Artemisinin
 | |
| Clinical data | |
|---|---|
| Other names | Artemisinine, Qinghaosu |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.458 |
| Chemical and physical data | |
| Formula | C15H22O5 |
| Molar mass | 282.332 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.24 ± 0.1 g/cm3 |
| Melting point | 152 to 157 °C (306 to 315 °F) |
| Boiling point | decomposes |
| |
| |
| | |
Artemisinin /ɑːrt[invalid input: 'ɨ']ˈmɪs[invalid input: 'ɨ']n[invalid input: 'ɨ']n/, also known as Qinghaosu (Chinese: 青蒿素), and its derivatives are a group of drugs that possess the most rapid action of all current drugs against Plasmodium falciparum malaria.[1] Treatments containing an artemisinin derivative (artemisinin-combination therapies, ACTs) are now standard treatment worldwide for P. falciparum malaria. Artemisinin is isolated from the plant Artemisia annua, sweet wormwood, a herb employed in Chinese traditional medicine. It can now also be produced using genetically engineered yeast.
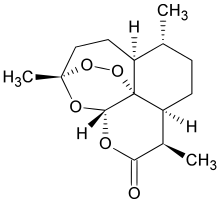
Chemically, artemisinin is a sesquiterpene lactone containing an unusual peroxide bridge. This peroxide is believed to be responsible for the drug's mechanism of action. Few other natural compounds with such a peroxide bridge are known.[2]
Use of the drug by itself as a monotherapy is explicitly discouraged by the World Health Organization, as there have been signs that malarial parasites are developing resistance to the drug. Therapies that combine artemisinin with some other antimalarial drug are the preferred treatment for malaria and are both effective and well tolerated in patients. The drug is also increasingly being used in Plasmodium vivax malaria,[3] as well as being a topic of research in cancer treatment.
History
Etymology
Artemisinin is an antimalarial lactone derived from qing hao 青蒿 (Artemisia annua or sweet wormwood). The medicinal value of this plant has been known to the Chinese for at least 2,000 years. In 1596, Li Shizhen recommended tea made from qing hao specifically to treat malaria symptoms in his "Compendium of Materia Medica". The genus name is derived from the Greek goddess Artemis and, more specifically, may have been named after Queen Artemisia II of Caria, a botanist and medical researcher in the fourth century bce.[4]
Discovery
This section needs additional citations for verification. (June 2014) |
Artemisia annua (A. annua) is a common herb found in many parts of the world, and has been used by Chinese herbalists for more than two thousand years in the treatment of malaria. The earliest record dates back to 200 BC, in the "Fifty-two Prescriptions" unearthed from the Mawangdui Han Dynasty tombs.[5] Its antimalarial application was first described, in Zhouhou Beiji Fang ("The Handbook of Prescriptions for Emergencies", Chinese: 肘后备急方), edited in the middle of the fourth century by Ge Hong; in that book, 43 malaria treatment methods were recorded.[6] Images of the original scientific papers that record the history of the discovery, have been available online since 2006.[7]

In 1967, a plant screening research program, under the name Project 523, was set up by the Chinese army to find an adequate treatment for malaria; the program and early clinical work were ordered of Chairman Mao Zedong at the request of North Vietnamese leaders to provide assistance for their malaria-ridden army.[8] In the course of this research, Tu Youyou 屠呦呦 discovered artemisinin in the leaves of Artemisia annua (annual wormwood; 1972).[9] The drug is named Qinghaosu (Chinese: 青蒿素) in Chinese.[citation needed] It was one of many candidates tested as possible treatments for malaria by Chinese scientists, from a list of nearly 5000 traditional Chinese medicines.[citation needed] Tu Youyou also discovered that a low-temperature extraction process could be used to isolate an effective antimalarial substance from the plant; Tu says she was influenced by a traditional Chinese herbal medicine source saying that this herb should be steeped in cold water.[10] The extracted substance, once subject to purification, proved to be a usable antimalarial drug.[11] A 2012 review reported that artemisinin-based therapies were the most effective drugs for treatment of malaria at that time;[12] it was also reported to clear malaria parasites from patients' bodies faster than other drugs. In addition to artemisinin, Project 523 developed a number of products that can be used in combination with artemisinin, including lumefantrine, piperaquine, and pyronaridine.[9]
For many years after the discovery, access to the purified drug and the plant from which it was extracted were restricted by the Chinese government.[citation needed] It was not until the Chinese economic reform in the late 1970s and early 1980s that news of the discovery reached scientists outside China via results published in the Chinese Medical Journal (in 1979).[13][non-primary source needed] Ying Lee, one of the scientists involved in the research into artemisinin, said the Chinese had distrusted the West at the time.[This quote needs a citation] The research was met with skepticism at first, partly because the chemical structure of artemisinin, particularly the peroxide portion, appeared to be too unstable to be a viable drug.[2]
In the late 1990s, Novartis bought a new Chinese patent for a combination treatment with artemether and lumefantrine, providing the first artemisinin-based combination therapies (ACTs) (Coartem) at reduced prices to the World Health Organisation.[14] In 2006, after artemisinin had become the treatment of choice for malaria, the WHO called for an immediate halt to single-drug artemisinin preparations in favor of combinations of artemisinin with another malaria drug, to reduce the risk of parasites developing resistance.[15]
In 2011, Tu Youyou was awarded the prestigious Lasker-DeBakey Clinical Medical Research Award for her role in the discovery and development of artemisinin.[9][16] The New York Times notes that the discovery of artemisinin is under consideration for a future Nobel Prize in Physiology or Medicine.[17]
Artemisinin derivatives
Because artemisinin itself has physical properties such as poor bioavailability that limit its effectiveness, semisynthetic derivatives of artemisinin have been developed. These include:
- Artesunate (water-soluble: for oral, rectal, intramuscular, or intravenous use)
- Artemether (lipid-soluble: for oral, rectal or intramuscular use)
- Dihydroartemisinin
- Artelinic acid
- Artemotil
There are also simplified analogs in preclinical research.[18]
A synthetic compound with a similar trioxolane structure (ring containing three oxygen atoms) named arterolane[19] showed promise in in vitro testing. Phase II testing in patients with malaria was not as successful as hoped, but the manufacturer decided to start Phase III testing anyway.[20] A combination with piperaquine is also in development.[citation needed]
Indications
Uncomplicated malaria
Artemisinins can be used alone, but this leads to a high rate of recrudescence (return of parasites) and other drugs are required to clear the body of all parasites and prevent recurrence. The World Health Organization (WHO) is pressuring manufacturers to stop making the uncompounded drug available to the medical community at large, aware of the catastrophe that would result if the malaria parasite developed resistance to artemisinins.[21]
The WHO has recommended artemisinin combination therapies (ACT) be the first-line therapy for P. falciparum malaria worldwide.[22] Combinations are effective because the artemisinin component kills the majority of parasites at the start of the treatment, while the more slowly eliminated partner drug clears the remaining parasites.[23]
Several fixed-dose ACTs are now available containing an artemisinin component and a partner drug which has a long half-life, such as mefloquine (ASMQ[24]), lumefantrine (Coartem), amodiaquine (ASAQ), piperaquine (Duo-Cotecxin), and pyronaridine (Pyramax). Increasingly, these combinations are being made to GMP standard. A separate issue concerns the quality of some artemisinin-containing products being sold in Africa and Southeast Asia.[25][26]
Artemisinins are not used for malaria prophylaxis (prevention) because of the extremely short activity (half-life) of the drug. To be effective, it would have to be administered multiple times each day.
Severe malaria
Artesunate administered by intravenous or intramuscular injection has proven superior to quinine in large, randomised controlled trials in both adults [27] and children.[28] Combining all trials comparing these two drugs, artesunate is associated with a mortality rate that is approximately 30% lower than that of quinine.[28] Reasons for this difference include reduced incidence of hypoglycaemia, easier administration and more rapid action against circulating and sequestered parasites. Artesunate is now recommended by the WHO for treatment of all cases of severe malaria.
Cancer treatment
Artemisinin is undergoing early research and testing for the treatment of cancer.[29] Artemisinin has anticancer effects in experimental models of hepatocellular carcinoma.[30] Artemisinin has a peroxide lactone group in its structure, and it is thought that when the peroxide comes into contact with high iron concentrations (common in cancerous cells), the molecule becomes unstable and releases reactive oxygen species. It has been shown to reduce angiogenesis and the expression of vascular endothelial growth factor in some tissue cultures. Recent pharmacological evidence demonstrates the artemisinin derivative dihydroartemisinin targets human metastatic melanoma cells in vitro with induction of phorbol-12-myristate-13-acetate-induced protein 1 dependent mitochondrial apoptosis that occurs downstream of iron-dependent generation of cytotoxic oxidative stress.[31]
Helminth parasites
Serendipitous discovery was made in China while searching for novel anthelmintics for schistosomiasis. Artemisinin was effective against schistosomes, the human blood flukes, which are the second-most prevalent parasitic infections, after malaria. Artemisinin and its derivatives are all potent anthelmintics.[32] Artemisinins were later found to possess a broad spectrum of activity against a wide range of trematodes, including Schistosoma japonicum, S. mansoni, S. haematobium, Clonorchis sinensis, Fasciola hepatica, and Opisthorchis viverrini. Clinical trials were also successfully conducted in Africa among patients with schistosomiasis.[33] A randomized, double-blind, placebo-controlled trial also revealed the efficacy against schistosome infection in Côte d'Ivoire[34] and China.[35]
Resistance
Clinical evidence for artemisinin resistance in southeast Asia was first reported in 2008,[36] and was subsequently confirmed by a detailed study from western Cambodia.[37] Resistance in neighbouring Thailand was reported in 2012,[38] and in Northern Cambodia, Vietnam and Eastern Myanmar in 2014.[39][40] Emerging resistance was reported in Southern Laos, central Myanmar and North-Eastern Cambodia in 2014.[39][40] The parasite's kelch gene on chromosome 13 appears to be a reliable molecular marker for clinical resistance in southeast Asia.[41]
In April 2011, the WHO stated that resistance to the most effective antimalarial drug, artemisinin, could unravel national (India) malaria control programs, which have achieved significant progress in the last decade. WHO advocates the rational use of antimalarial drugs and acknowledges the crucial role of community health workers in reducing malaria in the region.[42]
Adverse effects
Artemisinins are generally well tolerated at the doses used to treat malaria.[43] The side effects from the artemisinin class of medications are similar to the symptoms of malaria: nausea, vomiting, anorexia, and dizziness. Mild blood abnormalities have also been noted. A rare but serious adverse effect is allergic reaction.[43][44] One case of significant liver inflammation has been reported in association with prolonged use of a relatively high-dose of artemisinin for an unclear reason (the patient did not have malaria).[45] The drugs used in combination therapies can contribute to the adverse effects experienced by those undergoing treatment. Adverse effects in patients with acute P. falciparum malaria treated with artemisinin derivatives tend to be higher.[46]
Mechanism of action
Most artemisinins used today are prodrugs of the biologically active metabolite dihydroartemisinin, which is active during the stage when the parasite is located inside red blood cells. Although there is no consensus regarding the mechanism through which artemisinin derivatives kill the parasites,[47] several lines of evidence indicate that artemisinins exert their antimalarial action by radical formation that depends on their endoperoxide bridge. When the parasite that causes malaria infects a red blood cell, it consumes hemoglobin within its digestive vacuole, a process that generates oxidative stress.[48] In one theory of the mechanism of action the iron of the heme directly reduces the peroxide bond in artemisinin, generating high-valent iron-oxo species and resulting in a cascade of reactions that produce reactive oxygen radicals which damages the parasite and lead to its death.[49] However, the likelihood of this mechanism has been debated.[50] A more recently described alternative is that artemisinins disrupt cellular redox cycling.[51] Artesunate has been shown to potently inhibit the essential Plasmodium falciparum exported protein 1 (EXP1), a membrane glutathione S-transferase.[52]
Numerous studies have investigated the type of damage oxygen radicals may induce. For example, Pandey et al. have observed inhibition of digestive vacuole cysteine protease activity of malarial parasites by artemisinin.[53] These observations were supported by ex vivo experiments showing accumulation of hemoglobin in the parasites treated with artemisinin and inhibition of hemozoin formation by malaria parasites, although this inhibition was not seen in an in vitro B-hematin inhibition assay.[54] Electron microscopic evidence linking artemisinin action to the parasite's digestive vacuole has been obtained showing that the digestive vacuole membrane suffers damage soon after parasites are exposed to artemisinin.[55] This would also be consistent with data showing that the digestive vacuole is already established by the mid-ring stage of the parasite's blood cycle,[56] a stage that is sensitive to artemisinins but not other antimalarials. However, fluorescently tagged artemisinin was seen in the Golgi, ER and mitochondria, rather than the digestive vacuole, suggesting that the vacuolar damage may be a downstream effect, and also that tiny ring stages (containing minimal digested material) are highly susceptible to artemisinins.[57][58][59]
Other suggested targets include the mitochondrial electron transport chain [60] and the parasite's SERCA pump (PfATP6/PfSERCA)[61]; neither theory has gained widespread acceptance.
Dosing
Artemisinin derivatives have half-lives of the order of an hour, and therefore require at least daily dosing over several days. For example, the WHO-approved adult dose of co-artemether (artemether-lumefantrine) is 4 tablets at 0, 8, 24, 36, 48 and 60 hours (six doses).[62][63]
Artemisinin is not soluble in water, therefore Artemisia annua tea was postulated not to contain pharmacologically significant amounts of artemesinin.[64] However, this conclusion was rebuked by several experts who stated that hot water (85 °C), and not boiling water, should be used to prepare the tea. Although Artemisia tea is not recommended as a substitute for the ACT (artemisinin combination therapies), more clinical studies on its tea preparation have been suggested.[65]
Production and price
China and Vietnam provide 70% and East Africa 20% of the raw plant material. Seedlings are grown in nurseries and then transplanted into fields. It takes about 8 months for them to reach full size. The plants are harvested, the leaves are dried and sent to facilities where the artemisinin is extracted using solvent, typically hexane. Alternative extraction methods have been proposed.[66] The market price for artemisinin has fluctuated widely, between $120 and $1200 per kilogram from 2005 to 2008.[67]
After negotiation with the WHO, Novartis and Sanofi-Aventis provide ACT drugs at cost on a nonprofit basis; however, these drugs are still more expensive than other malaria treatments.[68] Artesunate injection for severe malaria treatment is made by the Guilin Factory in China where production has received WHO prequalification,[69] an indicator of drug quality.
High-yield varieties of Artemisia are being produced by the Centre for Novel Agricultural Products at the University of York using molecular breeding techniques.[67]
Using seed supplied by Action for Natural Medicine (ANAMED), the World Agroforestry Centre (ICRAF) has developed a hybrid, dubbed A3, which can grow to a height of 3 m and produce 20 times more artemisinin than wild varieties. In northwestern Mozambique, ICRAF is working together with a medical organisation, Médecins sans frontières, ANAMED and the Ministry of Agriculture and Rural Development to train farmers on how to grow the shrub from cuttings, and to harvest and dry the leaves to make artemisia tea.
In April 2013, Sanofi announced the launch[70] of a production facility in Garessio, Italy, to manufacture the anti-plasmodial drug on a large scale. The partnership to create a new pharmaceutical manufacturing process was led by PATH’s Drug Development program (through an affiliation with OneWorld Health), with funding from the Bill & Melinda Gates Foundation and based on a modified biosynthetic process for artemisinic acid, initially designed by Jay Keasling at the University of California, Berkeley and optimized by Amyris. The reaction is followed by a photochemical process creating singlet oxygen to obtain the end product. Sanofi expects to produce 25 tons of artemisinin in 2013, ramping up the production to 55-60 tons in 2014. The price per kg will be $350–400, roughly the same as the botanical source.[71] Despite concerns that this equivalent source would lead to the demise of companies, which produce this substance conventionally through extraction of A. annua biomass, an increased supply of this drug will likely produce lower prices and therefore increase the availability for ACTs treatment. In August 2014, Sanofi announced the release of the first batch of semisynthetic artemisinin. 1.7 million doses of Sanofi's ArteSunate AmodiaQuine Winthrop (ASAQ Winthrop), a fixed-dose artemisinin-based combination therapy will be shipped to half a dozen African countries over the next few months. [72]
Synthesis
Biosynthesis in A. annua
The biosynthesis of artemisinin is believed to involve the mevalonate pathway (MVA) and the cyclization of farnesyl diphosphate (FDP). It is not clear whether the non-mevalonate pathway pathway can also contribute 5-carbon precursors (IPP or/and DMAPP), as occurs in other sesquiterpene biosynthetic systems. The routes from artemisinic alcohol to artemisinin remain controversial, and they differ mainly in when the reduction step takes place. Both routes suggested dihydroartemisinic acid as the final precursor to artemisinin. Dihydroartemisinic acid then undergoes photo-oxidation to produce dihydroartemisinic acid hydroperoxide. Ring expansion by the cleavage of hydroperoxide and a second oxygen-mediated hydroperoxidation finish the biosynthesis of artemisinin.

Chemical synthesis
The total synthesis of the sesquiterpene lactone, artemisinin (quinghaosu) has been performed from available organic starting materials, using basic organic reagents, many times. The first two total syntheses were a "remarkable... stereoselective synthesis" by Schmid and Hofheinz at Hoffmann-La Roche in Basel starting from (−)-isopulegol (13 steps, ≈5% overall yield) and a concurrent synthesis by Zhou and coworkers at the Shanghai Institute of Organic Chemistry from R-(+)-citronellal (20 steps, ≈0.3% overall yield).[73] Key steps of the Schmid-Hofheinz approach included an initial Ohrloff stereoselective hydroboration/oxidation to establish the "off-ring" methyl stereocenter on the propene side chain; two sequential lithium-reagent mediated alkylations that introduced all needed carbon atoms and that were, together highly diasteroselective; and further reduction, oxidation, and desilylation steps performed on this mono-carbocyclic intermediate, including a final singlet oxygen-utilizing photooxygenation and ene reaction, which, after acidic workup closed the three remaining oxacyclic rings of the desired product, artemisinin, in a single step.[73][74] (In essence, the final oxidative ring closing operation in these syntheses accomplishes the closing three biosynthetic steps shown above.)
A wide variety of further routes continue to be explored, from early days until today, including total synthesis routes from R-(+)-pulegone, isomenthene,[73] and even 2-cyclohexen-1-one,[75] as well as routes better described as partial or semisyntheses from a more plentiful biosynthetic precursor, artemisinic acid—in the latter case, including some very short and very high yielding biomimetic synthesis examples (of Roth and Acton, and Haynes et al., e.g., 3 steps, 30% yield), which again feature the singlet oxygen ene chemsitry.[73][76][77]
Synthesis in engineered organisms
The partnership to develop semisynthetic artemisinin was led by PATH’s Drug Development program (through an affiliation with OneWorld Health), with funding from the Bill & Melinda Gates Foundation. The project began in 2004, and initial project partners included the University of CA, Berkeley (which provided the technology on which the project was based – a process that genetically altered yeast to produce artemisinic acid); and Amyris, Inc. (a biotechnology firm in California, which refined the process to enable large-scale production and developed scalable processes for transfer to an industrial partner).
In 2006, a team from UC Berkeley reported they had engineered Saccharomyces cerevisiae yeast to produce small amount of the precursor artemisinic acid. The synthesized artemisinic acid can then be transported out, purified and chemically converted into artemisinin that they claim will cost roughly $0.25 per dose. In this effort of synthetic biology, a modified mevalonate pathway was used, and the yeast cells were engineered to express the enzyme amorphadiene synthase and a cytochrome P450 monooxygenase (CYP71AV1), both from A. annua. A three-step oxidation of amorpha-4,11-diene gives the resulting artemisinic acid.[78]
The Berkeley method was augmented using technology from various other organizations. The final successful technology is based on inventions licensed from UC Berkeley and the National Research Council (NRC) Plant Biotechnology Institute of Canada.
Commercial production of semisynthetic artemisinin is now underway at Sanofi's site in Garessio, Italy. This second source of artemisinin is poised to enable a more stable flow of key antimalarial treatments to those who need them most.[70] The production goal is set at 35 tons for 2013. It is expected to increase to 50-60 tons per year in 2014, supplying approximately 1/3 of the global annual need for artemisinin.
On May 8, 2013, WHO’s Prequalification of Medicines Programme announced the acceptability of semisynthetic artemisinin for use in the manufacture of active pharmaceutical ingredients submitted to WHO for prequalification, or that have already been qualified by WHO.[79] Sanofi’s API produced from semisynthetic artemisinin (artesunate) was also prequalified by WHO on May 8, 2013, making it the first semisynthetic artemisinin derivative prequalified.
In 2010, a team from Wageningen University reported they had engineered a close relative of tobacco, Nicotiana benthamiana, that can also produce the precursor artemisinic acid.[80]
References
This article contains public domain text from the CDC as cited
- ^ White NJ (July 1997). "Assessment of the pharmacodynamic properties of antimalarial drugs in vivo". Antimicrob. Agents Chemother. 41 (7): 1413–22. PMC 163932. PMID 9210658.
- ^ a b Royal Society of Chemistry (July 2006). "Artemisinin and a new generation of antimalarial drugs". Education in Chemistry.
- ^ Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN (June 2010). "Artemisinin combination therapy for vivax malaria". Lancet Infect Dis. 10 (6): 405–16. doi:10.1016/S1473-3099(10)70079-7. PMC 3350863. PMID 20510281.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Various (Jul 2014). "Etymologia: Artemisinin". Emerg Infect Dis [Internet]. 20 (7). CDC. doi:10.3201/eid2007.ET2007. Retrieved July 4, 2014.
- ^ "Medical manuscripts from Ma Wang Dui". Qingaosu Project.
- ^ "Zhou Hou Bei Ji Fang". Qingaosu Project.
- ^ Burns, William. Qingaosu Project http://qinghaosu.blogspot.com/. Retrieved 8 August 2014.
{{cite web}}: Missing or empty|title=(help) - ^ Jianfang, Zhang (2006). A Detailed Chronological Record of Project 523 and the Discovery and Development of Qinghaosu (Artemisinin).
- ^ a b c Miller L.H. and Su X. (September 16, 2011). "Artemisinin: discovery from the Chinese herbal garden". Cell. 146 (6). CAMBRIDGE, MA 02139, USA: Cell Press: 855–858. doi:10.1016/j.cell.2011.08.024. ISSN 0092-8674. PMC 3414217. PMID 21907397.
{{cite journal}}: CS1 maint: location (link) - ^ "Lasker Award Rekindles Debate Over Artemisinin's Discovery | Science/AAAS | News". News.sciencemag.org. Retrieved 2014-01-07.
- ^ Miller, Louis H.; Su, Xinzhuan (2011). "Artemisinin: Discovery from the Chinese Herbal Garden". Cell. 146 (6): 855–8. doi:10.1016/j.cell.2011.08.024. PMC 3414217. PMID 21907397.
- ^ Fairhurst, RM; Nayyar, GM; Breman, JG; Hallett, R; Vennerstrom, JL; Duong, S; Ringwald, P; Wellems, TE; Plowe, CV; Dondorp, AM (2012). "Artemisinin-resistant malaria: Research challenges, opportunities, and public health implications". The American journal of tropical medicine and hygiene. 87 (2): 231–41. doi:10.4269/ajtmh.2012.12-0025. PMC 3414557. PMID 22855752.
- ^ "Antimalaria studies on Qinghaosu". Chin. Med. J. 92 (12): 811–6. December 1979. PMID 117984.
- ^ D. MNeil (2012). "For Intrigue, Malaria Drug Gets the Prize". NYTimes, Health. Retrieved 20. April 2013.
- ^ "WHO calls for an immediate halt to provision of single-drug artemisinin malaria pills". WHO. 19 January 2006.
- ^ Elizabeth Weise, "'America's Nobel' awarded to Chinese scientist", USA Today, 12 September 2011, accessed September 12, 2011.
- ^ "For Intrigue, Malaria Drug Gets the Prize". New York Times. 16 January 2012.
- ^ Gary H. Posner; Michael H. Parker; Northrop, John; Elias, Jeffrey S.; Ploypradith, Poonsakdi; Xie, Suji; Shapiro, Theresa A. (1999). "Orally Active, Hydrolytically Stable, Semisynthetic, Antimalarial Trioxanes in the Artemisinin Family". J. Med. Chem. 42 (2): 300–304. doi:10.1021/jm980529v. PMID 9925735.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vennerstrom JL, Arbe-Barnes S, Brun R; et al. (August 2004). "Identification of an antimalarial synthetic trioxolane drug development candidate". Nature. 430 (7002): 900–4. doi:10.1038/nature02779. PMID 15318224.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ C.H. Unnikrishnan (September 21, 2007). "Blow to Ranbaxy drug research plans". livemint.com.
- ^ Rehwagen C (May 2006). "WHO ultimatum on artemisinin monotherapy is showing results". BMJ. 332 (7551): 1176. doi:10.1136/bmj.332.7551.1176-b. PMC 1463909. PMID 16709988.
- ^ Guidelines for the Treatment of Malaria. Geneva: World Health Organization. 2006. ISBN 92-4-154694-8.
- ^ White NJ (April 2004). "Antimalarial drug resistance". J. Clin. Invest. 113 (8): 1084–92. doi:10.1172/JCI21682. PMC 385418. PMID 15085184.
- ^ Krudsood S, Looareesuwan S, Tangpukdee N; et al. (June 2010). "New Fixed-Dose Artesunate-Mefloquine Formulation against Multidrug-Resistant Plasmodium falciparum in Adults: a Comparative Phase IIb Safety and Pharmacokinetic Study with Standard-Dose Nonfixed Artesunate plus Mefloquine". Antimicrob Agents Chemother. 54 (9): 3730–7. doi:10.1128/AAC.01187-09. PMC 2935027. PMID 20547795.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Malaria drugs recalled in Kenya". BBC News. 17 August 2007.
- ^ Newton P, Proux S, Green M; et al. (June 2001). "Fake artesunate in southeast Asia". Lancet. 357 (9272): 1948–50. doi:10.1016/S0140-6736(00)05085-6. PMID 11425421.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Dondorp A, Nosten F, Stepniewska K, Day N, White N (2005). "Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial". Lancet. 366 (9487): 717–25. doi:10.1016/S0140-6736(05)67176-0. PMID 16125588.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Dondorp AM, Fanello CI, Hendriksen IC; et al. (November 2010). "Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial". Lancet. 376 (9753): 1647–57. doi:10.1016/S0140-6736(10)61924-1. PMC 3033534. PMID 21062666.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ University of Washington: Artemisinin
- ^ Hou J, Wang D, Zhang R, Wang H (2008). "Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action". Clin Cancer Res. 14 (7): 5519–5530. doi:10.1158/1078-0432.CCR-08-0197. PMID 18765544.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cabello CM, Lamore SD, Bair WB 3rd, Qiao S, Azimian S, Lesson JL, Wondrak GT (2011). "The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis". Invest. New Drugs. 30 (4): 1289–301. doi:10.1007/s10637-011-9676-7. PMC 3203350. PMID 21547369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Xiao SH. (2005). "Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins". Acta Trop. 96 (2–3): 153–167. doi:10.1016/j.actatropica.2005.07.010. PMID 16112072.
- ^ Keiser J, Utzinger J. (2007). "Artemisinins and synthetic trioxolanes in the treatment of helminth infections". Current Opinion in Infectious Diseases. 20 (6): 605–612. doi:10.1097/QCO.0b013e3282f19ec4. PMID 17975411.
- ^ Utzinger J, N'Goran EK, N'Dri A, Lengeler C, Xiao S, Tanner M. (2000). "Oral artemether for prevention of Schistosoma mansoni infection: randomised controlled trial". Lancet. 355 (9212): 1320–5. doi:10.1016/S0140-6736(00)02114-0. PMID 10776745.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li YS, Chen HG, He HB, Hou XY, Ellis M, McManus DP. (2005). "A double-blind field trial on the effects of artemether on Schistosoma japonicum infection in a highly endemic focus in southern China". Acta Trop. 96 (23): 184–190. doi:10.1016/j.actatropica.2005.07.013. PMID 16112071.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19064625, please use {{cite journal}} with
|pmid=19064625instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19641202, please use {{cite journal}} with
|pmid=19641202instead. - ^ Phyo AP, Nkhoma S, Stepniewska K; et al. (2012). "Emergence of artemisinin-resistant malaria on the Western border of Thailand: a longitudinal study". Lancet. 379 (9830): 1960–6. doi:10.1016/S0140-6736(12)60484-X. PMC 3525980. PMID 22484134.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Briggs, Helen (30 July 2014) Call for 'radical action' on drug-resistant malaria BBC News, health, Retrieved 30 July 2013
- ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1056/NEJMoa1314981, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1056/NEJMoa1314981instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 24352242, please use {{cite journal}} with
|pmid=24352242instead. - ^ Drugs immunity ‘may’ fail malaria fight. The Jakarta Post, April 23, 2011.
- ^ a b Taylor WR, White NJ (2004). "Antimalarial drug toxicity: a review". Drug Saf. 27 (1): 25–61. doi:10.2165/00002018-200427010-00003. PMID 14720085.
- ^ Leonardi E, Gilvary G, White NJ, Nosten F (2001). "Severe allergic reactions to oral artesunate: a report of two cases". Trans. R. Soc. Trop. Med. Hyg. 95 (2): 182–3. doi:10.1016/S0035-9203(01)90157-9. PMID 11355556.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Hepatitis Temporally Associated with an Herbal Supplement Containing Artemisinin — Washington, 2008". CDC.
- ^ R. Price; et al. (1999). "Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives". American Journal of Tropical Medicine and Hygiene. 60 (4): 547–555. PMID 10348227.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Kappe SH, Vaughan AM, Boddey JA, Cowman AF (May 2010). "That was then but this is now: malaria research in the time of an eradication agenda". Science. 328 (5980): 862–6. doi:10.1126/science.1184785. PMID 20466924.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9140469, please use {{cite journal}} with
|pmid=9140469instead. - ^ Cumming JN; Ploypradith P; Posner GH (1997). "Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action". Adv. Pharmacol. 37: 253–97. doi:10.1016/S1054-3589(08)60952-7. PMID 8891104.
- ^ Haynes, RK; Cheu, KW; N'Da, D; Coghi, P; Monti, D (Aug 2013). "Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses". Infectious disorders drug targets. 13 (4): 217–77. doi:10.2174/1871526513666131129155708. PMID 24304352.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21994127, please use {{cite journal}} with
|pmid= 21994127instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 25126794, please use {{cite journal}} with
|pmid= 25126794instead. - ^ Pandey AV, Tekwani BL, Singh RL, Chauhan VS (July 1999). "Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite". J. Biol. Chem. 274 (27): 19383–8. doi:10.1074/jbc.274.27.19383. PMID 10383451.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Haynes, RK; Monti, D; Taramelli, D; Basilico, N; Parapini, S; Olliaro, P (March 2003). "Artemisinin antimalarials do not inhibit hemozoin formation". Antimicrob Agents Chemother. 47 (3): 1175. doi:10.1128/aac.47.3.1175.2003. PMC 149339. PMID 12604568.
- ^ del Pilar Crespo M, Avery TD, Hanssen E; et al. (January 2008). "Artemisinin and a Series of Novel Endoperoxide Antimalarials Exert Early Effects on Digestive Vacuole Morphology". Antimicrob. Agents Chemother. 52 (1): 98–109. doi:10.1128/AAC.00609-07. PMC 2223901. PMID 17938190.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Abu Bakar N, Klonis N, Hanssen E, Chan C, Tilley L (February 2010). "Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum". J. Cell. Sci. 123 (Pt 3): 441–50. doi:10.1242/jcs.061499. PMID 20067995.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Liu, Y; Lok, CN; Ko, BC; Shum, TY; Wong, MK; Che, CM (April 2, 2010). "Subcellular localization of a fluorescent artemisinin derivative to endoplasmic reticulum". Org Lett. 12 (7): 1420–1423. doi:10.1021/ol902890j. PMID 20192248.
- ^ Golenser, J; Waknine, JH; Krugliak, M; Hunt, NH; Grau, GE (Dec 2006). "Current perspectives on the mechanism of action of artemisinins". Int J Parasitol. 36 (14): 1427–1441. doi:10.1016/j.ijpara.2006.07.011. PMID 17005183.
- ^ Eckstein-Ludwig, U; Webb, RJ; Van Goethem, ID; East, JM; Lee, AG; Kimura, M; O'Neill, PM; Bray, PG; Ward, SA; Krishna, S (Aug 21, 2003). "Artemisinins target the SERCA of Plasmodium falciparum". Nature. 424 (6951): 957–961. doi:10.1038/nature01813. PMID 12931192.
- ^ Li W, Mo W, Shen D; et al. (September 2005). "Yeast Model Uncovers Dual Roles of Mitochondria in the Action of Artemisinin". PLoS Genet. 1 (3): e36. doi:10.1371/journal.pgen.0010036. PMC 1201371. PMID 16170412.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12931192, please use {{cite journal}} with
|pmid=12931192instead. - ^ Vugt MV, Wilairatana P, Gemperli B; et al. (1999). "Efficacy of six doses of artemether-lumefantrine (benflumetol) in multidrug-resistant Plasmodium falciparum malaria". Am J Trop Med Hyg. 60 (6): 936–42. PMID 10403324.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Lefevre G, Looareesuwan S, Treeprasertsuk S; et al. (2001). "A clinical and pharmacokinetic trial of six doses of artemether-lumefantrine for multidrug-resistant Plasmodium falciparum malaria in Thailand". Am J Trop Med Hyg. 64 (5–6): 247–56. PMID 11463111.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Jansen FH (2006). "The herbal tea approach for artemesinin as a therapy for malaria?". Trans R Soc Trop Med Hyg. 100 (3): 285–6. doi:10.1016/j.trstmh.2005.08.004. PMID 16274712.
- ^ RITAM Artemisia annua Task Force (2007). "Artemisia Annua as a Herbal Tea for Malaria". Afr J Tradit Complement Altern Med. 4 (1): 121–3. ISSN 0189-6016. PMC 2816434. PMID 20162081.
- ^ Lapkin, Alexei A.; Peters, Martina; Greiner, Lasse; Chemat, Smain; Leonhard, Kai; Liauw, Marcel A.; Leitner, Walter (1 January 2010). "Screening of new solvents for artemisinin extraction process using ab initio methodology". Green Chemistry. 12 (2): 241. doi:10.1039/b922001a. and literature cited therein
- ^ a b "Report of the Artemisinin Enterprise Conference 2008" (PDF).
- ^ Artemisinin combination therapies, CNAP Artemisia Project
- ^ http://www.mmv.org/partnering/guilin-pharmaceutical-─-achieving-prequalification-price-affordable-in-africa
- ^ a b Pantjushenko, Elena. "Sanofi and PATH announce the launch of large-scale production of semisynthetic artemisinin against malaria". PATH. Cite error: The named reference "Sanofi and PATH announce the launch of large-scale production of semisynthetic artemisinin against malaria" was defined multiple times with different content (see the help page).
- ^ Mark Peplow (April 2013). "Sanofi launches malaria drug production to maintain stability in Artemisinin availability". chemistryworld online (RSC). Retrieved April 19, 2013.
- ^ Palmer, Eric (19 August 2014). "Sanofi shipping new malaria treatment manufactured from 'semisynthetic artemisinin'". fiercepharmamanufacturing.com. Retrieved 14 September 2014.
- ^ a b c d Michael C. Pirrung & Andrew T. Morehead, Jr. (1997) A Sesquidecade of Sesquiterpenes, 1980-1994: Part A. Acyclic and Monocyclic Sesquiterpenes, Part 1, in The Total Synthesis of Natural Products, Vol 10 (D Goldsmith, Ed.) New York:John Wiley & Sons, pp. 90-96. see [books.google.com/books?isbn=047012962X], accessed 19 June 2014.
- ^ Schmid G., Hofheinz W. (1983). "Total Synthesis of qinghaosu". J. Am. Chem. Soc. 105 (3): 624–5. doi:10.1021/ja00341a054.
- ^ Chunyin Zhu & Silas P. Cook (2012) A Concise Synthesis of (+)-Artemisinin, J. Amer. Chem. Soc. 134(33):13577-13579, see [1], accessed 19 June 2014.
- ^ Lévesque F, Seeberger PH (January 2012). "Continuous-Flow Synthesis of the Anti-Malaria Drug Artemisinin". Angewandte Chemie International Edition 51 (7) 1706-1709. doi:10.1002/anie.201107446
- ^ Joël Turconi, Frédéric Griolet, Ronan Guevel, Gilles Oddon, Roberto Villa, Andrea Geatti, Massimo Hvala, Kai Rossen, Rudolf Göller, and Andreas Burgard (2014) Semisynthetic artemisinin, the chemical path to industrial production, Org. Proc. Research Devel., 18(3):417-422, see [2], accessed 19 June 2014.
- ^ Ro DK, Paradise EM, Ouellet M; et al. (April 2006). "Production of the antimalarial drug precursor artemisinic acid in engineered yeast". Nature. 440 (7086): 940–3. doi:10.1038/nature04640. PMID 16612385.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Pantjushenko, Elena. "Semisynthetic artemisinin achieves WHO prequalification". PATH. Retrieved 8 February 2014.
- ^ van Herpen, TW.; Cankar, K.; Nogueira, M.; Bosch, D.; Bouwmeester, HJ.; Beekwilder, J. (3 December 2010). Yang, Haibing (ed.). "Nicotiana benthamiana as a Production Platform for Artemisinin Precursors". PLoS ONE. 5 (12): e14222. doi:10.1371/journal.pone.0014222. PMC 2997059. PMID 21151979.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- History, Aetiology, Pathophysiology, Clinical Features, Diagnosis, Treatment, Complications And Control Of Malaria: Artemisinin Derivatives
- Design and synthesis of antimalarial endoperoxides
- van Vugt M, Looareesuwan S, Wilairatana P; et al. (2000). "Artemether-lumefantrine for the treatment of multidrug-resistant falciparum malaria". Trans. R. Soc. Trop. Med. Hyg. 94 (5): 545–8. doi:10.1016/S0035-9203(00)90082-8. PMID 11132386.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Daviss B (2005). "Malaria, Science, and Social Responsibility: Nonprofit drug-development partnership seeks to cure the ills of developing nations". The Scientist. 19 (6): 42.
- Research on the use of artemisinin for cancer treatment
- Artemisinin — Researchers blend folk treatment, high tech for promising anti-cancer compound
- BBC Horizon documentary about artemisinin
- Artemisia Annua, by Memorial-Sloan Kettering Cancer Center
- Use of Artemisinin for Cancer Treatment and Bacterial Infection, Henry Lai, Ph.D., University of Washington (streaming video, Spring 2005)
- Assured Artemisinin Supply System, support the global production of sufficient Artemisia/artemisinin to meet the expanded needs
- Artemisinin latest patents, covering use in fighting infections, including viral infections; fighting cancer; various novel derivatives and ACTs; high-yielding Artemisia plants; and extraction methods.
