PMID 12439689.
- ^ Jain S, Proudlock F, Constantinescu CS, Gottlob I (2002). "Combined pharmacologic and surgical approach to acquired nystagmus due to multiple sclerosis". Am. J. Ophthalmol. 134 (5): 780–2. doi:10.1016/S0002-9394(02)01629-X. PMID 12429265.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pikoulas, TE (2012 Jul-Aug). "Dalfampridine: a medication to improve walking in patients with multiple sclerosis". The Annals of pharmacotherapy. 46 (7–8): 1010–5. PMID 22764324.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cohen JA (2009). "Emerging therapies for relapsing multiple sclerosis". Arch. Neurol. 66 (7): 821–8. doi:10.1001/archneurol.2009.104. PMID 19597083.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e Cite error: The named reference
pmid21425270was invoked but never defined (see the help page). - ^ Jeffrey, susan (09 Aug 2012). "CONCERTO: A Third Phase 3 Trial for Laquinimod in MS". Medscape Medical News. Retrieved 21 May 2013.
{{cite news}}: Check date values in:|date=(help) - ^ Kieseier BC, Calabresi PA (2012). "PEGylation of interferon-β-1a: a promising strategy in multiple sclerosis". CNS Drugs. 26 (3): 205–14. doi:10.2165/11596970-000000000-00000. PMID 22201341.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Biogen Idec (24 Jan 2013). "Biogen Idec Announces Positive Top-Line Results from Phase 3 Study of Peginterferon Beta-1a in Multiple Sclerosis-Press release". Retrieved 21 May 2013.
- ^ Saidha S, Eckstein C, Calabresi PA (2012). "New and emerging disease modifying therapies for multiple sclerosis". Ann. N. Y. Acad. Sci. 1247: 117–37. doi:10.1111/j.1749-6632.2011.06272.x. PMID 22224673.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Milo R, Panitch H (2011). "Combination therapy in multiple sclerosis". J. Neuroimmunol. 231 (1–2): 23–31. doi:10.1016/j.jneuroim.2010.10.021. PMID 21111490.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Luessi F, Siffrin V, Zipp F (2012). "Neurodegeneration in multiple sclerosis: novel treatment strategies". Expert Rev Neurother. 12 (9): 1061–76, quiz 1077. doi:10.1586/ern.12.59. PMID 23039386.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zamboni P, Galeotti R, Menegatti E; et al. (April 2009). "Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis". J. Neurol. Neurosurg. Psychiatr. 80 (4): 392–9. doi:10.1136/jnnp.2008.157164. PMC 2647682. PMID 19060024.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Pullman D, Zarzeczny A, Picard A (2013). ""Media, politics and science policy: MS and evidence from the CCSVI Trenches"". BMC Med Ethics. 14: 6. doi:10.1186/1472-6939-14-6. PMC 3575396. PMID 23402260.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Qiu J (2010). "Venous abnormalities and multiple sclerosis: another breakthrough claim?". Lancet Neurol. 9 (5): 464–5. doi:10.1016/S1474-4422(10)70098-3. PMID 20398855.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Ghezzi A, Comi G, Federico A (2011). "Chronic cerebro-spinal venous insufficiency (CCSVI) and multiple sclerosis". Neurol. Sci. 32 (1): 17–21. doi:10.1007/s10072-010-0458-3. PMID 21161309.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dorne H, Zaidat OO, Fiorella D, Hirsch J, Prestigiacomo C, Albuquerque F, Tarr RW. (October 2010). "Chronic cerebrospinal venous insufficiency and the doubtful promise of an endovascular treatment for multiple sclerosis". J NeuroIntervent Surg. 2 (4): 309–311. doi:10.1136/jnis.2010.003947. PMID 21990639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khan O, Filippi M, Freedman MS, Barkhof F, Dore-Duffy P, Lassmann H, Trapp B, Bar-Or A, Zak I, Siegel MJ, Lisak R (2010-02-12). "Chronic cerebrospinal venous insufficiency and multiple sclerosis". Annals of neurology. 67 (3). Annals of Neurology: 286–90. doi:10.1002/ana.22001. PMID 20373339.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Namaka M, Crook A, Doupe A; et al. (2008). "Examining the evidence: complementary adjunctive therapies for multiple sclerosis". Neurol Res. 30 (7): 710–9. doi:10.1179/174313208X325038. PMID 18631428.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Farinotti M, Simi S, Di Pietrantonj C; et al. (2007). Farinotti, Mariangela (ed.). "Dietary interventions for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD004192. doi:10.1002/14651858.CD004192.pub2. PMID 17253500.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Swank RL (1991). "Multiple sclerosis: fat-oil relationship". Nutrition (Burbank, Los Angeles County, Calif.). 7 (5): 368–76. PMID 1804476.
- ^ Swank RL, Goodwin J (2003). "Review of MS patient survival on a Swank low saturated fat diet". Nutrition (Burbank, Los Angeles County, Calif.). 19 (2): 161–2. doi:10.1016/S0899-9007(02)00851-1. PMID 12591551.
- ^ Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E (2006). "Cannabis use in patients with multiple sclerosis". Mult. Scler. 12 (5): 646–51. doi:10.1177/1352458506070947. PMID 17086912.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zajicek JP, Sanders HP, Wright DE; et al. (2005). "Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up". J. Neurol. Neurosurg. Psychiatr. 76 (12): 1664–9. doi:10.1136/jnnp.2005.070136. PMC 1739436. PMID 16291891.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bohuslavizki KH, Hänsel W, Kneip A, Koppenhöfer E, Reimers A (1992). "Potassium channel blockers from Ruta--a new approach for the treatment of multiple sclerosis". Gen. Physiol. Biophys. 11 (5): 507–12. PMID 1291451.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bodendiek SB, Mahieux C, Hänsel W, Wulff H (2008). "4-Phenoxybutoxy-substituted Heterocycles - a Structure-Activity Relationship Study of Blockers of the Lymphocyte Potassium Channel Kv1.3". Eur J Med Chem. 44 (5): 1838–52. doi:10.1016/j.ejmech.2008.10.033. PMC 2662044. PMID 19056148.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Beeton C, Chandy KG (2005). "Potassium channels, memory T cells, and multiple sclerosis". Neuroscientist. 11 (6): 550–62. doi:10.1177/1073858405278016. PMID 16282596.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wulff H, Pennington M (2007). "Targeting effector memory T-cells with Kv1.3 blockers". Curr Opin Drug Discov Devel. 10 (4): 438–45. PMID 17659485.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bennett M, Heard R (2004). Bennett, Michael H (ed.). "Hyperbaric oxygen therapy for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD003057. doi:10.1002/14651858.CD003057.pub2. PMID 14974004.
- ^ Oken BS, Kishiyama S, Zajdel D; et al. (2004). "Randomized controlled trial of yoga and exercise in multiple sclerosis". Neurology. 62 (11): 2058–64. PMID 15184614.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Wang C, Collet JP, Lau J (2004). "The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review". Arch Intern Med. 164 (5): 493–501. doi:10.1001/archinte.164.5.493. PMID 15006825.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Searching for Better Treatments for Multiple Sclerosis
- MS Society Natural Treatments for Multiple Sclerosis
