Graves' disease
| Graves' disease | |
|---|---|
| Specialty | Endocrinology |
Graves' disease is an autoimmune disease where the thyroid is overactive, producing an excessive amount of thyroid hormones (a serious metabolic imbalance known as hyperthyroidism and thyrotoxicosis). This is caused by thyroid autoantibodies (TSHR-Ab) that activate the TSH-receptor (TSHR), thereby stimulating thyroid hormone synthesis and secretion, and thyroid growth (causing a diffusely enlarged goiter). The resulting state of hyperthyroidism can cause a dramatic constellation of neuropsychological and physical signs and symptoms.[1]
Graves' disease is the most common cause of hyperthyroidism, and usually presents itself during early adolescence.[2] It has a powerful hereditary component, affects up to 2% of the female population, and is between five and ten times as common in females as in males.[3] Graves’ disease is also the most common cause of severe hyperthyroidism, which is accompanied by more clinical signs and symptoms and laboratory abnormalities as compared with milder forms of hyperthyroidism.[4] About 25-30% of people with Graves' disease will also suffer from Graves' ophthalmopathy (a protrusion of one or both eyes), caused by inflammation of the eye muscles by attacking autoantibodies.[5]
Diagnosis is usually made on the basis of symptoms, although thyroid hormone tests may be useful.[6] Graves’ thyrotoxicosis frequently builds over an extended period, sometimes reaching years, before being diagnosed.[7] This is partially because symptoms can develop so insidiously that they go unnoticed; when they do get reported, they are often confused with other health problems. Thus, diagnosing thyroid disease clinically can be challenging.[8] Nevertheless, patients can experience a wide range of symptoms and suffer major impairment in most areas of health-related quality of life.[9]
There is no cure for Graves’ disease. There are, however, treatments for its consequences: hyperthyroidism, ophthalmopathy and mental symptoms.[10] The Graves’ disease itself - as defined, for example, by high serum TSHR-Ab concentrations or ophthalmopathy - often persists after its hyperthyroidism has been successfully treated.[10]
Symptoms and signs
The symptoms and signs of Graves' disease virtually all result from the direct and indirect effects of hyperthyroidism, with main exceptions being Graves' ophthalmopathy, goitre and pretibial myxedema (which are caused by the autoimmune processes of Graves' disease). Symptoms of the resultant hyperthyroidism are mainly insomnia, hand tremor, hyperactivity, hair loss, excessive sweating, heat intolerance, weight loss despite increased appetite, diarrhea, frequent defecation, palpitations, muscle weakness and skin being warm and moist.[11] Further signs that may be seen on physical examination are most commonly a diffusely enlarged (usually symmetric) nontender thyroid, lid lag, excessive lacrimation due to Graves' ophthalmopathy, arrhythmias of the heart such as sinus tachycardia, atrial fibrillation and premature ventricular contractions, as well as hypertension.[11] It is important to note that thyrotoxic patients may experience behavioral and personality changes, such as psychosis, agitation, and depression. In milder hyperthyroidism, patients will rather experience less overt manifestations like anxiety, restlessness, irritability, and emotional lability.[12]
Cause
The trigger for autoantibody production is unknown.
Some people may have a genetic predisposition to develop TSH receptor autoantibodies (HLADR, especially DR3, appears to play a significant role.[13] Some of the eye symptoms of hyperthyroidism are believed to result from heightened sensitivity of receptors to sympathetic nervous system activity, possibly mediated by increased alpha-adrenergic receptors in some tissues.[14]
Neuropsychological manifestations
Hyperthyroidism plays a major role in psychiatric morbidity in Graves' disease, and is associated with long-term mood disturbances.[15][16] Although hyperthyroidism has been considered to induce psychiatric symptoms by enhancement of the sensitivity and turnover in catecholaminergic neurotransmission, the precise mechanism of cognitive and behavioral dysfunction in hyperthyroidism is not known.[17] According to Gonen, the direct influence of thyroid hormones on brain functions stems from the wide distribution of T3 receptors throughout the brain.[18] Improvement of some clinical features (attention and concentration) with beta-blocker therapy suggests a role for a hyperthyroid-induced hyperactivity of the adrenergic nervous system, possibly disrupting the adrenergic pathways between the locus ceruleus and frontal lobe that subserve attention and vigilance, and thereby accounting for many physical and mental symptoms.[19] Another possibility is that hyperthyroidism may cause oxidative stress, resulting in neuronal injury and hastening a presentation of degenerative or vascular dementia.[20] A study of 2002 suggests another possible mechanism, involving activational and translational regulation of functional proteins in the brain.[17]
Whatever the precise mechanisms, it is clear that thyroid hormones influence adult brain functioning, and may interact with mood regulation via targets in specific brain circuits.[16] Singh et al. formulate that "differential thyroidal status is known to cause decrease in cell number and induces irreversible morphometric changes in adult brain resulting in different neuronal abnormalities".[21] This is underscored by recent studies, who document a thyroid hormone effect on the neurotransmitters serotonin and norepinephrine, with changes in neurotransmitter synthesis and receptor sensitivity being noted.[22] De Groot points out that, in spite of the fact that epinephrine levels and catecholamine excretion are actually not elevated, propranolol (it is presumed, acting by inhibition of alpha-adrenergic sympathetic activity) reduces anxiety and tremor in a very useful manner, indicating that some of the central nervous system irritability is a manifestation of elevated sensitivity to circulating epinephrine (though this has not been proved).[3] Thompson mentions that T3 can increase the activity of serotonin in the brain, while serotonin has been shown to inhibit thyroid function. Thus, although a complex system of interaction between thyroid hormone and neurotransmitters has been recognized and examined, no clear-cut explanation for the effect of thyroid hormone on depression has emerged.[22]
A literature study of 2006 mentions that ophthalmopathy may also contribute to psychiatric morbidity, it is presumed through the psychosocial consequences of a changed appearance.[10] However, the observation that a substantial proportion of patients have an altered mental state even after successful treatment of hyperthyroidism, has led some researchers to suggest that the automimmune process itself may play a role in the presentation of mental symptoms and psychiatric disorders in Graves’ disease, whether or not ophthalmopathy is present.[10] Persistent stimulation of TSH-Rs may be involved. In Graves’ disease, the TSH-R gives rise to antibodies and in some patients these antibodies persist after restoration of euthyroidism. The cerebral cortex and hippocampus are rich in TSH-Rs. Antibody stimulation of these brain receptors may result in increased local production of T3.[10]
Thus, despite ongoing research, a complete understanding of the causes of mental disability in Graves’ disease awaits a full description of the effects on neural tissue of thyroid hormones as well as of the underlying autoimmune process.[15]
Pathophysiology
Graves' disease is an autoimmune disorder, in which the body produces antibodies to the receptor for thyroid-stimulating hormone (TSHR). (Antibodies to thyroglobulin and to the thyroid hormones T3 and T4 may also be produced.) These antibodies (TSHR-Ab) bind to the TSH receptors, which are located on the cells that produce thyroid hormone in the thyroid gland (follicular cells), and chronically stimulate them, resulting in an abnormally high production of T3 and T4. This causes the clinical symptoms of hyperthyroidism, and the enlargement of the thyroid gland (visible as goitre).
The infiltrative exophthalmos that is frequently encountered, has been explained by postulating that the thyroid gland and the extraocular muscles share a common antigen that is recognized by the antibodies. Antibodies binding to the extraocular muscles would cause swelling behind the eyeball. This swelling has also been postulated to be the consequence of mucopolysacharide deposition posterior to the eyes, a symptom tangentially related to Graves'. The "orange peel" skin has been explained by the infiltration of antibodies under the skin, causing an inflammatory reaction and subsequent fibrous plaques.
There are 3 types of autoantibodies to the TSH receptor currently recognized:
- TSI, Thyroid-stimulating immunoglobulins: these antibodies (mainly Immunoglobulin G) act as LATS (Long-Acting Thyroid Stimulants), activating the cells in a longer and slower way than the normal thyroid-stimulating hormone (TSH), leading to an elevated production of thyroid hormone.
- TGI, Thyroid growth immunoglobulins: these antibodies bind directly to the TSH-receptor and have been implicated in the growth of thyroid follicles.
- TBII, Thyrotropin Binding-Inhibiting Immunoglobulins: these antibodies inhibit the normal union of TSH with its receptor. Some will actually act as if TSH itself is binding to its receptor, thus inducing thyroid function. Other types may not stimulate the thyroid gland, but will prevent TSI and TSH from binding to and stimulating the receptor.
In their study of thyrotoxic patients, Sensenbach et al. found the cerebral blood flow to be increased, the cerebral vascular resistance decreased, arteriovenous oxygen difference decreased, and oxygen consumption unchanged. They found that during treatment, brain size was shown to decrease significantly, and ventricular size increased. The cause of this remarkable change is unknown, but may involve osmotic regulation.[23] A study by Singh et al. showed for the first time that differential thyroidal status induces apoptosis in adult cerebral cortex. T3 acts directly on cerebral cortex mitochondria and induces release of cytochrome c to induce apoptosis. They note that adult cerebellum seems to be less responsive to changes in thyroidal status.[21]
Hyperthyroidism causes lower levels of apolipoprotein (A), HDL, and ratio of total/HDL cholesterol.[3] The processes and pathways mediating the intermediary metabolism of carbohydrates, lipids, and proteins are all affected by thyroid hormones in almost all tissues.[24] Protein formation and destruction are both accelerated in hyperthyroidism. The absorption of vitamin A is increased and conversion of carotene to vitamin A is accelerated (the requirements of the body are likewise increased, and low blood concentrations of vitamin A may be found). Requirements for thiamine and vitamin B6 and B12 are increased. Lack of the B vitamins has been implicated as a cause of liver damage in thyrotoxicosis.[3] Hyperthryoidism can also augment calcium levels in the blood by as much as 25% (known as hypercalcaemia). An increased excretion of calcium and phosphorus in the urine and stool can result in bone loss from osteoporosis.[25] Also, parathyroid hormone (PTH) levels tend to be suppressed in hyperthyroidism, possibly in response to elevated calcium levels.[26]
Diagnosis

The onset of Graves' disease symptoms is often insidious: the intensity of symptoms can increase gradually for a long time before the patient is correctly diagnosed with Graves’ disease, which may take months or years.[7] (Not only Graves' disease but most endocrinological diseases have an insidious, subclinical onset.[27]) One study puts the average time for diagnosis at 2.9 years, having observed a range from 3 months to 20 years in their sample population.[15] A 1996 study offers a partial explanation for this generally late diagnosis, suggesting that the psychiatric symptoms cause delays in seeking treatment as well as delays in receiving appropriate diagnosis.[28] Also, earlier symptoms of nervousness, hyperactivity, and a decline in school performance, may easily be attributed to other causes. Many symptoms may occasionally be noted, at times, in otherwise healthy individuals who do not have thyroid disease (e.g., everyone feels anxiety and tension to some degree), and many thyroid symptoms are similar to those of other diseases.[29] Thus, clinical findings may be full blown and unmistakable or insidious and easily confused with other disorders.[30] The results of overlooking the thyroid can however be very serious.[31] Also noteworthy and problematic, is that in a 1996 survey study respondents reported a significant decline in memory, attention, planning, and overall productivity from the period 2 years prior to Graves' symptoms onset to the period when hyperthyroid.[28] Also, hypersensitivity of the central nervous system to low-grade hyperthyroidism can result in an anxiety disorder before other Graves’ disease symptoms emerge. E.g., panic disorder has been reported to precede Graves’ hyperthyroidism by 4 to 5 years in some cases, although it is not known how frequently this occurs.[32]
The resulting hyperthyroidism in Graves' disease causes a wide variety of symptoms. The two signs that are truly 'diagnostic' of Graves' disease (i.e., not seen in other hyperthyroid conditions) are exophthalmos (protuberance of one or both eyes) and pretibial myxedema, a rare skin disorder with an occurrence rate of 1-4%, that causes lumpy, reddish skin on the lower legs. Graves' disease also causes goitre (an enlargement of the thyroid gland) that is of the diffuse type (i.e., spread throughout the gland). This phenomenon also occurs with other causes of hyperthyroidism, though Graves' disease is the most common cause of diffuse goitre. A large goitre will be visible to the naked eye, but a smaller goitre may be detectable only by a physical exam. On occasion, goitre is not clinically detectable but may be seen only with CT or ultrasound examination of the thyroid.
A highly suggestive symptom of hyperthyroidism, is a change in reaction to external temperature. A hyperthyroid person will usually develop a preference for cold weather, a desire for less clothing and less bed covering, and a decreased ability to tolerate hot weather.[3] When thyroid disease runs in the family, the physician should be particularly wary: Studies of twins suggest that the genetic factors account for 79% of the liability to the development of Graves’ disease (whereas environmental factors, it is presumed, account for the remainder).[3] Other nearly pathognomonic signs of hyperthyroidism are excessive sweating, high pulse during sleep, and a pattern of weight loss with increased appetite (although this may also occur in diabetes mellitus and malabsorption or intestinal parasitism).[3][33]

Hyperthyroidism in Graves' disease is confirmed, as with any other cause of hyperthyroidism, by a blood test. Elevated blood levels of the principal thyroid hormones (i.e. free T3 and T4), and a suppressed thyroid-stimulating hormone (low due to negative feedback from the elevated T3 and T4), point to hyperthyroidism. However, a 2007 study makes clear that diagnosis depends to a considerable extent on the position of the patient’s unique set point for T4 and T3 within the laboratory reference range (an important issue that is further elaborated below).[34]
Differentiating Graves' hyperthyroidism from the other causes of hyperthyroidism (thyroiditis, toxic multinodular goiter, toxic thyroid nodule, and excess thyroid hormone supplementation) is important to determine proper treatment. Thus, when hyperthyroidism is confirmed, or when blood results are inconclusive, thyroid antibodies should be measured (almost all patients with Graves' hyperthyroidism have detectable TSHR-Ab).[35] Measurement of thyroid-stimulating immunoglobulin (TSI) is the most accurate measure of thyroid antibodies. They will be positive in 60 to 90% of children with Graves' disease. If TSI is not elevated, then a radioactive iodine uptake should be performed; an elevated result with a diffuse pattern is typical of Graves' disease.[36] Biopsy to obtain histological testing is not normally required but may be obtained if thyroidectomy is performed.
Treatment
It is yet unknown how to interrupt the autoimmune processes of Graves' disease, which means treatment has to be indirect. The link that is targeted is the thyroid gland, via three different methods (which have not changed fundamentally since the 1940s). These are the use of antithyroid drugs (which reduce the production of thyroid hormone), partial or complete destruction of the thyroid gland by ingestion of radioactive iodine (radioiodine), and partial or complete surgical removal of the thyroid gland (thyroidectomy).
There is no standard choice for treating Graves' hyperthyroidism; it is not straightforward and often involves complex decision making. The physician must weigh the advantages and disadvantages of the different treatment options and help the patient arrive at an individualized therapeutic strategy that is appropriate and cost-effective. Kaplan summarizes that "the choice of therapy varies according to nonbiological factors - physicians' training and personal experience; local and national practice patterns; patient, physician, and societal attitudes toward radiation exposure; and biological factors including age, reproductive status, and severity of the disease".[37]
Therapy with radioiodine is the most common treatment in the United States, whilst antithyroid drugs and/or thyroidectomy is used more often in Europe, Japan, and most of the rest of the world. However, due to the varying success of every treatment option, patients are often subjected to more than one of these, when the first attempted treatment didn't prove entirely successful; the risk of relapse or subsequent hypothyroidism is substantial.[2]
In the short term, treatment of hyperthyroidism usually produces a parallel decrease in endocrine symptoms and in psychiatric symptoms. When prolonged treatment normalizes thyroid function, some psychiatric symptoms and somatic complaints may persist (as has been thoroughly clarified above).[15] A 2009 study shows that in spite of modern therapeutic modalities, Graves' disease is accompanied by seriously impaired quality of life. Several recent studies stress the importance of early prevention, speedy rehabilitation, and a thorough follow-up of hyperthyroid patients.[38] Patients who do not have a spontaneous remission with the use of antithyroid drugs, become lifelong thyroid patients.
Symptomatic
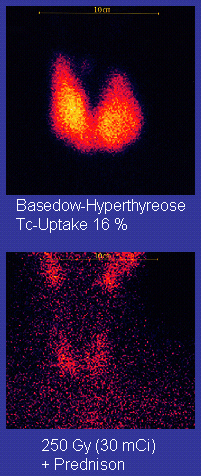
Beta blockers (such as propranolol) may be used to inhibit the sympathetic nervous system symptoms of rapid heart rate and nausea until such time as antithyroid treatments start to take effect.
Antithyroid drugs
The main antithyroid drugs are carbimazole (in the UK), methimazole (in the US), and propylthiouracil/PTU. These drugs block the binding of iodine and coupling of iodotyrosines. The most dangerous side-effect is agranulocytosis. Others include granulocytopenia (dose dependent, which improves on cessation of the drug) and aplastic anemia, and for propylthiouracil severe, fulminant liver failure.[39] Patients on these medications should see a doctor if they develop sore throat or fever.
Treatment with antithyroid medications must be given for six months to two years, in order to be effective. Success rates vary from 34% to 64%, but even then the hyperthyroid state may recur, sometimes upon cessation of the drugs, somtimes months or years later.[2] If treatment with antithyroid drugs fails to induce remission, RAI or surgery must be considered.
Radioiodine
Radioiodine (radioactive iodine-131, abbreviated as RAI) was developed in the early 1940s at the Mallinckrodt General Clinical Research Center. This modality is suitable for most patients, although some doctors prefer to use it mainly for older patients. Indications for RAI are failed medical therapy or surgery, or where medical or surgical therapy are contraindicated. Contraindications to RAI are pregnancy (absolute), ophthalmopathy (relative; it can aggravate thyroid eye disease), and solitary thyoid nodules.
The radio-iodine treatment acts slowly (over months to years) to partially or completely destroy the thyroid gland (depending on the administered dose). Patients must therefore be monitored regularly with thyroid blood tests to ensure that they do not evolve to hypothyroidism (incidence rate of 80%), in which case they will become lifelong thyroid patients. Graves' disease-associated hyperthyroidism is not cured in all persons by radioiodine, but has a relapse rate that depends on the administered dose of radioiodine.
Surgery

This modality is suitable for young patients and pregnant patients. Indications are: a large goitre (especially when compressing the trachea), suspicious nodules or suspected cancer (to pathologically examine the thyroid) and patients with ophthalmopathy. As operating on a frankly hyperthyroid patient is dangerous, prior to thyroidectomy preoperative treatment with antithyroid drugs is given to render the patient "euthyroid". Preoperative administration of (not radioactive) iodine, usually by Lugol's iodine solution, decreases intraoperative blood loss during thyroidectomy in patients with Grave's disease.[40] However, it appears ineffective in patients who are already euthyroid due to treatment with anti-thyroid drugs and T4.[41]
Doctors can opt for partial or total removal of the thyroid gland (subtotal thyroidectomy versus total thyroidectomy). A total removal excludes the difficulty in determining how much thyroid tissue must be removed. More aggressive surgery has a higher likelihood of inducing hypothyroidism; less aggressive surgery has a higher likelihood of recurrent hyperthyroidism.[42] Around 10–15% of patients who had a subtotal thyrodectomy will develop underactive thyroids many years after their operation. This is not counting those who develop underactive thyroids immediately after the operation (within 6 weeks).[43] Thyroid remnants smaller than 4 grams are associated with postoperative hypothyroidism in 27 to 99 percent of patients. Patients who have thyroid remnants of 7 to 8 g become euthyroid, but may have subclinical hyperthyroidism. In addition, 9 to 12 percent develop recurrent overt hyperthyroidism.[44] As repeat surgery is associated with a high risk of complications, further permanent treatment should be with radioiodine.

In a study of 380 patients undergoing a 98% subtotal thyroidectomy, the complications were as follows[45]:
- Transient vocal cord paralysis in 3%
- Prolonged postoperative hypocalcemia in 3%
- Permanent hypoparathyroidism in 1% (due to removal of one or more parathyroid glands)
- Recurrent hyperthyroidism in 2%
A scar is created across the neck just above the collar bone line. However, the scar is very thin, and eventually recedes to appear as nothing more than a crease in the neck. Patients may spend one or more nights in hospital after the surgery, and endure the effects of general anesthesia (i.e., vomiting), as well as a sore throat, a raspy voice, and a cough from having an endotracheal tube inserted in the trachea during surgery.[citation needed]
Removal of the gland enables complete biopsy to be performed to have definite evidence of thyroid cancer, since needle biopsies are not as accurate at predicting a benign state of the thyroid. No further treatment of the thyroid is required, unless cancer is detected. Radioiodine treatment may be done after surgery, to ensure that all remaining (potentially cancerous) thyroid cells are destroyed (i.e., those near the nerves to the vocal cords, which cannot be surgically removed without damage to those cords). Besides this, the only remaining treatment will be thyroid replacement pills (to be taken for the rest of the patient's life), if the surgery results in hypothyroidism.
Thyroid hormones
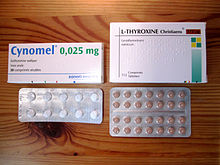
Many Graves' disease patients will become lifelong thyroid patients, due to the surgical removal or radioactive destruction of their thyroid. In effect, they are then hypothyroid patients, requiring perpetual intake of artificial thyroid hormones.[46] Given the one-week plasma half-life of levothyroxine (T4), it takes about five-six weeks (half-lives) before a steady state is attained after the dosage is initiated or changed. After the optimal thyroxine dose has been defined, long-term monitoring of patients with an annual clinical evaluation and serum TSH measurement is appropriate.[46] However, the difficulty lies in determining and controlling the proper dosage for a particular patient, which can be an intricate process. Because levothyroxine has a very narrow therapeutic index, the margin between overdosing and underdosing can be quite small.[47] Being treated with too much or too little thyroid hormone can lead to a chronic state of (possibly subclinical) hypo- or hyperthyroidism. Several studies show that this is not an uncommon occurrence.[8][48][49]
Neuropsychiatric symptoms
A substantial proportion of patients have an altered mental state, even after successful treatment of hyperthyroidism. When psychiatric disorders remain after restoration of euthyroidism and after treatment with beta blockers, specific treatment for the psychiatric symptoms, especially psychotropic drugs, may be needed.[10] A literature study concluded in 2006, found that, after being diagnosed with Graves’ hyperthyroidism, approximately one-third of patients are prescribed psychotropic drugs. Sometimes these drugs are given to treat mental symptoms of hyperthyroidism, sometimes to treat mental symptoms remaining after amelioration of hyperthyroidism, and sometimes when the diagnosis of Graves’ hyperthyroidism has been missed and the patient is treated as having a primary psychiatric disorder. There are no systematic data on the general efficacy of psychotropic drugs in the treatment of mental symptoms in patients with hyperthyroidism, although many reports describe the use of individual agents.[10] De Groot mentions that a mild sedative or tranquilizer is often helpful.[50] German research of 2004 reported that 35 percent of treated Graves' disease patients (with normal thyroid tests for at least six months after treatment), suffered from psychological distress, and had high levels of anxiety. Almost all these patients had clear-cut depression.[51]
Eye disease
Mild cases are treated with lubricant eye drops or nonsteroidal anti-inflammatory drops. Severe cases threatening vision (corneal exposure or optic nerve compression) are treated with steroids or orbital decompression. In all cases cessation of smoking is essential. Double vision can be corrected with prism glasses and surgery (the latter only when the process has been stable for a while).
Eyelid muscles can become tight, making it impossible to completely close the eyes. This can be treated with lubricant gel at night, or with tape on the eyes to enable full sleep. Eyelid surgery can be performed on upper and/or lower eyelids to reverse the effects of Graves' disease on the eyelids. This surgery involves an incision along the natural crease of the eyelid, and a scraping away of the muscle that holds the eyelid open. The muscle then becomes weaker, which allows the eyelid to extend over the eyeball more effectively. Eyelid surgery helps reduce or eliminate dry eye symptoms.
Orbital decompression can be performed to enable bulging eyes to be retracted again. In this procedure, bone is removed from the skull behind the eyes, and space is made for the enlarged muscles and fatty tissue to be moved back into the skull.
General measurements
Graves' disease patients are nutritionally depleted in proportion to the duration and severity of their illness. Until metabolism is restored to normal, and for some time afterward, caloric and protein requirements may be well above normal. Specific deficiencies may exist, and multivitamin supplementation is indicated. The intake of calcium should be above normal. All in all, the physician should pay heed to the patient's emotional needs, as well as to his or her requirements for rest, nutrition, and specific (anti)thyroid medication.[50]
Prognosis
The disease typically begins gradually, and is progressive unless treated.[3] If left untreated, more serious complications could result, including bone loss and fractures, inanition, birth defects in pregnancy, and increased risk of a miscarriage.[52][53][54][3] Graves disease is often accompanied by an increase in heart rate, which may lead to cardiovascular damage and further heart complications including loss of the normal heart rhythm (atrial fibrillation), which may lead to stroke.[3] If the eyes are bulging severely enough that the lids do not close completely at night, severe dryness will occur with a very high risk of a secondary corneal infection, which could lead to blindness. Pressure on the optic nerve behind the globe can lead to visual field defects and vision loss as well. In severe thyrotoxicosis, a condition frequently referred to as thyroid storm, the neurologic presentations are more fulminant, progressing if untreated through an agitated delirium to somnolence and ultimately to coma. All in all, untreated Graves' disease can lead to significant morbidity, disability and even death. However, the long-term history also includes spontaneous remission in some cases and eventual spontaneous development of hypothyroidism if autoimmune thyroiditis coexists and destroys the thyroid gland.[3]
When effective thyroid treatment is begun, the general response is quite favorable: physical symptoms resolve, vitality returns and the mental processes become efficient again.[31] However, symptom relief is usually not immediate and is achieved over time as the treatments take effect and thyroid levels reach stability. In addition, not all symptoms may resolve at the same time. Prognosis also depends on the duration and severity of the disease before treatment. Swedish research of 2005 reports a lower quality of life for 14 to 21 years after treatment of Graves' disease, with lower mood and lower vitality, regardless of the choice of treatment.[55]
Remission and relapses
A literature study in 2006 found that patients who have residual mental symptoms have a significantly higher chance of relapse of hyperthyroidism. Patients with recurrent Graves’ hyperthyroidism, compared with patients in remission and healthy subjects, had significantly higher scores on scales related to depression and anxiety, as well as less tolerance of stress.[10][16] According to a 2010 publication, a total thyroidectomy offers the best chance of preventing recurrent hyperthyroidism.[56]
Mental impairment
A literature review in 2006, whilst noting methodology issues in the consistency of Graves' disease diagnostic criteria, found many reports about residual complaints in patients who were euthyroid after treatment with a high prevalence of anxiety disorders and bipolar disorder, as well as elevated scores on scales of anxiety, depression and psychological distress.[10] Bunevicius et al. point out that this "substantial mental disability" is more severe in patients with residual hyperthyroidism but is present even in euthyroid patients.[15] Delay in therapy markedly worsens the prognosis for recovery, but complications can be prevented by early treatment.[57] In rare cases, patients will experience psychosis-like symptoms only after they have been treated for hypo- or hyperthyroidism, due to a rapid normalisation of thyroid hormone levels in a patient who has partly adapted to abnormal values.[33]
Thyroid replacement treatment after thyroidectomy or radioiodine
Several studies find a high frequency of TSH level abnormalities in patients who take thyroid hormone supplementation for long periods of time, and stress the importance of periodic assessment of serum TSH.[48][49]
Coping with Graves' disease & the patient-physician relationship
Mentally, Graves' disease can be very disturbing. Mood swings, thinking impairment and other mental symptoms can be difficult to handle, and make it appear that the patient is suffering from a severe mental disorder. There have even been cases where patients have been placed in mental institutions.[1][29] Given the sometimes dramatic impact and long duration of the disease and its treatment, identifying and maintaining emotional support systems (which are frequently affected) can help patients and their families cope.[50][28] Because emotional lability of the thyrotoxic patient may create interpersonal problems (often producing significant marital stress and conflict), thorough explanation of the disease can be invaluable.[50][28] In Graves' disease, the accent should lie on written information, as a host of mental problems, such as decreased attention span and memory problems, can impair a patient’s ability to absorb details of doctor visits. In a complicated and difficult illness like Graves' disease, physicians should therefore furnish patients with educational materials or resources such as handouts, website links and community support groups.[58]
However, many patients indicate they are not getting the information they need from the general medical community, and are concerned that they have not obtained a full understanding of their condition.[1][59] De Groot et al. feel that sympathetic discussion by the physician, possibly together with assistance in environmental manipulation, is an important part of the general attack on Graves' disease.[50] Patient education is the "drug of choice" for prevention and treatment of every medical condition, and open communication with health care professionals can be highly beneficial in maximizing health and outlook on life.[50][58] During the initial and subsequent interviews, the physician must determine the level of the psychic and physical stresses. Frequently, major emotional problems come to light after the patient recognizes the sincere interest of the physician. Personal problems can strongly affect therapy by interfering with rest or by causing economic hardship.[50] It is therefore recommended that physicians implement a social questionnaire as part of the initial intake, allowing the patient to communicate essential, non-medical information about their lives.[58]
The communication and health management skills of Graves' disease patients can be seriously impaired. This is something physicians should be conscious of while dealing with these patients, as mounting evidence demonstrates that the effectiveness of the patient-physician relationship directly relates to health outcomes. The report of a large 2003 summit of physicians and patients notes a number of barriers to achieving desired patient-centered outcomes. It mentions insufficient or unreliable clinical information, lack of communication or inability to communicate effectively, lack of trust between patient and physician, lack of appropriate coordination of care, lack of physician cooperation, and the need to work with too many caregivers, all of which can be very relevant to Graves' disease.[58]
Epidemiology
Recent studies in England put the incidence of Graves' disease at 1 to 2 cases per 1,000 population per year (in England). It occurs much more frequently in women than in men. The disease frequently presents itself during early adolescence or begins gradually in adult women, often after childbirth, and is progressive until treatment. It has a powerful hereditary component.[3]
Graves' disease tends to be more severe in men, even though it is rarer. It appears less likely to go into permanent remission and the eye disease tends to be more severe, but men are less likely to have large goitres.[60] In a statistical study of symptoms and signs of 184 thyrotoxic patients (52 men, 132 women), the male patients were somewhat older than the females, and there were more severe cases among men than among women. Cardiac symptoms were more common in women, even though the men were older and more often had a severe form of the disease; palpitations and dyspnea were more common and severe in women.[3]
Cigarette smoking, which is associated with many autoimmune diseases, raises the incidence of Graves' ophthalmopathy 7.7-fold.[61]
History
Graves' disease owes its name to the Irish doctor Robert James Graves, who described a case of goitre with exophthalmos in 1835.[62][63] However, the German Karl Adolph von Basedow independently reported the same constellation of symptoms in 1840.[64][65] As a result, the term Basedow's syndrome/disease is more common on the European continent than Graves' disease.[66][67] It has also been called exophthalmic goitre.[68] It has been known less commonly as Parry's disease, Begbie's disease, Flajani's disease, Flajani-Basedow syndrome, and Marsh's disease, in honor of other pioneer investigators of the disorder, whose earlier reports were not widely circulated: Caleb Hillier Parry, James Begbie, Giuseppe Flajani, and Henry Marsh.[66][68] For example, cases of goitre with exophthalmos were published by the Italians Giuseppe Flajani and Antonio Giuseppe Testa, in 1802 and 1810, respectively.[69][70][71] Prior to these, Caleb Hillier Parry, a notable provincial physician in England of the late 18th century, first noted the condition in 1786.[72][73] This case was not published until 1825, but still 10 years ahead of Graves.[74] However, fair credit for the first description of Graves' disease goes to the 12th century Persian physician Sayyid Ismail al-Jurjani, who noted the association of goitre and exophthalmos in his "Thesaurus of the Shah of Khwarazm", the major medical dictionary of its time.[62]
One of the first reports of the adverse effects of hyperthyroidism on the skeleton dates from 1891, when von Recklinghausen described the "worm eaten" appearance of the long bones of a young woman who died from hyperthyroidism.[75] With Graves Disease affecting the immune system the body also has potential to be affected by Pneumocystis carinii pneumonia
Notable cases

- John Adams, Second President of the United States (possible case) [76]
- Ayaka, Japanese singer/songwriter[77]
- George H. W. Bush, U.S. president, developed new atrial fibrillation and was diagnosed in 1991 with hyperthyroidism due to the disease, and treated with radioactive iodine. The president's wife Barbara Bush also developed the disease about the same time, which in her case produced severe infiltrative exophthalmos. Scientists said that the odds of both George and Barbara Bush having Graves’ disease might be 1 in 100,000 or as low as 1 in 3,000,000, presuming that the disease was independently caused.[78]
- Maggie Cheung Ho-Yee, Hong Kong actress[79]
- Toni Childs, American singer/songwriter[80]
- Gail Devers, Athletic champion[81]
- Melissa Arnette "Missy" Elliott, American songwriter, producer and recording artist[82]
- Marty Feldman, British comedian[83]
- Diane Finley, Canadian cabinet minister[84]
- Faith Ford, American actress[85]
- Sia Furler, Australian singer[86]
- Sammy "The Bull" Gravano, Former Gambino Family Underboss[87]
- Herbert Norman Howells, British Composer[88]
- Nadezhda Krupskaya, the wife of Lenin[89]
- Barbara Leigh, an American former actress and fashion model, now spokeswomen for the National Graves' Disease Foundation[90]
- Yūko Miyamura, Japanese voice actress[91]
- Christopher Monckton, 3rd Viscount Monckton of Brenchley[92]
- Claire Rayner, UK Nurse, Agony Aunt and Broadcaster[93]
- Christina Georgina Rossetti, British Victorian Poet[94]
- Cecil Spring-Rice, British Ambassador to the USA from 1912 to 1918[95]
- Mary Webb, English author and poet, descendant of Sir Walter Scott[96]
See also
Notes
- ^ a b c Patterson, Nancy Ruth; Jake George (2002). Graves' Disease In Our Own Words. Blue Note Pubns. ISBN 1-878398-20-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c eMedicine - Hyperthyroidism, Robert J Ferry Jr emedicine.medscape.com
- ^ a b c d e f g h i j k l m Graves' Disease and the Manifestations of Thyrotoxicosis - Leslie l. De Groot, Thyroid Disease Manager, Chapter 10 (http://www.thyroidmanager.org/Chapter10/10-frame.htm)
- ^ Iglesias P, Dévora O, García J, Tajada P, García-Arévalo C, Díez JJ (2009). "Severe hyperthyroidism: aetiology, clinical features and treatment outcome". Clin. Endocrinol. (Oxf). 72 (4): 551–7. doi:10.1111/j.1365-2265.2009.03682.x. PMID 19681915.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ [1], Graves Disease.
- ^ Brent GA (2008). "Clinical practice. Graves' disease". N. Engl. J. Med. 358 (24): 2594–605. doi:10.1056/NEJMcp0801880. PMID 18550875.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Elberling TV, Rasmussen AK, Feldt-Rasmussen U, Hørding M, Perrild H, Waldemar G (2004). "Impaired health-related quality of life in Graves' disease. A prospective study" (PDF). Eur. J. Endocrinol. 151 (5): 549–55. doi:10.1530/eje.0.1510549. PMID 15538931.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Canaris GJ, Manowitz NR, Mayor G, Ridgway EC (2000). "The Colorado thyroid disease prevalence study". Arch. Intern. Med. 160 (4): 526–34. doi:10.1001/archinte.160.4.526. PMID 10695693.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid10695693" was defined multiple times with different content (see the help page). - ^ Watt T, Groenvold M, Rasmussen AK; et al. (2006). "Quality of life in patients with benign thyroid disorders. A review". Eur. J. Endocrinol. 154 (4): 501–10. doi:10.1530/eje.1.02124. PMID 16556711.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i Bunevicius R, Prange AJ (2006). "Psychiatric manifestations of Graves' hyperthyroidism: pathophysiology and treatment options". CNS Drugs. 20 (11): 897–909. PMID 17044727.
- ^ a b page 157 in:Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ A survey study of neuropsychiatric complaints in patients with Graves' disease. Stern RA, Robinson B, Thorner AR, Arruda JE, Prohaska ML, Prange AJ Jr - J Neuropsychiatry Clin Neurosci. 1996;8(2):181.
- ^ Tomer Y, Davies T (1993). "Infection, thyroid disease, and autoimmunity" (PDF). Endocr Rev. 14 (1): 107–20. doi:10.1210/er.14.1.107. PMID 8491150.
- ^ Bilezekian, JP, Loeb, JN. The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocrinol Rev 1983; 4:378.
- ^ a b c d e Bunevicius R, Velickiene D, Prange AJ (2005). "Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves' disease". Gen Hosp Psychiatry. 27 (2): 133–9. doi:10.1016/j.genhosppsych.2004.10.002. PMID 15763125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Thomsen AF, Kvist TK, Andersen PK, Kessing LV (2005). "Increased risk of affective disorder following hospitalisation with hyperthyroidism - a register-based study". Eur. J. Endocrinol. 152 (4): 535–43. doi:10.1530/eje.1.01894. PMID 15817908.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Nibuya M, Sugiyama H, Shioda K, Nakamura K, Nishijima K (2002). "ECT for the treatment of psychiatric symptoms in Basedow's disease". J ECT. 18 (1): 54–7. doi:10.1097/00124509-200203000-00014. PMID 11925523.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gonen; Kisakol, G; Savas Cilli, A; Dikbas, O; Gungor, K; Inal, A; Kaya, A (2004). "Assessment of anxiety in subclinical thyroid disorders". Endocrine journal. 51 (3): 311–315. doi:10.1507/endocrj.51.311. PMID 15256776.
- ^ Robertas Bunevicius and Arthur J. Prange Jr (2006). "Psychiatric Manifestations of Graves' Hyperthyroidism Pathophysiology and Treatment Options". CNS Drugs. 20 (11): 897–909. PMID 17044727.
- ^ Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. van Osch LA, Hogervorst E, Combrinck M, Smith AD - Neurology. 2004;62(11):1967.
- ^ a b Hyperthyroidism Induces Apoptosis in the Adult Cerebral Cortex: Direct Action of T3 on Mitochondria R. Singh, G. Upadhyay, A. Kapoor, S. Kumar, A. Kumar, M. Tiwari, M.M. Godbole
- ^ a b Frank King Thompson (2007). "Is There A Thyroid-Cortisol-Depression Axis?". Thyroid Science. 2 (10): 1.
- ^ Sensenbach W, Madison L, Eisenberg S, Ochs L (1954). "The Cerebral Circulation and Metabolism in Hyperthyroidism and Myxedema". J Clin Invest. 33 (11): 1434. doi:10.1172/JCI103021. PMC 1072568. PMID 13211797.
{{cite journal}}: More than one of|pages=and|page=specified (help)CS1 maint: multiple names: authors list (link) - ^ Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. (2008). "Metabolic effects of thyroid hormone derivatives". Thyroid. 18 (2): 239–53. doi:10.1089/thy.2007.0248. PMID 18279024.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mathur R (12/7/2006). "Thyroid Disease, Osteoporosis, and Calcium". MedicineNet. Retrieved 2010-05-03.
{{cite web}}: Check date values in:|date=(help) - ^ Bouillon R, DeMoor P: Parathyroid function in patients with hyper- or hypothyroidism. J Clin Endocrinol Metab 38:999, 1974
- ^ Jadresic (1990). "Psychiatric aspects of hyperthyroidism". Journal of psychosomatic research. 34 (6): 603–615. doi:10.1016/0022-3999(90)90104-C. PMID 2290133.
- ^ a b c d Stern RA, Robinson B, Thorner AR, Arruda JE, Prohaska ML, Prange AJ (1996). "A survey study of neuropsychiatric complaints in patients with Graves' disease". J Neuropsychiatry Clin Neurosci. 8 (2): 181–5. PMID 9081554.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Druckerhttp D (2005). "Hyperthyroidism". MyThyroid.com. Retrieved 2010-05-03.
- ^ Felz, The many faces of Graves’ disease, -part 1, Postgraduate medicine online, 1999, 106(4), 57-64.
- ^ a b Awad AG (2000). "The Thyroid and the Mind and Emotions/Thyroid Dysfunction and Mental Disorders". Thyrobulletin, Thryoid Foundation of Canada. 7 (3). Retrieved 2010-05-06.
- ^ Matsubayashi, S; Tamai, H; Matsumoto, Y; Tamagawa, K; Mukuta, T; Morita, T; Kubo, C (1996). "Graves' disease after the onset of panic disorder". Psychother Psychosom. 65 (5): 277–80. doi:10.1159/000289088. PMID 8893330.
{{cite journal}}: Cite has empty unknown parameter:|author-name-separator=(help); Unknown parameter|author-separator=ignored (help) - ^ a b Psychische stoornissen bij endocriene zieken, 1983, C. van der Meer en W. van Tilburg (red.)
- ^ Andersen S, Pedersen KM, Bruun NH, Laurberg P (2002). "Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease". J. Clin. Endocrinol. Metab. 87 (3): 1068–72. doi:10.1210/jc.87.3.1068. PMID 11889165.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Clinical value of M22-based assays for TSH-receptor antibody (TRAb) in the follow-up of antithyroid drug treated Graves' disease: comparison with the second generation human TRAb assay. Massart C, Gibassier J, d'Herbomez M - Clin Chim Acta. 2009 Sep;407(1-2):62-6. Epub 2009 Jul 1.
- ^ Bioassay of thyrotropin receptor antibodies with Chinese hamster ovary cells transfected with recombinant human thyrotropin receptor: clinical utility in children and adolescents with Graves disease. Botero D, Brown RS - J Pediatr. 1998;132(4):612.
- ^ Endocrinology and Metabolism Clinics of North America, KAPLAN ed., Vol 27, no. 1, march 1998, 4.
- ^ Graves' Disease: Quality Of Life And Occupational Disability, Katharina Ponto et al., Dtsch Arztebl, Int 2009; 106(17): 283-9
- ^ Bahn, RS; Burch, HS; Cooper, DS; Garber, JR; Greenlee, CM; Klein, IL; Laurberg, P; McDougall, IR; Rivkees, SA (2009). "The Role of Propylthiouracil in the Management of Graves' Disease in Adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration". Thyroid : official journal of the American Thyroid Association. 19 (7): 673–4. doi:10.1089/thy.2009.0169. PMID 19583480.
{{cite journal}}: Cite has empty unknown parameter:|author-name-separator=(help); Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Erbil Y, Ozluk Y, Giriş M; et al. (2007). "Effect of lugol solution on thyroid gland blood flow and microvessel density in the patients with Graves' disease". J. Clin. Endocrinol. Metab. 92 (6): 2182–9. doi:10.1210/jc.2007-0229. PMID 17389702.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kaur S, Parr JH, Ramsay ID, Hennebry TM, Jarvis KJ, Lester E (1988). "Effect of preoperative iodine in patients with Graves' disease controlled with antithyroid drugs and thyroxine". Ann R Coll Surg Engl. 70 (3): 123–7. PMC 2498739. PMID 2457351.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thyroid function after surgical treatment of thyrotoxicosis. A report of 100 cases treated with propranolol before operation. Toft AD, Irvine WJ, Sinclair I, McIntosh D, Seth J, Cameron EH. N Engl J Med. 1978;298(12):643; The influence of remnant size, antithyroid antibodies, thyroid morphology, and lymphocyte infiltration on thyroid function after subtotal resection for hyperthyroidism. JörtsöE, Lennquist S, Lundström B, Norrby K, Smeds S, World J Surg. 1987;11(3):365.
- ^ Newsletter of Thyroid Australia Ltd Volume 3 No 3 July 2002
- ^ The influence of remnant size, antithyroid antibodies, thyroid morphology, and lymphocyte infiltration on thyroid function after subtotal resection for hyperthyroidism. JörtsöE, Lennquist S, Lundström B, Norrby K, Smeds S, World J Surg. 1987;11(3):365
- ^ Surgical treatment of hyperthyroidism: a ten-year experience. Werga-Kjellman P, Zedenius J, Tallstedt L, Träisk F, Lundell G, Wallin G, Thyroid. 2001;11(2):187.
- ^ a b Ayala AR, Danese MD, Ladenson PW (2000). "When to treat mild hypothyroidism". Endocrinol. Metab. Clin. North Am. 29 (2): 399–415. doi:10.1016/S0889-8529(05)70139-0. PMID 10874537.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ TSH levels are altered by the timing of levothyroxine administration Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J - J Clin Endocrinol Metab 2009; July 7 - Commentary by Jennifer Sipos, thyroid.org
- ^ a b Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR (2009). "High Frequency of and Factors Associated with Thyroid Hormone Over-Replacement and Under-Replacement in Men and Women Aged 65 and Over" (PDF). J. Clin. Endocrinol. Metab. 94 (4): 1342–5. doi:10.1210/jc.2008-1696. PMC 2682480. PMID 19126628.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Hollowell JG, Staehling NW, Flanders WD; et al. (2002). "Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III)". J. Clin. Endocrinol. Metab. 87 (2): 489–99. doi:10.1210/jc.87.2.489. PMID 11836274.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Chapter 11. Diagnosis and Treatment of Graves’ Disease Leslie J. De Groot, MD Professor of Medicine - http://www.thyroidmanager.org/Chapter11/chapter11.html Updated: March 5, 2008
- ^ Scheffer, Heckmann, Mijic, Rudorff. Chronic Distress Syndrome in Patients with Graves' Disease. Med Klin 99, no.10(2004), 578-84
- ^ Gleicher N, Weghofer A, Barad DH (2011). "Do chromosomally abnormal pregnancies really preclude autoimmune etiologies of spontaneous miscarriages?". Autoimmun Rev. 10 (6): 361–3. doi:10.1016/j.autrev.2010.12.004. PMID 21195806.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Researchers warn against premature dismissal of autoimmune causes in miscarriages"
- ^ Gleicher N (2010). "Does the immune system induce labor? Lessons from preterm deliveries in women with autoimmune diseases". Clin Rev Allergy Immunol. 39 (3): 194–206. doi:10.1007/s12016-009-8180-8. PMID 19844811.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Abraham-Nordling, Torring, Hamberger, Lundell, Tallstedt, Calissendorff, Wallin. Graves' Disease: A long-term quality-of-life follow-up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery, Thyroid 15, no. 11(2005), 1279-86
- ^ Could total thyroidectomy become the standard treatment for Graves’ disease? Ayhan Koyuncu, Cengiz Aydin, Ömer Topçu, Oruç Numan Gökçe, Şahande Elagöz and Hatice Sebila Dökmetaş - Surgery Today; Volume 40, Number 1 / January, 2010
- ^ Fahrenfort JJ, Wilterdink AM, van der Veen EA (2000). "Long-term residual complaints and psychosocial sequelae after remission of hyperthyroidism". Psychoneuroendocrinology. 25 (2): 201–11. doi:10.1016/S0306-4530(99)00050-5. PMID 10674283.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Defining the Patient-PhysicianRelationship for the 21st Century - 3rd Annual Disease Management - Outcomes Summit October 30 – November 2, 2003 - Phoenix, Arizona docs/PatientPhysician.pdf patient-physician.com
- ^ The Thyroid Sourcebook, Sara Rosenthal
- ^ Dayan C (July 2002). "Does Graves' disease in men tend to be more severe?" (PDF front cover). Thyroid Flyyer - Newsletter of Thyroid Australia. 3 (3). Retrieved 2010-05-06.
- ^ Bahn RS (2010). "Graves' ophthalmopathy". N. Engl. J. Med. 362 (8): 726–38. doi:10.1056/NEJMra0905750. PMID 20181974.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Mathew Graves at Who Named It?
- ^ Graves, RJ. New observed affection of the thyroid gland in females. (Clinical lectures.) London Medical and Surgical Journal (Renshaw), 1835; 7: 516-517. Reprinted in Medical Classics, 1940;5:33-36.
- ^ Von Basedow, KA. Exophthalmus durch Hypertrophie des Zellgewebes in der Augenhöhle. [Casper's] Wochenschrift für die gesammte Heilkunde, Berlin, 1840, 6: 197-204; 220-228. Partial English translation in: Ralph Hermon Major (1884-1970): Classic Descriptions of Disease. Springfield, C. C. Thomas, 1932. 2nd edition, 1939; 3rd edition, 1945.
- ^ Von Basedow, KA. Die Glotzaugen. [Casper's] Wochenschrift für die gesammte Heilkunde, Berlin, 1848: 769-777.
- ^ a b Basedow's syndrome or disease at Who Named It? - the history and naming of the disease
- ^ Goiter, Diffuse Toxic at eMedicine
- ^ a b Robinson, Victor, ed. (1939). "Exophthalmic goiter, Basedow's disease, Grave's disesase". The Modern Home Physician, A New Encyclopedia of Medical Knowledge. WM. H. Wise & Company (New York)., pages 82, 294, and 295.
- ^ Flajani, G. Sopra un tumor freddo nell'anterior parte del collo broncocele. (Osservazione LXVII). In Collezione d'osservazioni e reflessioni di chirurgia. Rome, Michele A Ripa Presso Lino Contedini, 1802;3:270-273.
- ^ Testa, AG. Delle malattie del cuore, loro cagioni, specie, segni e cura. Bologna, 1810. 2nd edition in 3 volumes, Florence, 1823; Milano 1831; German translation, Halle, 1813.
- ^ Giuseppe Flajani at Who Named It?
- ^ Parry, CH. Enlargement of the thyroid gland in connection with enlargement or palpitations of the heart. Posthumous, in: Collections from the unpublished medical writings of C. H. Parry. London, 1825, pp. 111-129. According to Garrison, Parry first noted the condition in 1786. He briefly reported it in his Elements of Pathology and Therapeutics, 1815. Reprinted in Medical Classics, 1940, 5: 8-30.
- ^ Hull G (1998). "Caleb Hillier Parry 1755-1822: a notable provincial physician". Journal of the Royal Society of Medicine. 91 (6): 335–8. PMC 1296785. PMID 9771526.
- ^ Caleb Hillier Parry at Who Named It?
- ^ von Recklinghausen, FD. Die Fibröse oder deformierende Ostitis, die Osteomalazie und die osteoplastische Carzinose in ihren gegenseitigen Beziehungen. Festchrift Rudolf Virchow. George Reimer, Berlin 1891. p.1.
- ^ Craig Nelson, "Thomas Paine: Enlightenment, Revolution, and the Birth of Modern Nations," p. 96.
- ^ "Hiro Mizushima, Ayaka married since February". Tokyograph. Retrieved 2009-06-03.
- ^ Altman L (May 28, 1991). "The Doctor's World; A White House Puzzle: Immunity Ailments". Science Section. New York Times Newspaper. Retrieved 2010-03-14.
- ^ The-sun.co.cc
- ^ "Toni Childs' comes back from the 'Graves' - ABC Adelaide (Australian Broadcasting Corporation)". Abc.net.au. 2008-10-01. Retrieved 2009-06-03.
- ^ "A Champion Battles Thyroid Disease: Gail Devers' Story". Wrongdiagnosis.healthology.com. Retrieved 2009-06-03.
- ^ "Missy Elliot Has Graves Disease". huffingtonpost.com. Retrieved 2011-06-23.
- ^ Kugler, R.N., Mary (December 9, 2003). "Graves' Disease and Research: Multiple Areas of Study". About.com. Retrieved 2009-06-03.
- ^ Gombu, Phinjo (January 5, 2007). "Immigration file a revolving door". Toronto Star. Retrieved 2009-06-10.
- ^ TVguide.com
- ^ Murfett, Andrew (2010-06-18). "Sia Furler: Fame does not become her". The Sydney Morning Herald.
- ^ Guart, Al (2002-03-31). "Rare Disease Could Whack Sammy Bull". New York Post. Retrieved 2010-11-03.
- ^ Herbert Howells
- ^ Dimitri Volkogonov "Lenin - A New Biography" p. 34.
- ^ Home.rmci.net
- ^ Miyamura, Yuko (September 10, 2007). "Blog Entry" (in Japanese). Yuko Miyamura blog. Retrieved 2009-06-10.
- ^ Monckton, Christopher. "Hitler Youth in Denmark – again by Christopher Monckton in Copenhagen".
- ^ Rayner, Jay (2003-03-02). "Tales my mother never told me". The Guardian. London.
- ^ Packer, Lona Mosk (1963). Christina Rossetti. University of California Press. p. 285. OCLC 362192.
- ^ Burton, David H. (1990). Cecil Spring Rice: A Diplomat's Life. Fairleigh Dickinson University Press. p. 147. ISBN 0838633951.
- ^ Coles, Gladys Mary (1978). The flower of light: a biography of Mary Webb. Duckworth. p. 52. ISBN 9780715611203.
External links
- Graves' Disease Foundation Conducts research about Graves' disease, and provides support via the largest internet forum on Graves' disease
- Thyroid Disease Manager Contains several very specialised chapters on Graves' disease, and related issues
- Patient information: Antithyroid drugs Article at UpToDate
- Drug Therapy: Antithyroid drugs Elaborate article published in 2005 in the medical magazine Medicina Interna
This template is no longer used; please see Template:Endocrine pathology for a suitable replacement
