Polyethylene: Difference between revisions
No edit summary |
→History: Full wording of 'ICI' in first instance |
||
| Line 130: | Line 130: | ||
[[File:First polythene pillbox.JPG|thumb|right|A [[pill organizer|pill box]] presented to a technician at ICI in 1936 made from the first pound of polyethylene]] |
[[File:First polythene pillbox.JPG|thumb|right|A [[pill organizer|pill box]] presented to a technician at ICI in 1936 made from the first pound of polyethylene]] |
||
The first industrially practical polyethylene synthesis (diazomethane is a notoriously unstable substance that is generally avoided in industrial application) was discovered in 1933 by Eric Fawcett and Reginald Gibson, again by accident, at the [[Imperial Chemical Industries |
The first industrially practical polyethylene synthesis (diazomethane is a notoriously unstable substance that is generally avoided in industrial application) was discovered in 1933 by Eric Fawcett and Reginald Gibson, again by accident, at the [[Imperial Chemical Industries]] (ICI) works in [[Northwich]], England.<ref>{{cite web |url=http://archive.thisischeshire.co.uk/2006/8/23/275808.html |archiveurl= http://web.archive.org/web/20100121071050/http://archive.thisischeshire.co.uk/2006/8/23/275808.html |archivedate=21 January 2010 |title=Winnington history in the making |date=23 August 2006 |work=This is Cheshire |accessdate=20 February 2014 |ref=harv}}</ref> Upon applying extremely high pressure (several hundred atmospheres) to a mixture of ethylene and [[benzaldehyde]] they again produced a white, waxy, material. Because the reaction had been initiated by trace [[oxygen]] contamination in their apparatus, the experiment was, at first, difficult to reproduce. It was not until 1935 that another ICI chemist, [[Michael Perrin]], developed this accident into a reproducible high-pressure synthesis for polyethylene that became the basis for industrial LDPE production beginning in 1939. Because polyethylene was found to have very low-loss properties at very high frequency radio waves, commercial distribution in Britain was suspended on the outbreak of World War II, secrecy imposed and the new process was used to produce insulation for UHF and SHF [[coaxial cable]]s of [[radar]] sets. During World War II, further research was done on the ICI process and in 1944 Bakelite Corporation at Sabine, Texas and Du Pont at Charleston, West Virginia, began large scale commercial production under license from ICI.<ref>{{cite journal |date=July 1949 |title=Poly – The All Star Plastic |journal=Popular Mechanics |url= http://books.google.com/books?id=GtkDAAAAMBAJ&pg=PA126 |pages=125–129 |volume=91 |issue=1 |accessdate=20 February 2014}}</ref> |
||
The breakthrough landmark in the commercial production of polyethylene began with the development of [[catalyst]] that promote the polymerization at mild temperatures and pressures. The first of these was a [[chromium trioxide]]–based catalyst discovered in 1951 by [[Robert Banks (chemist)|Robert Banks]] and [[J. Paul Hogan]] at [[Phillips Petroleum]].<ref>{{cite book |title=Handbook of Transition Metal Polymerization Catalysts |last1=Hoff |first1=Ray |last2=Mathers |first2=Robert T. |editor1-last=Hoff |editor1-first=Ray |editor2-last=Mathers |editor2-first=Robert T. |year=2010 |publisher=John Wiley & Sons |isbn=978-0-470-13798-7 |doi=10.1002/9780470504437.ch10 |chapter=Chapter 10. Review of Phillips Chromium Catalyst for Ethylene Polymerization}}</ref> In 1953 the German chemist [[Karl Ziegler]] developed a catalytic system based on [[titanium]] [[halide]]s and organoaluminium compounds that worked at even milder conditions than the Phillips catalyst. The Phillips catalyst is less expensive and easier to work with, however, and both methods are heavily used industrially. By the end of the 1950s both the Phillips- and [[Ziegler-Natta catalyst|Ziegler]]-type catalysts were being used for HDPE production. In the 1970s, the Ziegler system was improved by the incorporation of [[magnesium chloride]]. Catalytic systems based on soluble catalysts, the [[metallocene]]s, were reported in 1976 by [[Walter Kaminsky]] and [[Hansjörg Sinn]]. The Ziegler- and metallocene-based catalysts families have proven to be very flexible at copolymerizing ethylene with other [[olefin]]s and have become the basis for the wide range of polyethylene [[resin]]s available today, including [[very low density polyethylene]] and [[linear low-density polyethylene]]. Such resins, in the form of fibers like [[Dyneema]], have (as of 2005) begun to replace [[aramid]]s in many high-strength applications. |
The breakthrough landmark in the commercial production of polyethylene began with the development of [[catalyst]] that promote the polymerization at mild temperatures and pressures. The first of these was a [[chromium trioxide]]–based catalyst discovered in 1951 by [[Robert Banks (chemist)|Robert Banks]] and [[J. Paul Hogan]] at [[Phillips Petroleum]].<ref>{{cite book |title=Handbook of Transition Metal Polymerization Catalysts |last1=Hoff |first1=Ray |last2=Mathers |first2=Robert T. |editor1-last=Hoff |editor1-first=Ray |editor2-last=Mathers |editor2-first=Robert T. |year=2010 |publisher=John Wiley & Sons |isbn=978-0-470-13798-7 |doi=10.1002/9780470504437.ch10 |chapter=Chapter 10. Review of Phillips Chromium Catalyst for Ethylene Polymerization}}</ref> In 1953 the German chemist [[Karl Ziegler]] developed a catalytic system based on [[titanium]] [[halide]]s and organoaluminium compounds that worked at even milder conditions than the Phillips catalyst. The Phillips catalyst is less expensive and easier to work with, however, and both methods are heavily used industrially. By the end of the 1950s both the Phillips- and [[Ziegler-Natta catalyst|Ziegler]]-type catalysts were being used for HDPE production. In the 1970s, the Ziegler system was improved by the incorporation of [[magnesium chloride]]. Catalytic systems based on soluble catalysts, the [[metallocene]]s, were reported in 1976 by [[Walter Kaminsky]] and [[Hansjörg Sinn]]. The Ziegler- and metallocene-based catalysts families have proven to be very flexible at copolymerizing ethylene with other [[olefin]]s and have become the basis for the wide range of polyethylene [[resin]]s available today, including [[very low density polyethylene]] and [[linear low-density polyethylene]]. Such resins, in the form of fibers like [[Dyneema]], have (as of 2005) begun to replace [[aramid]]s in many high-strength applications. |
||
Revision as of 10:12, 26 October 2014

| |

| |
| Names | |
|---|---|
| IUPAC name
Polyethene or Poly(methylene)
| |
| Other names
Polyethene
| |
| Identifiers | |
| Abbreviations | PE |
| ChemSpider | |
| ECHA InfoCard | 100.121.698 |
| KEGG | |
| MeSH | Polyethylene |
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C2H4)n | |
| Density | 0.91-0.96 g/cm³[1] |
| Melting point | 115-135 °C[1] (239–275 °F) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

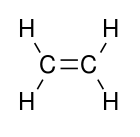
Polyethylene (abbreviated PE) or polythene (IUPAC name polyethene or poly(methylene)) is the most common plastic. The annual global production is approximately 80 million tonnes.[2] Its primary use is in packaging (plastic bag, plastic films, geomembranes, containers including bottles, etc.). Many kinds of polyethylene are known, with most having the chemical formula (C2H4)nH2. Thus PE is usually a mixture of similar organic compounds that differ in terms of the value of n.
Properties
Physical properties
Polyethylene is a thermoplastic polymer consisting of long hydrocarbon chains. Depending on the crystallinity and molecular weight, a melting point and glass transition may or may not be observable. The temperature at which these occur varies strongly with the type of polyethylene. For common commercial grades of medium- and high-density polyethylene the melting point is typically in the range 120 to 180 °C (248 to 356 °F). The melting point for average, commercial, low-density polyethylene is typically 105 to 115 °C (221 to 239 °F).
Chemical properties
Most LDPE, MDPE and HDPE grades have excellent chemical resistance, meaning that it is not attacked by strong acids or strong bases. It is also resistant to gentle oxidants and reducing agents. Polyethylene burns slowly with a blue flame having a yellow tip and gives off an odour of paraffin. The material continues burning on removal of the flame source and produces a drip.[3] Crystalline samples do not dissolve at room temperature. Polyethylene (other than cross-linked polyethylene) usually can be dissolved at elevated temperatures in aromatic hydrocarbons such as toluene or xylene, or in chlorinated solvents such as trichloroethane or trichlorobenzene.[4]
Process
Monomer
The ingredient or monomer is ethylene (IUPAC name ethene), a gaseous hydrocarbon with the formula C2H4, which can be viewed as a pair of methylene groups (=CH
2) connected to each other. Because the compound is highly reactive, the ethylene must be of high purity. Typical specifications are <5 ppm for water, oxygen, as well as other alkenes. Acceptable contaminants include N2, ethane (common precursor to ethylene), and methane. Ethylene is usually produced from petrochemical sources, but also is generated by dehydration of ethanol.[4]
Polymerization
Ethylene is a rather stable molecule that polymerizes only upon contact with catalysts. The conversion is highly exothermic. Coordination polymerization is the most pervasive technology, which means that metal chlorides or metal oxides are used. The most common catalysts consist of titanium(III) chloride, the so-called Ziegler-Natta catalysts. Another common catalyst is the Phillips catalyst, prepared by depositing chromium(VI) oxide on silica.[4] Ethylene can be produced through radical polymerization, but this route has only limited utility and typically requires high pressure apparatus.
Classification
Polyethylene is classified into several different categories based mostly on its density and branching. Its mechanical properties depend significantly on variables such as the extent and type of branching, the crystal structure and the molecular weight. With regard to sold volumes, the most important polyethylene grades are HDPE, LLDPE and LDPE.
- Ultra-high-molecular-weight polyethylene (UHMWPE)
- Ultra-low-molecular-weight polyethylene (ULMWPE or PE-WAX)
- High-molecular-weight polyethylene (HMWPE)
- High-density polyethylene (HDPE)
- High-density cross-linked polyethylene (HDXLPE)
- Cross-linked polyethylene (PEX or XLPE)
- Medium-density polyethylene (MDPE)
- Linear low-density polyethylene (LLDPE)
- Low-density polyethylene (LDPE)
- Very-low-density polyethylene (VLDPE)
- Chlorinated polyethylene (CPE)
Ultra-high-molecular-weight polyethylene (UHMWPE)
UHMWPE is polyethylene with a molecular weight numbering in the millions, usually between 3.1 and 5.67 million. The high molecular weight makes it a very tough material, but results in less efficient packing of the chains into the crystal structure as evidenced by densities of less than high density polyethylene (for example, 0.930–0.935 g/cm3). UHMWPE can be made through any catalyst technology, although Ziegler catalysts are most common. Because of its outstanding toughness and its cut, wear and excellent chemical resistance, UHMWPE is used in a diverse range of applications. These include can and bottle handling machine parts, moving parts on weaving machines, bearings, gears, artificial joints, edge protection on ice rinks and butchers' chopping boards. It competes with aramid in bulletproof vests, under the tradenames Spectra and Dyneema, and is commonly used for the construction of articular portions of implants used for hip and knee replacements.
High-density polyethylene (HDPE)

HDPE is defined by a density of greater or equal to 0.941 g/cm3. HDPE has a low degree of branching and thus low intermolecular forces and tensile strength. HDPE can be produced by chromium/silica catalysts, Ziegler-Natta catalysts or metallocene catalysts. The lack of branching is ensured by an appropriate choice of catalyst (for example, chromium catalysts or Ziegler-Natta catalysts) and reaction conditions. HDPE is used in products and packaging such as milk jugs, detergent bottles, butter tubs, garbage containers and water pipes. One third of all toys are manufactured from HDPE. In 2007 the global HDPE consumption reached a volume of more than 30 million tons.[5]
Cross-linked polyethylene (PEX or XLPE)
PEX is a medium- to high-density polyethylene containing cross-link bonds introduced into the polymer structure, changing the thermoplastic into a thermoset. The high-temperature properties of the polymer are improved, its flow is reduced and its chemical resistance is enhanced. PEX is used in some potable-water plumbing systems because tubes made of the material can be expanded to fit over a metal nipple and it will slowly return to its original shape, forming a permanent, water-tight, connection.
Medium-density polyethylene (MDPE)
MDPE is defined by a density range of 0.926–0.940 g/cm3. MDPE can be produced by chromium/silica catalysts, Ziegler-Natta catalysts or metallocene catalysts. MDPE has good shock and drop resistance properties. It also is less notch-sensitive than HDPE, stress cracking resistance is better than HDPE. MDPE is typically used in gas pipes and fittings, sacks, shrink film, packaging film, carrier bags and screw closures.
Linear low-density polyethylene (LLDPE)
LLDPE is defined by a density range of 0.915–0.925 g/cm3. LLDPE is a substantially linear polymer with significant numbers of short branches, commonly made by copolymerization of ethylene with short-chain alpha-olefins (for example, 1-butene, 1-hexene and 1-octene). LLDPE has higher tensile strength than LDPE, it exhibits higher impact and puncture resistance than LDPE. Lower thickness (gauge) films can be blown, compared with LDPE, with better environmental stress cracking resistance but is not as easy to process. LLDPE is used in packaging, particularly film for bags and sheets. Lower thickness may be used compared to LDPE. Cable covering, toys, lids, buckets, containers and pipe. While other applications are available, LLDPE is used predominantly in film applications due to its toughness, flexibility and relative transparency. Product examples range from agricultural films, saran wrap, and bubble wrap, to multilayer and composite films. In 2009 the world LLDPE market reached a volume of almost US$24 billion (€17 billion).[6]
Low-density polyethylene (LDPE)
LDPE is defined by a density range of 0.910–0.940 g/cm3. LDPE has a high degree of short and long chain branching, which means that the chains do not pack into the crystal structure as well. It has, therefore, less strong intermolecular forces as the instantaneous-dipole induced-dipole attraction is less. This results in a lower tensile strength and increased ductility. LDPE is created by free radical polymerization. The high degree of branching with long chains gives molten LDPE unique and desirable flow properties. LDPE is used for both rigid containers and plastic film applications such as plastic bags and film wrap. In 2009 the global LDPE market had a volume of circa US$22.2 billion (€15.9 billion).[7]
Very-low-density polyethylene (VLDPE)
VLDPE is defined by a density range of 0.880–0.915 g/cm3. VLDPE is a substantially linear polymer with high levels of short-chain branches, commonly made by copolymerization of ethylene with short-chain alpha-olefins (for example, 1-butene, 1-hexene and 1-octene). VLDPE is most commonly produced using metallocene catalysts due to the greater co-monomer incorporation exhibited by these catalysts. VLDPEs are used for hose and tubing, ice and frozen food bags, food packaging and stretch wrap as well as impact modifiers when blended with other polymers.
Recently much research activity has focused on the nature and distribution of long chain branches in polyethylene. In HDPE a relatively small number of these branches, perhaps 1 in 100 or 1,000 branches per backbone carbon, can significantly affect the rheological properties of the polymer.
Copolymers
In addition to copolymerization with alpha-olefins, ethylene can also be copolymerized with a wide range of other monomers and ionic composition that creates ionized free radicals. Common examples include vinyl acetate (the resulting product is ethylene-vinyl acetate copolymer, or EVA, widely used in athletic-shoe sole foams) and a variety of acrylates. Applications of acrylic copolymer include packaging and sporting goods, and superplasticizer, used for cement production.
History
Polyethylene was first synthesized by the German chemist Hans von Pechmann who prepared it by accident in 1898 while investigating diazomethane.[8][9] When his colleagues Eugen Bamberger and Friedrich Tschirner characterized the white, waxy substance that he had created, they recognized that it contained long -CH2- chains and termed it polymethylene.[10]

The first industrially practical polyethylene synthesis (diazomethane is a notoriously unstable substance that is generally avoided in industrial application) was discovered in 1933 by Eric Fawcett and Reginald Gibson, again by accident, at the Imperial Chemical Industries (ICI) works in Northwich, England.[11] Upon applying extremely high pressure (several hundred atmospheres) to a mixture of ethylene and benzaldehyde they again produced a white, waxy, material. Because the reaction had been initiated by trace oxygen contamination in their apparatus, the experiment was, at first, difficult to reproduce. It was not until 1935 that another ICI chemist, Michael Perrin, developed this accident into a reproducible high-pressure synthesis for polyethylene that became the basis for industrial LDPE production beginning in 1939. Because polyethylene was found to have very low-loss properties at very high frequency radio waves, commercial distribution in Britain was suspended on the outbreak of World War II, secrecy imposed and the new process was used to produce insulation for UHF and SHF coaxial cables of radar sets. During World War II, further research was done on the ICI process and in 1944 Bakelite Corporation at Sabine, Texas and Du Pont at Charleston, West Virginia, began large scale commercial production under license from ICI.[12]
The breakthrough landmark in the commercial production of polyethylene began with the development of catalyst that promote the polymerization at mild temperatures and pressures. The first of these was a chromium trioxide–based catalyst discovered in 1951 by Robert Banks and J. Paul Hogan at Phillips Petroleum.[13] In 1953 the German chemist Karl Ziegler developed a catalytic system based on titanium halides and organoaluminium compounds that worked at even milder conditions than the Phillips catalyst. The Phillips catalyst is less expensive and easier to work with, however, and both methods are heavily used industrially. By the end of the 1950s both the Phillips- and Ziegler-type catalysts were being used for HDPE production. In the 1970s, the Ziegler system was improved by the incorporation of magnesium chloride. Catalytic systems based on soluble catalysts, the metallocenes, were reported in 1976 by Walter Kaminsky and Hansjörg Sinn. The Ziegler- and metallocene-based catalysts families have proven to be very flexible at copolymerizing ethylene with other olefins and have become the basis for the wide range of polyethylene resins available today, including very low density polyethylene and linear low-density polyethylene. Such resins, in the form of fibers like Dyneema, have (as of 2005) begun to replace aramids in many high-strength applications.
Environmental issues

This section may require cleanup to meet Wikipedia's quality standards. The specific problem is: unsourced, poorly sourced, and innacurate statements. (September 2012) |
Although ethylene can be produced from renewables, polyethylene is mainly made from petroleum or natural gas.
Biodegrading plastics
One of the main problems of polyethelyne is that without special treatment it is not readily biodegradable, and thus accumulates. In Japan getting rid of plastics in an environmentally friendly way was the major problem discussed until the Fukushima disaster in 2011. It was listed as a $90 billion market for solutions. Since 2008 Japan has rapidly increased the recycling of plastics, but still has a large rate of plastic wrapping which goes to waste.[14]
In May 2008, Daniel Burd, a 16-year-old Canadian, won the Canada-Wide Science Fair in Ottawa after discovering that Pseudomonas fluorescens, with the help of Sphingomonas, can degrade over 40% of the weight of plastic bags in less than three months.[15]
The thermophilic bacterium Brevibaccillus borstelensis (strain 707) was isolated from a soil sample and found to use low-density polyethylene as a sole carbon source; when incubated together at 50 degrees Celsius. Biodegredation increased with time exposed to ultraviolet radiation.[16]
In 2010 a Japanese researcher Akinori Ito released the prototype of a machine which creates oil from Polyethylene using a small, self-contained vapor distillation process.[17]
Acinetobacter sp. 351 can degrade lower molecular weight PE oligomers. When PE is subjected to thermo and photo-oxidization, products including alkanes, alkenes, ketones, aldehydes, alcohols, carboxylic acid, keto-acids, dicarboxylic acids, lactones and esters are released.[18]
Bio-derived polyethylene
Braskem and Toyota Tsusho Corporation started Joint marketing activities for producing polyethylene from sugar cane. Braskem will build a new facility at their existing industrial unit in Triunfo, RS, Brazil with an annual production capacity of 200,000 short tons (180,000,000 kg), and will produce high-density polyethylene (HDPE) and low-density polyethylene (LDPE) from bioethanol derived from sugarcane.[19]
Polyethylene can also be made from other feedstocks, including wheat grain and sugar beet. Retrieved from cane sugar, i.e. plant biomass renewable feedstock; Brazil is the first country to develop the product.[citation needed]
These developments are using renewable resources rather than fossil fuel, although the issue of plastic source is currently negligible in the wake of plastic waste and in particular polyethylene waste as shown above.
Joining
Commonly used methods for joining polyethylene parts together include:[20]
- Hot gas welding
- Fastening
- Infrared welding
- Laser welding
- Ultrasonic welding
- Heat sealing
- Heat fusion
Adhesives and solvents are rarely used because polyethylene is nonpolar and has a high resistance to solvents. Pressure-sensitive adhesives (PSA) are feasible if the surface is flame treated or corona treated. Commonly used adhesives include:[20]
- Dispersion of solvent-type PSAs
- Polyurethane contact adhesives
- Two-part polyurethane or epoxy adhesives
- Vinyl acetate copolymer hot melt adhesives
Nomenclature and general description of the process
The name polyethylene comes from the ingredient and not the resulting chemical compound, which contains no double bonds. The scientific name polyethene is systematically derived from the scientific name of the monomer.[21][22] The alkene monomer converts to a long, sometimes very long, alkane in the polymerization process.[22] In certain circumstances it is useful to use a structure-based nomenclature; in such cases IUPAC recommends poly(methylene) (poly(methanediyl) is a non-preferred alternative).[21] The difference in names between the two systems is due to the opening up of the monomer's double bond upon polymerization.[23] The name is abbreviated to PE. In a similar manner polypropylene and polystyrene are shortened to PP and PS, respectively. In the United Kingdom the polymer is commonly called polythene, although this is not recognized scientifically.
References
- ^ a b Batra, Kamal (2014). ROLE OF ADDITIVES IN LINEAR LOW DENSITY POLYETHYLENE (LLDPE) FILMS. p. 9. Retrieved September 16, 2014.
- ^ Piringer & Baner 2008, p. 32.
- ^ "How to Identify Plastic Materials Using The Burn Test". Boedeker Plastics. Retrieved May 8, 2012.
- ^ a b c Kenneth S. Whiteley, T. Geoffrey Heggs, Hartmut Koch, Ralph L. Mawer, Wolfgang Immel, "Polyolefins" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a21_487
- ^ "Market Study: Polyethylene – HDPE". Ceresana Research. May 2012. Retrieved May 8, 2012.
- ^ "Market Study: Polyethylene – LLDPE". Ceresana Research. March 2010. Retrieved May 8, 2012.
- ^ "Market Study: Polyethylene – LDPE". Ceresana Research. April 2010. Retrieved May 8, 2012.
- ^ H. von Pechmann (1898) "Ueber Diazomethan und Nitrosoacylamine," Berichte der Deutschen chemischen Gesellschaft zu Berlin, 31 : 2640-2646; see especially page 2643. From page 2643: "Erwähnt sei noch, dass aus einer ätherischen Diazomethanlösung sich beim Stehen manchmal minimale Quantitäten eines weissen, flockigen, aus Chloroform krystallisirenden Körpers abscheiden; … " (It should be mentioned that from an ether solution of diazomethane, upon standing, sometimes small quantities of a white, flakey substance, which can be crystallized from chloroform, precipitate; … )
- ^ Bamberger claimed that one of his students, Hindermann, had noted the formation of polyethylene in 1897. Eug. Bamberger & Fred. Tschirner (1900) "Ueber die Einwirkung von Diazomethan auf β-Arylhydroxylamine" (On the effect of diazomethane on β-arylhydroxylamine), Berichte der Deutschen chemischen Gesellschaft zu Berlin, 33 : 955-959. From footnote 3 on page 956: "Die Abscheidung weisser Flocken aus Diazomethanlösungen erwähnt auch v. Pechmann (diese Berichte 31, 2643); er hat sie aber wegen Substanzmangel nicht untersucht. Ich hatte übrigens Hrn. v. Pechmann schon einige Zeit vor Erscheinen seiner Publication mitgetheilt, dass aus Diazomethan ein fester, weisser Körper entstehe, der sich bei der Analyse als (CH2)x erwiesen habe, worauf mir Hr. v. Pechmann schrieb, dass er den weissen Körper ebensfalls beobachtet, aber nicht untersucht habe. Zuerst erwähnt ist derselbe in der Dissertation meines Schülers Hindermann, Zürich (1897), S. 120." (Von Pechmann (these Reports, 31, 2643) also mentioned the precipitation of white flakes from diazomethane solutions; however, due to a scarcity of the material, he didn't investigate it. Incidentally, some time before the appearance of his publication, I had communicated to Mr. von Pechmann that a solid, white substance arose from diazomethane, which on analysis proved to be (CH2)x, whereupon Mr. von Pechmann wrote me that he had likewise observed the white substance, but not investigated it. It is first mentioned in the dissertation of my student Hindermann, Zürich (1897), p. 120.)
- ^ Eug. Bamberger & Fred. Tschirner (1900) "Ueber die Einwirkung von Diazomethan auf β-Arylhydroxylamine" (On the effect of diazomethane on β-arylhydroxylamine), Berichte der Deutschen chemischen Gesellschaft zu Berlin, 33 : 955-959. From page 956: "Eine theilweise — übrigens immer nur minimale — Umwandlung des Diazomethans in Stickstoff und Polymethylen vollzieht sich auch bei ganz andersartigen Reactionen; … " (A partial — incidentally, always only minimal — conversion of diazomethane into nitrogen and polymethylene takes place also during quite different reactions; … )
- ^ "Winnington history in the making". This is Cheshire. August 23, 2006. Archived from the original on January 21, 2010. Retrieved February 20, 2014.
{{cite web}}: Invalid|ref=harv(help) - ^ "Poly – The All Star Plastic". Popular Mechanics. 91 (1): 125–129. July 1949. Retrieved February 20, 2014.
- ^ Hoff, Ray; Mathers, Robert T. (2010). "Chapter 10. Review of Phillips Chromium Catalyst for Ethylene Polymerization". In Hoff, Ray; Mathers, Robert T. (eds.). Handbook of Transition Metal Polymerization Catalysts. John Wiley & Sons. doi:10.1002/9780470504437.ch10. ISBN 978-0-470-13798-7.
- ^ Prideaux, Eric (November 3, 2007). "Plastic incineration rise draws ire". The Japan Times Online. Retrieved May 8, 2012.[dead link]
- ^ "CanadaWorld – WCI student isolates microbe that lunches on plastic bags". The Record.com. Retrieved February 20, 2014.
- ^ Hadad, D.; Geresh, S.; Sivan, A. (2005). "Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis". journal of applied microbiology. pp. 1093–100. doi:10.1111/j.1365-2672.2005.02553.x. PMID 15836478.
{{cite web}}: Missing or empty|url=(help) - ^ Nguyen, Tuan (February 17, 2011). "New invention turns plastic bags into oil". smartplanet.com. Retrieved February 20, 2014.
- ^ Tokiwa, Yutaka; Calabia, Buenaventurada P.; Ugwu, Charles U.; Aiba, Seiichi (September 2009). "Biodegradability of Plastics". International Journal of Molecular Science. 9: 3722–3742. doi:10.3390/ijms10093722. PMC 2769161. PMID 19865515.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Braskem & Toyota Tsusho start joint marketing activities for green polyethylene from sugar cane" (Press release). yourindustrynews.com. September 26, 2008. Retrieved February 20, 2014.
- ^ a b Plastics Design Library, p. 326.
- ^ a b "A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993) IUPAC, Commission on Nomenclature of Organic Chemistry". Blackwell Scientific Publications. 1993. ISBN 0632037024. Retrieved February 20, 2014.
- ^ a b Kahovec, J.; Fox, R.B.; Hatada, K. (2002). "Nomenclature of regular single-strand organic polymers (IUPAC Recommendations 2002)". Pure and Applied Chemistry. 74 (10): 1921. doi:10.1351/pac200274101921.
{{cite journal}}: Invalid|ref=harv(help) - ^ "IUPAC Provisional Recommendations on the Nomenclature of Organic Chemistry". International Union of Pure and Applied Chemistry. October 27, 2004. Retrieved February 20, 2014.
Bibliography
- Piringer, Otto G.; Baner, Albert Lawrence (2008). Plastic packaging: interactions with food and pharmaceuticals (2nd ed.). Wiley-VCH. ISBN 978-3-527-31455-3. Retrieved February 20, 2014.
{{cite book}}: Invalid|ref=harv(help) - Plastics Design Library (1997). Handbook of Plastics Joining: A Practical Guide (Illustrated ed.). William Andrew. ISBN 978-1-884207-17-4. Retrieved February 20, 2014.
{{cite book}}: Invalid|ref=harv(help)
External links
- Polythene's story: The accidental birth of plastic bags
- Polythene Technical Properties & Applications
- Article describing the discovery of Sphingomonas as a biodegrader of plastic bags Kawawada, Karen, The Record (May 22, 2008).