Tetrapod
| Tetrapods Temporal range: Early Devonian - Holocene,
| |
|---|---|

| |
| Representatives of the four classes of extant tetrapods, (clockwise from upper left): a frog (an amphibian), a hoatzin (a bird), a skink (a reptile), and a mouse (a mammal) | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Teleostomi |
| Superclass: | Tetrapoda authority unknown, before 1912 (see talk page) |
| Subgroups | |
and see text | |
The superclass Tetrapoda (Ancient Greek τετραπόδηs tetrapodēs, "four-footed"), or the tetrapods, comprises the first four-limbed vertebrates and their descendants, including the living and extinct amphibians, reptiles, birds, and mammals. The tetrapods evolved from the lobe-finned fishes about 395 million years ago in the Devonian Period.[1] The specific aquatic ancestors of the tetrapods, and the process by which land colonization occurred, remain unclear, and are areas of active research and debate among palaeontologists at present.
While most species today are terrestrial, little evidence supports the idea that any of the earliest tetrapods could move about on land, as their limbs could not have held their midsections off the ground and the known trackways do not indicate they dragged their bellies around. Presumably, the tracks were made by animals walking along the bottoms of shallow bodies of water.[2] Amphibians today generally remain semiaquatic, living the first stage of their lives as fish-like tadpoles. Several groups of tetrapods, such as the snakes and cetaceans, have lost some or all of their limbs. In addition, many tetrapods have returned to partially aquatic or fully aquatic lives throughout the history of the group (modern examples of fully aquatic tetrapods include cetaceans and sirenians). The first returns to an aquatic lifestyle may have occurred as early as the Carboniferous Period[3] whereas other returns occurred as recently as the Cenozoic, as in cetaceans, pinnipeds,[4] and several modern amphibians.[5]
A quadruped actually uses four limbs for locomotion; not all tetrapods are quadrupeds.
Biodiversity
Tetrapoda includes four classes: amphibians, reptiles, mammals, and birds. Overall, the biodiversity of lissamphibians,[6] as well as of tetrapods generally,[7] has grown exponentially over time; more than 30,000 species living today are descended from a single amphibian group in the Early to Middle Devonian. However, that diversification process was interrupted at least a few times by major biological crises such as the Permian–Triassic extinction event, which at least affected amniotes.[8] The overall composition of biodiversity was driven primarily by amphibians in the Palaeozoic, dominated by reptiles in the Mesozoic and expanded by the explosive growth of birds and mammals in the Cenozoic. As biodiversity has grown so has the number of niches tetrapods have occupied. The first tetrapods were aquatic and fed primarily on fish. Today, the Earth supports a great diversity of tetrapods that live in many habitats and subsist on a variety of diets.[7]
Evolution
Origin
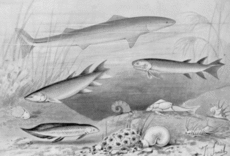
Fish ancestors
Tetrapods evolved from early bony fishes (Osteichthyes), living in freshwater and brackish environments at the beginning of the Devonian period. These fishes had many features in common with their cartilaginous ancestors, but also important differences, most notably a swim bladder/lung, a feature lacking in sharks and rays. [9] [10]

Lungs before land
The lung/swim bladder originated as an outgrowth of the gut, forming a gas-filled bladder above the digestive system. The primary function of this organ in its primitive form is not entirely certain. It was probably used as a lung for breathing air, but it may have been used for buoyancy instead, or both functions may have been important.
Lobe fins

Fleshy lobe-fins supported on bones seem to have been an ancestral trait of all bony fishes (Osteichthyes). The ancestors of the ray-finned fishes (Actinopterygii) evolved their fins in a different direction and developed a swim bladder dedicated to controlling buoyancy. The Tetrapodomorph ancestors of the Tetrapods retained a lung-like swim bladder and further developed their lobe fins. The paired fins had bones distinctly homologous to the humerus, ulna, and radius in the fore-fins and to the femur, tibia, and fibula in the pelvic fins.[11]
Into the shallows
While most Tetrapodomorphs were open-water fishes, one group, the Elpistostegalians, adapted to life in the shallows. They evolved flat bodies for movement in very shallow water, and the pectoral and pelvic fins took over as the main propulsion organs. Since the shallows were subject to occasional oxygen deficiency, the ability to breath atmospheric air with the swim bladder became increasingly important.[12]
From fins to feet

The first tetrapods probably evolved towards the end of the Devonian from Tetrapodomorph fish such as these living in shallow water environments.[13][14] The very earliest tetrapods would have been animals similar to Acanthostega, with legs and lungs as well as gills, but still primarily aquatic and unsuited to life on land.
Palaeozoic tetrapods
Devonian tetrapods

Tetrapods first appeared towards the end of the Devonian period. These early tetrapods would have been animals similar to Acanthostega, with legs and lungs as well as gills, but still primarily aquatic and unsuited to life on land. The Devonian tetrapods went through two major bottlenecks during what is known as the Late Devonian extinction. These extinction events led to the disappearance of primitive tetrapods with fish-like features. [15] When tetrapods reappear in the fossil record in early Carboniferous deposits, some 20 million years later, the adult forms are all fully adapted to a terrestrial existence.[16] Why they went to land in the first place is still debated.
Carboniferous tetrapods

During the early Carboniferous the number of digits on hands and feet became standardized at five, as lineages with more digits died out. By mid-Carboniferous times the early tetrapods had radiated into at least three main branches. Modern amphibians are derived from the either the temnospondyls or the lepospondyls (or possibly both) whereas the anthracosaurs were the relatives and ancestors of the amniotes.
The first amniotes are known from the early part of the Late Carboniferous. Amphibians must return to water to lay eggs; in contrast, amniote eggs have a membrane ensuring gas exchange out of water and can therefore be laid on land. A reptile is defined as any amniote that is neither a mammal nor a bird,[17] so these early amniotes were primitive reptiles.
Amphibians and reptiles were affected by the Carboniferous Rainforest Collapse (CRC), an extinction event that occurred ~300 million years ago. The sudden collapse of a vital ecosystem shifted the diversity and abundance of major groups. Reptiles were more suited to the new conditions. They invaded new ecological niches and began diversifying their diets to include plants and other tetrapods, previously having been limited to insects and fish.[18]
Permian tetrapods

In the Permian period, in addition to temnospondyl and anthracosaur clades, there were two important clades of amniotes, the sauropsids and the synapsids. The latter were the most important and successful Permian animals.
The end of the Permian saw a major turnover in fauna during the Permian–Triassic extinction event. There was a protracted loss of species, due to multiple extinction pulses.[19] Many of the once large and diverse groups died out or were greatly reduced.
Mesozoic tetrapods
The diapsids (a subgroup of the sauropsids) began to diversify during the Triassic, leading to the turtles, crocodiles and dinosaurs. In the Jurassic, birds first appeared as a derived clade of theropod dinosaurs, and lizards developed from other diapsids. In the Cretaceous, snakes developed from lizards. By the late Mesozoic, the large labyrinthodont groups that first appeared during the Paleozoic such as temnospondyls and reptile-like amphibians had gone extinct. Many groups of synapsids such as anomodonts and therocephalians that once comprised the dominant terrestrial fauna of the Permian also became extinct during the Mesozoic; however, during the Triassic one group (Cynodontia) gave rise to the mammals, which survived through the Mesozoic to later diversify during the Cenozoic.
Extant (living) tetrapods
Following the great faunal turnover at the end of the Mesozoic, only six major groups of tetrapods were left, all of which also include many extinct groups:
- Lissamphibia: frogs and toads, newts and salamanders, and caecilians
- Testudines: turtles and tortoises
- Lepidosauria: tuataras, lizards, amphisbaenians and snakes
- Crocodilia: crocodiles, alligators, caimans and gharials
- Neornithes: modern birds
- Mammalia: mammals
Classification

Classification of tetrapods has a long history. Traditionally, tetrapods are divided into four classes based on gross anatomical and physiological traits.[20] Snakes and other legless reptiles are considered tetrapods because they are sufficiently like other reptiles that have a full complement of limbs. Similar considerations apply to caecilians and aquatic mammals. Newer taxonomy is frequently based on cladistics instead, giving a variable number of major "branches" (clades) of the tetrapod family tree.
As is the case throughout evolutionary biology today, there is debate over how to properly classify the groups within Tetrapoda. Traditional biological classification recognizes evolutionary transitions between older groups and descendant groups with markedly different characteristics. For example, the birds, which evolved from the dinosaurs, are defined as a separate group from them, because they represent a distinct new type of physical form and functionality. In phylogenetic nomenclature, in contrast, the newer group is always included in the old. For this school of taxonomy, dinosaurs and birds are not groups in contrast to each other, but rather birds are a sub-type of dinosaurs.
History of classification
The tetrapods, including all large- and medium-sized land animals, have been among the best understood animals since earliest times. Already in Aristotle's days, the basic division between mammals, birds and egg-laying tetrapods (the "herptiles") was well known, and the inclusion of the legless snakes into this group was likewise recognized.[21] With the birth of modern biological classification in the 18th century, Linnaeus used the same division, with the tetrapods occupying the first three of his six classes of animals.[22] While reptiles and amphibians can be quite similar externally, the French zoologist Pierre André Latreille recognized the large physiological differences at the beginning of the 19th century and split the herptiles into two classes, giving the four familiar classes of tetrapods: amphibians, reptiles, birds and mammals.[23]
Modern classification
With the basic classification of tetrapods settled, a half a century followed where the classification of living and fossil groups predominately was done by experts working within classes. In the early 1930s, American vertebrate palaeontologist Alfred Romer (1894–1973) produced an overview, drawing together taxonomic work from the various subfields to create an orderly taxonomy in his Vertebrate Paleontology.[24] This classical scheme with minor variations is still used in works where systematic overview is essential, e.g. Benton (1998) and Knobill and Neill (2006).[25][26] While mostly seen in general works, it is also still used in some specialist works like Fortuny & al. (2011).[27] The taxonomy down to subclass level shown here is from Hildebrand and Goslow (2001):[28]
- Superclass Tetrapoda - four-limbed vertebrates
- Class Amphibia - amphibians
- Subclass Ichthyostegalia - early fish-like amphibians
- Subclass Anthracosauria - reptile-like amphibians (ancestors of the amniotes)
- Subclass Temnospondyli - large-headed Paleozoic and Mesozoic amphibians
- Subclass Lissamphibia - modern amphibians
- Class Reptilia - reptiles, (ancestors of both birds and mammals)
- Subclass Anapsida - early reptiles
- Subclass Synapsida - mammal-like reptiles, including the ancestors of mammals
- Subclass Testudinata - turtles (may belong with diapsids)[29]
- Subclass Diapsida - diapsids, including crocodiles, dinosaurs, lizards, and snakes
- Class Aves - birds
- Subclass Archaeornithes - ancestral tailed birds, e.g. Archaeopteryx
- Subclass Enantiornithes - the most abundant Mesozoic birds, most with claws and teeth, but lacking a long tail
- Subclass Neornithes - modern birds
- Class Mammalia - mammals
- Subclass Prototheria - monotremes
- Subclass Allotheria - multituberculates
- Subclass Theria - live bearing mammals
- Class Amphibia - amphibians
This classification is the one most commonly encountered in school textbooks and popular works. While orderly and easy to use, has come under critique from cladistics. The earliest tetrapods are grouped under Class Amphibia, although several of the groups are more closely related to amniotes than to modern day amphibians. Traditionally, birds are not considered a type of reptile, but crocodiles are more closely related to birds than they are to other reptiles like lizards. Birds themselves are thought to be descendents of theropod dinosaurs. Basal non-mammalian synapsids ("mammal-like reptiles") traditionally also sort under Class Reptilia as a separate subclass[20] but they are more closely related to mammals than to living reptiles. Considerations like these have led some authors to argue for a new classification based purely on phylogeny, disregarding the anatomy and physiology.
Phylogeny of early tetrapod diversification
Cladogram modified after Ruta, Jeffery & Coates (2003).[14]
All branches are extinct except for Lissamphibia (all modern amphibians) and Amniota (all reptiles, birds, and mammals).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Note: The origin of the subclass Lissamphibia, to which all extant amphibians belong, is disputed. This cladogram is the result of one analysis conducted by Ruta, Jeffery & Coates (2003) that placed Lissamphibia within Lepospondyli, with the latter clade being within the crown group Tetrapoda. A second analysis by the authors placed Lissamphibia within Temnospondyli, thus placing Lepospondyli outside crown group Tetrapoda and Temnospondyli within. Another prevailing theory not represented by either of the cladograms is a diphyletic grouping of Lissamphibia with both Lepospondyli and Temnospondyli, with caecilians belonging to the former clade and frogs and salamanders belonging to the latter.
Anatomical features of early tetrapods
The tetrapod's ancestral fish must have possessed similar traits to those inherited by the early tetrapods, including internal nostrils (to separate the breathing and feeding passages) and a large fleshy fin built on bones that could give rise to the tetrapod limb. The rhipidistian crossopterygians fulfill every requirement for this ancestry. Their palatal and jaw structures were identical to those of early tetrapods, and their dentition was identical too, with labyrinthine teeth fitting in a pit-and-tooth arrangement on the palate. The crossopterygian paired fins were smaller than tetrapod limbs, but the skeletal structure was very similar in that the crossopterygian had a single proximal bone (analogous to the humerus or femur), two bones in the next segment (forearm or lower leg), and an irregular subdivision of the fin, roughly comparable to the structure of the carpus / tarsus and phalanges of a hand.
The major difference between crossopterygians and early tetrapods was in relative development of front and back skull portions; the snout is much less developed than in most early tetrapods and the post-orbital skull is exceptionally longer than an amphibian's.
A great many kinds of early tetrapods lived during the Carboniferous period. Therefore, their ancestor would have lived earlier, during the Devonian period. Devonian ichthyostegans were among the earliest of the tetrapods, with a skeleton that is directly comparable to that of rhipidistian ancestors. Early temnospondyls (Late Devonian to Early Mississippian) still had some ichthyostegid features such as similar skull bone patterns, labyrinthine tooth structure, the fish skull-hinge, pieces of gill structure between the cheek and shoulder, and the vertebral column. They had, however, lost several other fish features such as the fin rays in the tail.
To propagate in the terrestrial environment, animals had to overcome certain challenges. Their bodies needed additional support, because buoyancy was no longer a factor. They needed a new method of respiration to extract atmospheric oxygen, instead of oxygen dissolved in water. Animals had to develop new means of locomotion to traverse distances between waterholes. Water retention was now important, since it was no longer the living matrix, and could be lost easily to the environment. Finally, animals needed new sensory input systems to have any ability to function reasonably on land.
Skull
The most notable characteristics that make a tetrapod's skull different from a fish's are the relative frontal and rear portion lengths. The fish had a long rear portion while the front was short; the orbital vacuities were thus located towards the anterior end. In the tetrapod, the front of the skull lengthened, positioning the orbits farther back on the skull. The lacrimal bone was not in contact with the frontal anymore, having been separated from it by the prefrontal bone. Also of importance is that the skull was now free to rotate from side to side, independent of the spine, on the newly forming neck.
A diagnostic character of temnospondyls is that the tabular bones (which formed the posterior corners of the skull-table) were separated from the respective left and right parietals by a sutural junction between the postparietals and supratemporals. Also at the rear of the skull, all bones dorsal to the cleithrum were lost.
The lower jaw of, for example, Eryops resembled its crossopterygian ancestors in that on the outer surface lay a long dentary that bore teeth. There were also bones below the dentary on the jaw: two splenials, the angular and the surangular. On the inside were usually three coronoids that bore teeth and lay close to the dentary. On the upper jaw was a row of marginal labyrinthine teeth, located on the maxilla and premaxilla. In Eryops, as in all early amphibians, the teeth were replaced in waves that traveled from the front of the jaw to the back in such a way that every other tooth was mature, and the ones in between were young.
Dentition
The "labyrinthodonts" had a peculiar tooth structure, from which their name derives—and though not exclusive to the group, the labyrinthine dentition is a useful indicator as to proper classification. The important feature of the tooth is that the enamel and dentine fold into a complicated corrugated pattern when viewed in cross section. This infolding strengthened the tooth and increased wear resistance. Such teeth survived for 100 Ma, first among crossopterygian fish, then stem reptiles. Modern amphibians no longer have this type of dentition, but rather pleurodont teeth, in fewer numbers of the whole group.
Sensory organs
The difference in density between air and water causes smells (certain chemical compounds detectable by chemoreceptors) to behave differently. An animal first venturing out onto land would have difficulty in locating such chemical signals if its sensory apparatus was designed for aquatic detection.
Fish have a lateral line system that detects pressure fluctuations in the water. Such pressure is non-detectable in air, but grooves for the lateral line sense organs were found on the skull of labyrinthodonts, suggesting a partially aquatic habitat. Modern amphibians, which are semi-aquatic, exhibit this feature whereas it has been retired by the higher vertebrates. The olfactory epithelium would also have to change to detect airborne odors.
In addition to the lateral line organ system, the eye had to change. This change came about because the refractive index of light differs between air and water, so the focal length of the lens altered to function in air. The eye was now exposed to a relatively dry environment rather than being bathed by water, so eyelids developed and tear ducts evolved to produce a liquid to moisten the eyeball.
Hearing
Animals retained the balancing function of the middle ear from fish ancestry. However, delicate air vibrations could not set up pulsations through the skull as in a proper auditory organ. Typical of most labyrinthodonts, the spiracular gill pouch was retained as the otic notch, closed in by the tympanum, a thin, tight membrane.
The hyomandibula of fish migrated upwards from its jaw supporting position, and was reduced in size to form the stapes. Situated between the tympanum and braincase in an air-filled cavity, the stapes was now capable of transmitting vibrations from the exterior of the head to the interior. Thus the stapes became an important element in an impedance matching system, coupling airborne sound waves to the receptor system of the inner ear. This system had evolved independently within several different amphibian lineages.
The impedance matching ear had to meet certain conditions to work. The stapes had to be perpendicular to the tympanum, small and light enough to reduce its inertia, and suspended in an air-filled cavity. In modern species that are sensitive to over 1 kHz frequencies, the footplate of the stapes is 1/20th the area of the tympanum. However, in early amphibians the stapes was too large, making the footplate area oversized, preventing the hearing of high frequencies. So it appears they could only hear high intensity, low frequency sounds—and the stapes more probably just supported the brain case against the cheek.
Girdles
The pectoral girdle of early tetrapods such as Eryops was highly developed, with a larger size for both increased muscle attachment to it and to the limbs. Most notably, the shoulder girdle was disconnected from the skull, resulting in improved terrestrial locomotion. The crossopterygian cleithrum was retained as the clavicle, and the interclavicle was well-developed, lying on the underside of the chest. In primitive forms, the two clavicles and the interclavical could have grown ventrally in such a way as to form a broad chest plate, although such was not the case in Eryops. The upper portion of the girdle had a flat, scapular blade, with the glenoid cavity situated below performing as the articulation surface for the humerus, while ventrally there was a large, flat coracoid plate turning in toward the midline.
The pelvic girdle also was much larger than the simple plate found in fishes, accommodating more muscles. It extended far dorsally and was joined to the backbone by one or more specialized sacral ribs. The hind legs were somewhat specialized in that they not only supported weight, but also provided propulsion. The dorsal extension of the pelvis was the ilium, while the broad ventral plate was composed of the pubis in front and the ischium in behind. The three bones met at a single point in the center of the pelvic triangle called the acetabulum, providing a surface of articulation for the femur.
The main strength of the ilio-sacral attachment of Eryops was by ligaments, a condition structurally, but not phylogenetically, intermediate between that of the most primitive embolomerous amphibians and early reptiles. The condition that is more usually found in higher vertebrates is that cartilage and fusion of the sacral ribs to the blade of the ilium are utilized in addition to ligamentous attachments.
Limbs
The humerus was the largest bone of the arm, its head articulating with the glenoid cavity of the pectoral girdle, distally with the radius and ulna. The radius resided on the inner side of the forearm and rested directly under the humerus, supporting much of the weight, while the ulna was located to the outside of the humerus. The ulna had a head, which muscles pulled on to extend the limb, called the olecranon that extended above the edge of the humerus.
The radius and the ulna articulated with the carpus, which was a proximal row of three elements: the radiale underlying the radius, the ulnare underneath the ulna and an intermedium between the two. A large central element was beneath the last and may have articulated with the radius. There were also three smaller centralia lying to the radial side. Opposite the head of each toe lay a series of five distal carpals. Each digit had a first segment, the metacarpal, lying in the palm region.
The pelvic limb bones were essentially the same as in the pectoral limb, but with different names. The analogue to the humerus was the femur, which was longer and slimmer. The two lower arm bones corresponded to the tibia and fibula of the hind leg, the former being the innermost and the latter the outermost bones. The tarsus is the hind version of the carpus and its bones correspond as well.
Locomotion
In typical early tetrapod posture the upper arm and upper leg extended nearly straight horizontal from its body, and the forearm and the lower leg extended downward from the upper segment at a near right angle. The body weight was not centered over the limbs, but was rather transferred 90 degrees outward and down through the lower limbs, which touched the ground. Most of the animal's strength was used to just lift its body off the ground for walking, which was probably slow and difficult. With this sort of posture, it could only make short broad strides. This has been confirmed by fossilized footprints found in Carboniferous rocks.
Feeding
Early tetrapods had a wide gaping jaw with weak muscles to open and close it. In the jaw were fang-like palatal teeth that, when coupled with the gape, suggests an inertial feeding habit. This is when the amphibian would grasp the prey and, lacking any chewing mechanism, toss the head up and backwards, throwing the prey farther back into the mouth. Such feeding is seen today in the crocodile and alligator. A study of these jaws shows they fed underwater, not on land.[30] As it is taken that early tetrapods were not very active, this suggests that they were not predatory. It is more likely that it fed on fish either in the water or on those that became stranded at the margins of lakes and swamps.
The tongue of modern adult amphibians is quite fleshy and attached to the front of the lower jaw, so it is reasonable to speculate that it was fastened in a similar fashion in primitive forms, although it was probably not specialized like it is in a frog.
Respiration
Modern amphibians breathe by inhaling air into lungs, where oxygen is absorbed. They also breathe through the moist lining of the mouth and skin, known as cutaneous respiration. Eryops also inhaled, but its ribs were too closely spaced to suggest that it did this by expanding the rib cage. More likely, it breathed by buccal pumping in which it opened its mouth and nostrils, depressed the hyoid apparatus to expand the oral cavity, closed its mouth and nostrils finally and elevated the floor of the mouth to force air back into the lungs — in other words, it gulped, then swallowed. It probably exhaled by contraction of the elastic tissue in the lung walls. Other special respiratory methods probably existed.
Circulation
Early tetrapods probably had a three-chambered heart, as do modern amphibians and reptiles, in which oxygenated blood from the lungs and de-oxygenated blood from the respiring tissues enters by separate atria, and is directed via a spiral valve to the appropriate vessel — aorta for oxygenated blood and pulmonary vein for deoxygenated blood. The spiral valve is essential to keeping the mixing of the two types of blood to a minimum, enabling the animal to have higher metabolic rates, and be more active than otherwise.
Ligamentous attachments within the limbs were present in Eryops, being important because they were the precursor to bony and cartilaginous variations seen in modern terrestrial animals that use their limbs for locomotion.
Of all body parts, the spine was the most affected by the move from water to land. It now had to resist the bending caused by body weight and had to provide mobility where needed. Previously, it could bend along its entire length. Likewise, the paired appendages had not been formerly connected to the spine, but the slowly strengthening limbs now transmitted their support to the axis of the body.
See also
References
This article needs additional citations for verification. (July 2009) |
- ^ Clack 2012
- ^ Clack, Jennifer A. (1997). "Devonian tetrapod trackways and trackmakers; a review of the fossils and footprints". Palaeogeography, Palaeoclimatology, Palaeoecology 130 (1997) 227 250. 130: 227–250. doi:10.1016/S0031-0182(96)00142-3.
{{cite journal}}: Invalid|ref=harv(help) - ^ Laurin, M. (2010). How Vertebrates Left the Water. Berkeley, California, USA.: University of California Press. ISBN 978-0-520-26647-6.
- ^ Canoville, Aurore; Laurin, Michel (2010). "Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on paleobiological inferences". Biological Journal of the Linnean Society. 100 (2): 384–406. doi:10.1111/j.1095-8312.2010.01431.x.
{{cite journal}}: Invalid|ref=harv(help) - ^ Laurin, Michel; Canoville, Aurore; Quilhac, Alexandra (2009). "Use of paleontological and molecular data in supertrees for comparative studies: the example of lissamphibian femoral microanatomy". Journal of Anatomy. 215 (2): 110–123. doi:10.1111/j.1469-7580.2009.01104.x. PMC 2740958. PMID 19508493.
{{cite journal}}: Invalid|ref=harv(help) - ^ Marjanović, D.; Laurin, M. (2008). "Assessing confidence intervals for stratigraphic ranges of higher taxa: the case of Lissamphibia" (PDF). Acta Palaeontologica Polonica. 53 (3): 413–432. doi:10.4202/app.2008.0305.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|author-separator=ignored (help) - ^ a b Sahney, S., Benton, M.J. and Ferry, P.A. (2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land" (PDF). Biology Letters. 6 (4): 544–547. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Ward, P.D.; Botha, J.; Buick, R.; Kock, M.O.; Erwin, D.H.; Garrisson, G.H.; Kirschvink, J.L.; Smith, R. (2005). "Abrupt and gradual extinction among late Permian land vertebrates in the Karoo Basin, South Africa". Science. 307 (5710): 709–714. Bibcode:2005Sci...307..709W. doi:10.1126/science.1107068. PMID 15661973.
{{cite journal}}: Invalid|ref=harv(help) - ^ Colbert, Edwin H. (1969). Evolution of the Vertebrates (2nd ed.). John Wiley & Sons. pp. 49–53.
- ^ Benton 2005, p. 67
- ^ Meunier, François J. (January 2012). "A microanatomical and histological study of the fin long bones of the Devonian sarcopterygian Eusthenopteron foordi". Acta Zoologica. 93 (1): 88–97. doi:10.1111/j.1463-6395.2010.00489.x.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Long JA, Gordon MS (2004). "The greatest step in vertebrate history: a paleobiological review of the fish-tetrapod transition". Physiol. Biochem. Zool. 77 (5): 700–19. doi:10.1086/425183. PMID 15547790.
{{cite journal}}: Invalid|ref=harv(help) as PDF - ^ Clack 2002, pp. 86–7
- ^ a b Ruta, M. (2003). "A supertree of early tetrapods". Proceedings of the Royal Society B. 270 (1532): 2507–16. doi:10.1098/rspb.2003.2524. PMC 1691537. PMID 14667343.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "RJC03" was defined multiple times with different content (see the help page). - ^ When the Invasion of Land Failed: The Legacy of the Devonian Extinctions
- ^ Research project: The Mid-Palaeozoic biotic crisis: Setting the trajectory of Tetrapod evolution
- ^ Tudge, Colin (2000). The Variety of Life. Oxford University Press. ISBN 0-19-860426-2.
- ^ Sahney, S., Benton, M.J. & Falcon-Lang, H.J. (2010). "Rainforest collapse triggered Pennsylvanian tetrapod diversification in Euramerica" (PDF). Geology. 38 (12): 1079–1082. doi:10.1130/G31182.1.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Sahney, S., Benton, M.J. (2008). "Recovery from the most profound mass extinction of all time" (PDF). Proceedings of the Royal Society: Biological. 275 (1636): 759–65. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ a b Romer, A.S. (1949). The Vertebrate Body. Philadelphia: W.B. Saunders. (2nd ed. 1955; 3rd ed. 1962; 4th ed. 1970)
- ^ Lloyd, G.E.R. (1961). "The Development of Aristotle's Theory of the Classification of Animals". Phronesis. 6 (1): 59–81. doi:10.1163/156852861X00080. JSTOR 4181685.
{{cite journal}}: Invalid|ref=harv(help) - ^ Linnaeus, Carolus (1758). Systema naturae per regna tria naturae :secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis (in Latin) (10th edition ed.). Stockholm: Laurentius Salvius.
- ^ Latreielle, P.A. (1804): Nouveau Dictionnaire à Histoire Naturelle, xxiv., cited in Latreille's Familles naturelles du règne animal, exposés succinctement et dans un ordre analytique, 1825
- ^ Smith, C.H. (2005): Romer, Alfred Sherwood (United States 1894-1973), homepage from Western Kentucky University
- ^ Benton, M. J. (1998) The quality of the fossil record of vertebrates. Pp. 269-303, in Donovan, S. K. and Paul, C. R. C. (eds), The adequacy of the fossil record, Fig. 2. Wiley, New York, 312 pp.
- ^ Neill, J.D. (ed.) (2006): Knobil and Neill’s Physiology of Reproduction, Vol 2, Academic Press, 3rd edition (p. 2177)
- ^ Fortuny, J., Bolet, A., Sellés, A.G., J. Cartanyà, J. & À. Galobart, À. (2011): New insights on the Permian and Triassic vertebrates from the Iberian Peninsula with emphasis on the Pyrenean and Catalonian basins. Journal of Iberian Geology no 37 (1): pp 65-86 doi:10.5209/rev_JIGE.2011.v37.n1.5
- ^ Hildebrand, M. & G. E. Goslow, Jr. Principal ill. Viola Hildebrand. (2001). Analysis of vertebrate structure. New York: Wiley. p. 429. ISBN 0-471-29505-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Rieppel O, DeBraga M (1996). "Turtles as diapsid reptiles". Nature. 384 (6608): 453–5. Bibcode:1996Natur.384..453R. doi:10.1038/384453a0.
{{cite journal}}: Invalid|ref=harv(help) - ^ http://phys.org/news/2014-02-jaw-mechanics-tetrapods-fed-underwater.html#jCp
Literature
- Clack, J.A. (2012). Gaining ground: the origin and evolution of tetrapods (2nd ed.). Bloomington, Indiana, USA.: Indiana University Press.
{{cite book}}: Invalid|ref=harv(help)
Further reading
- Clack, Jennifer A. (2009). "The Fin to Limb Transition: New Data, Interpretations, and Hypotheses from Paleontology and Developmental Biology". Annual Review of Earth and Planetary Sciences. 37 (1): 163–179. doi:10.1146/annurev.earth.36.031207.124146.
- Hall, Brian K. (ed) (2007). Fins Into Limbs: Evolution, Development, and Transformation. Chicago: University of Chicago Press. pp. 344 p. ISBN 9780226313405.
{{cite book}}:|first=has generic name (help) - Long JA, Young GC, Holland T, Senden TJ, Fitzgerald EM (November 2006). "An exceptional Devonian fish from Australia sheds light on tetrapod origins". Nature. 444 (7116): 199–202. Bibcode:2006Natur.444..199L. doi:10.1038/nature05243. PMID 17051154.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - Benton, Michael (2005). Vertebrate Palaeontology (3rd ed.). Blackwell Publishing.
{{cite book}}: Invalid|ref=harv(help)

