Drosophila melanogaster
| Drosophila melanogaster | |
|---|---|

| |
| Male Drosophila melanogaster | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Subgenus: | |
| Species group: | |
| Species subgroup: | |
| Species complex: | melanogaster complex
|
| Species: | D. melanogaster
|
| Binomial name | |
| Drosophila melanogaster | |
Drosophila melanogaster (Greek for dark-bellied dew lover : δρόσος = dew, φίλος = intimate friend, lover, μέλας = dark-coloured, γαστήρ = belly [2]) is a species of Diptera, or the order of flies, in the family Drosophilidae. The species is commonly known as the common fruit fly or vinegar fly. Starting from Charles W. Woodworth, this species is one of the most commonly used model organisms in biology, including studies in genetics, physiology, microbial pathogenesis and life history evolution because they are easy to take care of, breed quickly, and lay many eggs.[3]
Flies belonging to the family Tephritidae are also called fruit flies, which can lead to confusion, especially in Australia where the term fruit fly refers to the Tephritidae, economic pests in fruit production.
Physical appearance Are you sure this is accurate Mr Iverach?

Wildtype fruit flies have brick red eyes, are yellow-brown in color, and have transverse black rings across their abdomen. They exhibit sexual dimorphism: females are about 2.5 millimeters (0.098 in) long; males are slightly smaller and the back of their bodies is darker. Males are easily distinguished from females based on color differences, with a distinct black patch at the abdomen, less noticeable in recently emerged flies (see fig), and the sexcombs (a row of dark bristles on the tarsus of the first leg). Furthermore, males have a cluster of spiky hairs (claspers) surrounding the reproducing parts used to attach to the female during mating. There are extensive images at FlyBase.[4]
Life cycle and reproduction
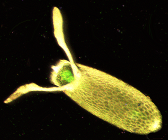
The D. melanogaster lifespan is about 30 days at 29 °C (84 °F).
The developmental period for Drosophila melanogaster varies with temperature, as with many ectothermic species. The shortest development time (egg to adult), 7 days, is achieved at 28 °C (82 °F).[5][6] Development times increase at higher temperatures (11 days at 30 °C or 86 °F) due to heat stress. Under ideal conditions, the development time at 25 °C (77 °F) is 8.5 days,[5][6][7] at 18 °C (64 °F) it takes 19 days[5][6] and at 12 °C (54 °F) it takes over 50 days.[5][6] Under crowded conditions, development time increases,[8] while the emerging flies are smaller.[8][9] Females lay some 400 eggs (embryos), about five at a time, into rotting fruit or other suitable material such as decaying mushrooms and sap fluxes. The eggs, which are about 0.5 millimetres long, hatch after 12–15 hours (at 25 °C or 77 °F).[5][6] The resulting larvae grow for about 4 days (at 25 °C) while molting twice (into 2nd- and 3rd-instar larvae), at about 24 and 48 h after hatching.[5][6] During this time, they feed on the microorganisms that decompose the fruit, as well as on the sugar of the fruit itself. Then the larvae encapsulate in the puparium and undergo a four-day-long metamorphosis (at 25 °C), after which the adults eclose (emerge).[5][6]

Females become receptive to courting males at about 8–12 hours after emergence.[10] Males perform a sequence of five behavioral patterns to court females. First, males orient themselves while playing a courtship song by horizontally extending and vibrating their wings. Soon after, the male positions itself at the rear of the female's abdomen in a low posture to tap and lick the female genitalia. Finally, the male curls its abdomen, and attempts copulation. Females can reject males by moving away, kicking and extruding their ovipositor. Copulation lasts around 15–20 minutes,[11] during which males transfer a few hundred very long (1.76 mm) sperm cells in seminal fluid to the female.[12] Females store the sperm in a tubular receptacle and in two mushroom-shaped spermathecae, sperm from multiple matings compete for fertilization. A last male precedence is believed to exist in which the last male to mate with a female sires approximately 80% of her offspring. This precedence was found to occur through displacement and incapacitation.[13] The displacement is attributed to sperm handling by the female fly as multiple matings are conducted and is most significant during the first 1–2 days after copulation. Displacement from the seminal receptacle is more significant than displacement from the spermathecae.[13] Incapacitation of first male sperm by second male sperm becomes significant 2–7 days after copulation. The seminal fluid of the second male is believed to be responsible for this incapacitation mechanism (without removal of first male sperm) which takes effect before fertilization occurs.[13] The delay in effectiveness of the incapacitation mechanism is believed to be a protective mechanism that prevents a male fly from incapacitating its own sperm should it mate with the same female fly repetitively.[13]
History of use in genetic analysis

Drosophila melanogaster was among the first organisms used for genetic analysis, and today it is one of the most widely used and genetically best-known of all eukaryotic organisms. All organisms use common genetic systems; therefore, comprehending processes such as transcription and replication in fruit flies helps in understanding these processes in other eukaryotes, including humans.[14]
Charles W. Woodworth is credited with being the first to breed Drosophila in quantity and for suggesting to W. E. Castle that they might be used for genetic research during his time at Harvard University.
Thomas Hunt Morgan began using fruit flies in experimental studies of heredity at Columbia University in 1910. His laboratory was located on the top floor of Schermerhorn Hall, which became known as the Fly Room. The Fly Room was cramped with eight desks, each occupied by students and their experiments. They started off experiments using milk bottles to rear the fruit flies and handheld lenses for observing their traits. The lenses were later replaced by microscopes, which enhanced their observations. The Fly Room was the source of some of the most important research in the history of biology. Morgan and his students eventually elucidated many basic principles of heredity, including sex-linked inheritance, epistasis, multiple alleles, and gene mapping.[14]
"Thomas Hunt Morgan and colleagues extended Mendel's work by describing X-linked inheritance and by showing that genes located on the same chromosome do not show independent assortment. Studies of X-linked traits helped confirm that genes are found on chromosomes, while studies of linked traits led to the first maps showing the locations of genetic loci on chromosomes" (Freman 214). The first maps of Drosophila chromosomes were completed by Alfred Sturtevant.
Model organism in genetics

Drosophila melanogaster is one of the most studied organisms in biological research, particularly in genetics and developmental biology. There are several reasons:
- The care and culture requires little equipment and use little space even when using large cultures, and the overall cost is low.
- It is small and easy to grow in the laboratory and their morphology is easy to identify once they are anesthetized (usually with ether, carbon dioxide gas, by cooling them, or with products like FlyNap)
- It has a short generation time (about 10 days at room temperature) so several generations can be studied within a few weeks.
- It has a high fecundity (females lay up to 100 eggs per day, and perhaps 2000 in a lifetime).[3]
- Males and females are readily distinguished and virgin females are easily isolated, facilitating genetic crossing.
- The mature larvae show giant chromosomes in the salivary glands called polytene chromosomes—"puffs" indicate regions of transcription and hence gene activity.
- It has only four pairs of chromosomes: three autosomes, and one sex chromosome.
- Males do not show meiotic recombination, facilitating genetic studies.
- Recessive lethal "balancer chromosomes" carrying visible genetic markers can be used to keep stocks of lethal alleles in a heterozygous state without recombination due to multiple inversions in the balancer.
- Genetic transformation techniques have been available since 1987.
- Its complete genome was sequenced and first published in 2000.[15]
Genetic markers
Genetic markers are commonly used in Drosophila research, for example within balancer chromosomes or P-element inserts, and most phenotypes are easily identifiable either with the naked eye or under a microscope. In the list of example common markers below, the allele symbol is followed by the name of the gene affected and a description of its phenotype. (Note: Recessive alleles are in lower case, while dominant alleles are capitalised.)
- Cy1: curly; The wings curve away from the body, flight may be somewhat impaired.
- e1: ebony; Black body and wings (heterozygotes are also visibly darker than wild type).
- Sb1: stubble; Hairs are shorter and thicker than wild type.
- w1: white; Eyes lack pigmentation and appear white, vision may be somewhat impaired.
- y1: yellow; Body pigmentation and wings appear yellow.
Drosophila genes are traditionally named after the phenotype they cause when mutated. For example, the absence of a particular gene in Drosophila will result in a mutant embryo that does not develop a heart. Scientists have thus called this gene tinman, named after the Oz character of the same name.[16] This system of nomenclature results in a wider range of gene names than in other organisms.
Genome

The genome of D. melanogaster (sequenced in 2000, and curated at the FlyBase database[15]) contains four pairs of chromosomes: an X/Y pair, and three autosomes labeled 2, 3, and 4. The fourth chromosome is so tiny that it is often ignored, aside from its important eyeless gene. The D. melanogaster sequenced genome of 165 million base pairs has been annotated[17] and contains approximately 13,767 protein-coding genes, which comprise ~20% of the genome out of a total of an estimated 14,000 genes. More than 60% of the genome appears to be functional non-protein-coding DNA[18] involved in gene expression control. Determination of sex in Drosophila occurs by the ratio of X chromosomes to autosomes, not because of the presence of a Y chromosome as in human sex determination. Although the Y chromosome is entirely heterochromatic, it contains at least 16 genes, many of which are thought to have male-related functions.[19]
Similarity to humans
About 75% of known human disease genes have a recognizable match in the genome of fruit flies,[20] and 50% of fly protein sequences have mammalian homologs. An online database called Homophila is available to search for human disease gene homologues in flies and vice versa.[21] Drosophila is being used as a genetic model for several human diseases including the neurodegenerative disorders Parkinson's, Huntington's, spinocerebellar ataxia and Alzheimer's disease. The fly is also being used to study mechanisms underlying aging and oxidative stress, immunity, diabetes, and cancer, as well as drug abuse.
Development
Embryogenesis in Drosophila has been extensively studied, as its small size, short generation time, and large brood size makes it ideal for genetic studies. It is also unique among model organisms in that cleavage occurs in a syncytium.
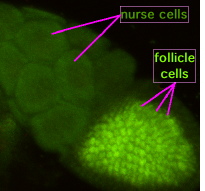
During oogenesis, cytoplasmic bridges called "ring canals" connect the forming oocyte to nurse cells. Nutrients and developmental control molecules move from the nurse cells into the oocyte. In the figure to the left, the forming oocyte can be seen to be covered by follicular support cells.
After fertilization of the oocyte the early embryo (or syncytial embryo) undergoes rapid DNA replication and 13 nuclear divisions until approximately 5000 to 6000 nuclei accumulate in the unseparated cytoplasm of the embryo. By the end of the 8th division most nuclei have migrated to the surface, surrounding the yolk sac (leaving behind only a few nuclei, which will become the yolk nuclei). After the 10th division the pole cells form at the posterior end of the embryo, segregating the germ line from the syncytium. Finally, after the 13th division cell membranes slowly invaginate, dividing the syncytium into individual somatic cells. Once this process is completed gastrulation starts.[22]
Nuclear division in the early Drosophila embryo happens so quickly there are no proper checkpoints so mistakes may be made in division of the DNA. To get around this problem, the nuclei that have made a mistake detach from their centrosomes and fall into the centre of the embryo (yolk sac), which will not form part of the fly.
The gene network (transcriptional and protein interactions) governing the early development of the fruit fly embryo is one of the best understood gene networks to date, especially the patterning along the antero-posterior (AP) and dorso-ventral (DV) axes (See under morphogenesis).[22]
The embryo undergoes well-characterized morphogenetic movements during gastrulation and early development, including germ-band extension, formation of several furrows, ventral invagination of the mesoderm, posterior and anterior invagination of endoderm (gut), as well as extensive body segmentation until finally hatching from the surrounding cuticle into a 1st-instar larva.
During larval development, tissues known as imaginal discs grow inside the larva. Imaginal discs develop to form most structures of the adult body, such as the head, legs, wings, thorax and genitalia. Cells of the imaginal disks are set aside during embryogenesis and continue to grow and divide during the larval stages—unlike most other cells of the larva, which have differentiated to perform specialized functions and grow without further cell division. At metamorphosis, the larva forms a pupa, inside which the larval tissues are reabsorbed and the imaginal tissues undergo extensive morphogenetic movements to form adult structures.
Immunity
Unlike mammals, Drosophila only have innate immunity and lack an adaptive immune response. The D. melanogaster immune system can be divided into two responses: humoral and cell-mediated. The former is a systemic response mediated through the Toll and imd pathways, which are parallel systems for detecting microbes. The Toll pathway in Drosophila is known as the homologue of Toll-Like pathways in mammals. Spatzle, a known ligand for the Toll pathway in flies, is produced in response to Gram+ve bacteria, parasites, and fungal infection. Upon infection, pro-Spatzle will be cleaved by protease SPE (Spatzle processing enzyme) to become active Spatzle, which then binds to the Toll receptor located on the cell surface (Fat body, hemocytes) and dimerise for activation of downstream NF-κB signaling pathways. On the other hand, the imd pathway is triggered by Gram-ve bacteria through soluble and surface receptors (PGRP-LE and LC, respectively). D. melanogaster have a "fat body", which is thought to be homologous to the human liver. It is the primary secretory organ and produces antimicrobial peptides. These peptides are secreted into the hemolymph and bind infectious bacteria, killing them by forming pores in their cell walls. Years ago many drug companies wanted to purify these peptides and use them as antibiotics. Other than the fat body, hemocytes, the blood cells in drosophila, are known as the homologue of mammalian monocyte/macrophages, possessing a significant role in immune responses. It is known from the literature that in response to immune challenge, hemocytes are able to secret cytokines, for example Spatzle, to activate downstream signaling pathways in the fat body. However, the mechanism still remains unclear.
Behavioral genetics and neuroscience
In 1971, Ron Konopka and Seymour Benzer published "Clock mutants of Drosophila melanogaster", a paper describing the first mutations that affected an animal's behavior. Wild-type flies show an activity rhythm with a frequency of about a day (24 hours). They found mutants with faster and slower rhythms as well as broken rhythms—flies that move and rest in random spurts. Work over the following 30 years has shown that these mutations (and others like them) affect a group of genes and their products that comprise a biochemical or biological clock. This clock is found in a wide range of fly cells, but the clock-bearing cells that control activity are several dozen neurons in the fly's central brain.
Since then, Benzer and others have used behavioral screens to isolate genes involved in vision, olfaction, audition, learning/memory, courtship, pain and other processes, such as longevity.
The first learning and memory mutants (dunce, rutabaga etc.) were isolated by William "Chip" Quinn while in Benzer's lab, and were eventually shown to encode components of an intracellular signaling pathway involving cyclic AMP, protein kinase A and a transcription factor known as CREB. These molecules were shown to be also involved in synaptic plasticity in Aplysia and mammals.
Male flies sing to the females during courtship using their wing to generate sound, and some of the genetics of sexual behavior have been characterized. In particular, the fruitless gene has several different splice forms, and male flies expressing female splice forms have female-like behavior and vice-versa.
Furthermore, Drosophila has been used in neuropharmacological research, including studies of cocaine and alcohol consumption.
Vision

The compound eye of the fruit fly contains 760 unit eyes or ommatidia, and are one of the most advanced among insects. Each ommatidium contains 8 photoreceptor cells (R1-8), support cells, pigment cells, and a cornea. Wild-type flies have reddish pigment cells, which serve to absorb excess blue light so the fly isn't blinded by ambient light.
Each photoreceptor cell consists of two main sections, the cell body and the rhabdomere. The cell body contains the nucleus while the 100-μm-long rhabdomere is made up of toothbrush-like stacks of membrane called microvilli. Each microvillus is 1–2 μm in length and ~60 nm in diameter.[23] The membrane of the rhabdomere is packed with about 100 million rhodopsin molecules, the visual protein that absorbs light. The rest of the visual proteins are also tightly packed into the microvillar space, leaving little room for cytoplasm.
The photoreceptors in Drosophila express a variety of rhodopsin isoforms. The R1-R6 photoreceptor cells express Rhodopsin1 (Rh1), which absorbs blue light (480 nm). The R7 and R8 cells express a combination of either Rh3 or Rh4, which absorb UV light (345 nm and 375 nm), and Rh5 or Rh6, which absorb blue (437 nm) and green (508 nm) light respectively. Each rhodopsin molecule consists of an opsin protein covalently linked to a carotenoid chromophore, 11-cis-3-hydroxyretinal.[24]

As in vertebrate vision, visual transduction in invertebrates occurs via a G protein-coupled pathway. However, in vertebrates the G protein is transducin, while the G protein in invertebrates is Gq (dgq in Drosophila). When rhodopsin (Rh) absorbs a photon of light its chromophore, 11-cis-3-hydroxyretinal, is isomerized to all-trans-3-hydroxyretinal. Rh undergoes a conformational change into its active form, metarhodopsin. Metarhodopsin activates Gq, which in turn activates a phospholipase Cβ (PLCβ) known as NorpA.[25]
PLCβ hydrolyzes phosphatidylinositol (4,5)-bisphosphate (PIP2), a phospholipid found in the cell membrane, into soluble inositol triphosphate (IP3) and diacylgycerol (DAG), which stays in the cell membrane. DAG or a derivative of DAG causes a calcium selective ion channel known as TRP (transient receptor potential) to open and calcium and sodium flows into the cell. IP3 is thought to bind to IP3 receptors in the subrhabdomeric cisternae, an extension of the endoplasmic reticulum, and cause release of calcium, but this process doesn't seem to be essential for normal vision.[25]
Calcium binds to proteins such as calmodulin (CaM) and an eye-specific protein kinase C (PKC) known as InaC. These proteins interact with other proteins and have been shown to be necessary for shut off of the light response. In addition, proteins called arrestins bind metarhodopsin and prevent it from activating more Gq. A sodium-calcium exchanger known as CalX pumps the calcium out of the cell. It uses the inward sodium gradient to export calcium at a stoichiometry of 3 Na+/ 1 Ca++.[26]
TRP, InaC, and PLC form a signaling complex by binding a scaffolding protein called InaD. InaD contains five binding domains called PDZ domain proteins, which specifically bind the C termini of target proteins. Disruption of the complex by mutations in either the PDZ domains or the target proteins reduces the efficiency of signaling. For example, disruption of the interaction between InaC, the protein kinase C, and InaD results in a delay in inactivation of the light response.
Unlike vertebrate metarhodopsin, invertebrate metarhodopsin can be converted back into rhodopsin by absorbing a photon of orange light (580 nm).
Approximately two-thirds of the Drosophila brain is dedicated to visual processing.[27] Although the spatial resolution of their vision is significantly worse than that of humans, their temporal resolution is approximately ten times better.
Flight
The wings of a fly are capable of beating at up to 220 times per second. Flies fly via straight sequences of movement interspersed by rapid turns called saccades. During these turns, a fly is able to rotate 90 degrees in fewer than 50 milliseconds.
It was long thought that the characteristics of Drosophila flight were dominated by the viscosity of the air, rather than the inertia of the fly body. However, research in the lab of Michael Dickinson has indicated that flies perform banked turns, where the fly accelerates, slows down while turning, and accelerates again at the end of the turn. This indicates that inertia is the dominant force, as is the case with larger flying animals.[28][29] Recent work, however, has shown that while the viscous effects on the insect body during flight may be negligible, the aerodynamic forces on the wings themselves actually cause fruit flies' turns to be damped viscously.[30]
See also
References
- ^ Meigen JW (1830). Systematische Beschreibung der bekannten europäischen zweiflügeligen Insekten. (Volume 6) (PDF) (in German). Schulz-Wundermann.
- ^ Η Πύλη για την ελληνική γλώσσα
- ^ a b James H. Sang (2001-06-23). "Drosophila melanogaster: The Fruit Fly". In Eric C. R. Reeve (ed.). Encyclopedia of genetics. USA: Fitzroy Dearborn Publishers, I. p. 157. Retrieved 2009-07-01.
{{cite encyclopedia}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "FlyBase: A database of Drosophila genes and genomes". Genetics Society of America. 2009. Retrieved August 11, 2009.
- ^ a b c d e f g Ashburner M, Thompson JN (1978). "The laboratory culture of Drosophila". In Ashburner M, Wright TRF (ed.). The genetics and biology of Drosophila. Vol. 2A. Academic Press. 1–81.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) - ^ a b c d e f g Ashburner M, Golic KG, Hawley RS (2005). Drosophila: A Laboratory Handbook (2nd ed.). Cold Spring Harbor Laboratory Press. pp. 162–4. ISBN 0879697067.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Bloomington Drosophila Stock Center at Indiana University: Basic Methods of Culturing Drosophila
- ^ a b Chiang HC, Hodson AC (1950). "An analytical study of population growth in Drosophila melanogaster". Ecological Monographs. 20 (3): 173–206. doi:10.2307/1948580.
- ^ Bakker K (1961). "An analysis of factors which determine success in competition for food among larvae of Drosophila melanogaster". Archives Neerlandaises de Zoologie. 14: 200–281.
- ^ Pitnick S (1996). "Investment in testes and the cost of making long sperm in Drosophila". American Naturalist. 148: 57–80. doi:10.1086/285911.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1242/jeb.041566, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1242/jeb.041566instead. - ^ Gilbert SF (2006). "9: Fertilization in Drosophila". In 8th (ed.). Developmental Biology. Sinauer Associates. ISBN 978-0878932504.
{{cite book}}: CS1 maint: numeric names: editors list (link) - ^ a b c d Price C; et al. (1999). "Sperm competition between Drosophila males involves both displacement and incapacitation". Nature. 400 (6743): 449–452. doi:10.1038/22755. PMID 10440373.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Pierce, Benjamin A (2004). Genetics: A Conceptual Approach (2nd ed.). W. H. Freeman. ISBN 978-0716788812.
- ^ a b Adams MD, Celniker SE, Holt RA; et al. (2000). "The genome sequence of Drosophila melanogaster". Science. 287 (5461): 2185–95. doi:10.1126/science.287.5461.2185. PMID 10731132. Retrieved 2007-05-25.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Azpiazu N, Frasch M (1993). "tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila". Genes and Development. 7 (7b): 1325–1340. doi:10.1101/gad.7.7b.1325. PMID 8101173.
- ^ Manning, Gerard (Oct 1, 2006). "Introduction to Drosophila". The WWW Virtual Library: Drosophila. p. 1. Retrieved 2009-08-17.
- ^ Halligan DL, Keightley PD (2006). "Ubiquitous selective constraints in the Drosophila genome revealed by a genome-wide interspecies comparison". Genome Research. 16 (7): 875–84. doi:10.1101/gr.5022906. PMC 1484454. PMID 16751341.
{{cite journal}}:|access-date=requires|url=(help) - ^ Carvalho, AB (2002). "Origin and evolution of the Drosophila Y chromosome". Current Opinion in Genetics & Development. 12 (6852): 664–668. doi:10.1016/S0959-437X(02)00356-8.
- ^ Reiter, LT; Potocki, L; Chien, S; Gribskov, M; Bier, E (2001). "A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster". Genome Research. 11 (6): 1114–1125. doi:10.1101/gr.169101. PMC 311089. PMID 11381037.
- ^ Bier lab (2008). "Homophila: Human disease to Drosophila disease database". University of California, San Diego. Retrieved August 11, 2009.
- ^ a b Katrin Weigmann, Robert Klapper, Thomas Strasser, Christof Rickert, Gerd Technau, Herbert Jäckle, Wilfried Janning & Christian Klämbt (2003). "FlyMove – a new way to look at development of Drosophila". Trends in Genetics. 19 (6): 310–311. doi:10.1016/S0168-9525(03)00050-7. PMID 12801722.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hardie RC, Raghu P (2001). "Visual transduction in Drosophila". Nature. 413 (6852): 186–93. doi:10.1038/35093002. PMID 11557987.
{{cite journal}}:|access-date=requires|url=(help) - ^ Nichols R, Pak WL (1985). "Characterization of Drosophila melanogaster rhodopsin". Journal of Biological Chemistry. 260 (23): 12670–4. PMID 3930500.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b Raghu P, Colley NJ, Webel R; et al. (2000). "Normal phototransduction in Drosophila photoreceptors lacking an InsP(3) receptor gene". Molecular and Cellular Neuroscience. 15 (5): 429–45. doi:10.1006/mcne.2000.0846. PMID 10833300.
{{cite journal}}:|access-date=requires|url=(help); Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Wang T, Xu H, Oberwinkler J, Gu Y, Hardie R, Montell C; et al. (2005). "Light activation, adaptation, and cell survival Functions of the Na+/Ca2+ exchanger CalX". Neuron. 45 (3): 367–378. doi:10.1016/j.neuron.2004.12.046. PMID 15694299.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Rein, K. and Zockler, M. and Mader, M.T. and Grubel, C. and Heisenberg, M. (2002). "The Drosophila Standard Brain". Current Biology. 12 (3): 227–231. doi:10.1016/S0960-9822(02)00656-5. PMID 11839276.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Caltech Press Release 4/17/2003
- ^ S. Fry and M. Dickinson (2003). "The aerodynamics of free-flight maneuvers in Drosophila". Science. 300 (5618): 495–8. doi:10.1126/science.1081944. PMID 12702878.
- ^ T. Hesselberg and F.-O. Lehmann (2007). "Turning behaviour depends on frictional damping in the fruit fly "Drosophila"". The Journal of Experimental Biology. 210 (Pt 24): 4319–34. doi:10.1242/jeb.010389. PMID 18055621.
Further reading
- K. Haug-Collet; et al. (1999). "Cloning and characterization of a potassium-dependent sodium/calcium exchanger in Drosophila". J. Cell Biol. 147 (3): 659–70. doi:10.1083/jcb.147.3.659. PMC 2151195. PMID 10545508.
{{cite journal}}: Explicit use of et al. in:|author=(help) - R. Ranganathan; et al. (1995). "Signal transduction in Drosophila photoreceptors". Annu. Rev. Neurosci. 18: 283–317. doi:10.1146/annurev.ne.18.030195.001435. PMID 7605064.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Adams MD; et al. (2000). "The genome sequence of Drosophila melanogaster". Science. 287 (5461): 2185–95. doi:10.1126/science.287.5461.2185. PMID 10731132.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Kohler, Robert E. (1994). Lords of the Fly: Drosophila genetics and the experimental life. Chicago: University of Chicago Press. ISBN 0-226-45063-5.
Popular media
- "Inside the Fly Lab" — broadcast by WGBH and PBS, in the program series "Curious", January 2008.
- "How a Fly Detects Poison"[dead link] — WhyFiles.org article describes how the fruit fly tastes a larva-killing chemical in food.
External links
- A quick and simple introduction to Drosophila melanogaster
- FlyBase — A Database of Drosophila Genes & Genomes
- NCBI page on Drosophila melanogaster
- The WWW Virtual Library: Drosophila
- The Berkeley Drosophila Genome Project
- FlyMove
- The Interactive Fly — A guide to Drosophila genes and their roles in development
- Drosophila Nomenclature — naming of genes
- Make Your Own Fruit Fly Trap
- Illustrates a simple to make non-toxic Vinegar fly trap
- Measurement of Courtship Behavior in Drosophila melanogaster
- Maintenance of a Drosophila Laboratory: General Procedures
- Transcript In Situ Hybridization of Whole-Mount Embryos for Phenotype Analysis of RNAi-Treated Drosophila
- Injection of dsRNA into Drosophila Embryos for RNA Interference (RNAi)
