Phenibut
 | |
| Clinical data | |
|---|---|
| Other names | Fenibut, Phenybut, PhGABA |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.800 |
| Chemical and physical data | |
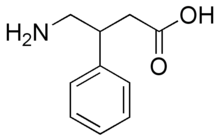
| Formula | C10H13NO2 |
| Molar mass | 179.216 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 253 °C (487 °F) |
| |
Beta-phenyl-gamma-aminobutyric acid, better known as Phenibut or less commonly Fenibut or Phenybut, is natural derivative of the inhibitory neurotransmitter GABA (Gamma amino butyric acid). Sold as a dietary supplement in the US while in Russia sold as a neuropsychotropic drug that is capable of passing the blood-brain barrier. Phenibut is cited as a nootropic for its ability to improve neurological functions. It was discovered in the Soviet Union in the 1960s, and has since been used there to treat a wide range of ailments including anxiety and insomnia.
The name Phenibut, along with many of the other names for the compound, comes directly from the chemical name for the compound, beta-phenyl-gamma-aminobutyric acid.
The d-isomer is the only biologically active isomer.
History
Phenibut was synthesized at the I. M. Herzen Leningrad Pedagogical Institute USSR and the Institute of Experimental Medicine, Academy of Medical Sciences USSR by Professor V. V. Perekalin's team.
Phenibut is mandated standard equipment in a cosmonaut's medkit. Conventional tranquilizers' use for stress and anxiety makes patients drowsy, which was deemed unacceptable for cosmonauts, while Phenibut lowered stress levels without affecting performance adversely.
In 1975 Phenibut was included in the cosmonauts' kit for those who participated in the Apollo-Soyuz joint mission.
General information
In chemical structure, Phenibut can be regarded as a phenyl derivative of GABA, as well as a derivative of phenethylamine.
Phenibut has nootropic activity and a tranquilizing effect, reduces stress and anxiety, improves sleep, and may potentiate the effects of tranquilizers, narcotics, and neuroleptics. It also has an anticonvulsant effect.
Physical properties
White crystalline powder. The taste is sour. Very easily soluble in water, soluble in alcohol, the pH of water (2.5%) solution is about 2,3-2,7.
Doses
Commonly recommended doses are 250-3,000 mg as needed or daily with weekly cycling off during weekends.
Pharmacology
Structurally, phenibut is gamma-aminobutyric acid with a phenyl group in the beta position. It has structural similarities to baclofen and phenethylamine. Phenibut is a GABAB receptor agonist, with slight activity at GABAA[clarification needed] receptors. It also is a beta-phenylethylamine antagonist[clarification needed] and thus reduces the effects of phenylethylamine and other compounds that raise phenlethylamine levels {{citation}}: Empty citation (help).
Furthermore, it has shown to stimulate dopamine receptors. The pharmacological effects of phenibut are similar to baclofen, but less potent per milligram of dosage.[citation needed]
Contraindications and side effects
Phenibut can have extremely unpleasant dose-dependent withdrawal symptoms. Withdrawal symptoms can include acute anxiety and insomnia that can last for up to three weeks afterwards. Withdrawal symptoms, however, are almost always associated with cessation after prolonged usage.
Phenibut is not to be mixed with alcohol, sedatives or prescription medication without consulting with a healthcare professional.
Persons on MAO inhibitors or epilepsy medications like carbamazepine or oxcarbazepine should consult with their physician or pharmacist prior to supplementation with phenibut. Clinical research has demonstrated that phenibut can potentiate or inhibit the function of some epilepsy medications.[citation needed]
References
- Shulgina GI (1986). "On neurotransmitter mechanisms of reinforcement and internal inhibition". Pavlov J Biol Sci. 21 (4): 129–40. PMID 2431377.
- Chojnacka-Wójcik E, Hano J, Sieroslawska J, Sypniewska M (1975). "Pharmacological properties of gamma-animobutyric acid and it derivatives. IV. Aryl gaba derivatives and their respective lactams". Arch. Immunol. Ther. Exp. (Warsz.). 23 (6): 733–46. PMID 1241266.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- Lapin I (2001). "Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug". CNS Drug Rev. 7 (4): 471–81. PMID 11830761.
- Full PDF
- Talalaenko AN, Pankrat'ev DV, Goncharenko NV (2003). "Neurochemical characteristics of the ventromedial hypothalamus in mediating the antiaversive effects of anxiolytics in different models of anxiety" (PDF). Neurosci. Behav. Physiol. 33 (3): 255–61. PMID 12762592.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
- Talalaenko AN, Pankrat'ev DV, Goncharenko NV (2002). "[Monoaminergic and aminoacidergic mechanisms of the posterior hypothalamus in realization of the antiaversive effects of anxiosedative and anxioselective agents in various anxiety models]". Eksp Klin Farmakol (in Russian). 65 (5): 22–6. PMID 12596508.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- Talalaenko AN, Pankrat'ev DV, Goncharenko NV (2001). "[Neurochemical characteristics of the ventromedial hypothalamus and anti-aversive effects of anxiolytic agents in various anxiety models]". Ross Fiziol Zh Im I M Sechenova (in Russian). 87 (9): 1217–26. PMID 11763535.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
- Kresiun VI (1984). "[Mg2+-ATPase activity of brain mitochondria fractions in chronic stress and its correction by psychotropic agents]". Ukr. Biokhim. Zh. (in Russian). 56 (6): 637–41. PMID 6151270.
- Karkishchenko NN, Dimitriadi NA (1991). "[The adequacy of a new method for assessing the vestibular protective effect of biologically active substances]". Farmakol Toksikol (in Russian). 54 (5): 14–6. PMID 1800137.
