User:Sandbh/sandbox
Group 3 and its elements in periods 6 and 7
Although scandium and yttrium are always the first two elements in group 3, the identity of the next two elements is not completely settled. They are commonly lanthanum and actinium, and less often lutetium and lawrencium. The two variants originate from historical difficulties in placing the lanthanides in the periodic table, and arguments as to where the f block elements start and end.[1]
The detachment of the lanthanides from the main body of the periodic table has been attributed to the Czech chemist Bohuslav Brauner who, in 1902, allocated all of them ("Ce etc.") to one position in group 4, below zirconium. This arrangement was referred to as the "asteroid hypothesis", in analogy to asteroids occupying a single orbit in the solar system. Before this time the lanthanides were generally (and unsuccessfully) placed throughout groups I to VIII of the older 8-column form of periodic table. Although predecessors of Brauner's 1902 arrangement are recorded from as early as 1895, he is known to have referred to the "chemistry of asteroids" in an 1881 letter to Mendeleev. Other authors assigned all of the lanthanides to either group 3, groups 3 and 4, or groups 2, 3 and 4. In 1922 Niels Bohr continued the detachment process by locating the lanthanides between the s- and d-blocks. In 1949 Glenn T. Seaborg (re)introduced the form of periodic table that is popular today, in which the lanthanides and actinides appear as footnotes. Seaborg first published his table in a classified report dated 1944. It was published again by him in 1945 in Chemical and Engineering News, and in the years up to 1949 several authors commented on, and generally agreed with, Seaborg's proposal. In that year he noted that the best method for presenting the actinides seemed to be by positioning them below, and as analogues of, the lanthanides.[2]
It has been claimed that such arguments are proof that, "it is a mistake to break the [periodic] system into sharply delimited blocks".[3] A third common variant shows the two positions below yttrium as being occupied by the lanthanides and the actinides. A fourth variant shows group 3 bifurcating after Sc-Y, into an La-Ac branch, and an Lu-Lr branch.[4]
In 1895, even before the discovery of lutetium, Hans Peter Jørgen Julius Thomsen considered lanthanum to ytterbium to form a 14-element series.[5] Since 1921, many chemical and physical arguments have been made in support of lutetium and lawrencium[6][7] but the majority of authors seem either unconvinced by them or unaware of them.[8][9] Most working chemists are not aware there is any controversy.[9] In December 2015 an IUPAC project was established to make a recommendation on the matter, considering only the first two alternatives as possibilities.[10]
Lanthanum and actinium
 La and Ac below Y |
Support
Lanthanum and actinium are commonly depicted as the remaining group 3 members.[11][n 1] It has been suggested that this layout originated in the 1940s, with the appearance of periodic tables relying on the ground-state electron configurations of the elements and the notion of the differentiating electron. The ground-state configurations of caesium, barium and lanthanum are [Xe]6s1, [Xe]6s2 and [Xe]5d16s2. Lanthanum thus emerges with a 5d differentiating electron and on these grounds it was considered to be "in group 3 as the first member of the d-block for period 6".[12] A consistent set of electron configurations is then seen in group 3: scandium [Ar]3d14s2, yttrium [Kr]4d15s2 and lanthanum [Xe]5d16s2. Still in period 6, ytterbium was assigned an electron configuration of [Xe]4f135d16s2 and lutetium [Xe]4f145d16s2, "resulting in a 4f differentiating electron for lutetium and firmly establishing it as the last member of the f-block for period 6".[12] Later spectroscopic work found that the electron configuration of ytterbium was in fact [Xe]4f146s2. This meant that ytterbium and lutetium—the latter with [Xe]4f145d16s2—both had 14 f-electrons, "resulting in a d- rather than an f- differentiating electron" for lutetium and making it an "equally valid candidate" with [Xe]5d16s2 lanthanum, for the group 3 periodic table position below yttrium.[12] Lanthanum has the advantage of incumbency since the 5d1 electron appears for the first time in its structure whereas it appears for the third time in lutetium, having also made a brief second appearance in gadolinium[13]
Support
In terms of chemical behaviour,[14] and trends going down group 3 (if Sc-Y-La is chosen) for properties such as melting point, electronegativity and ionic radius,[15][16] scandium, yttrium, lanthanum and actinium are similar to their group 1–2 counterparts, but at variance with the other groups in the early d-block. In this variant, the number of f electrons in the most common (trivalent) ions of the f-block elements consistently matches their position in the f-block.[17] For example, the f-electron counts for the trivalent ions of the first three f-block elements are Ce 1, Pr 2 and Nd 3.[18]
Oppose
- (though similar logic would also lead to thorium getting the 6d2 position, having incumbency over rutherfordium).[citation needed]
- Ground-state gas-phase configurations consider only isolated atoms as opposed to bonding atoms in compounds (the latter being more relevant for chemistry), which often show different configurations.[19] Moreover, the lowest levels of two different configurations often are separated by only very small energies, that are minuscule compared to the spreading of J-levels of each configuration (e.g. terbium, where the 285 cm−1 difference between [Xe]4f85d16s2 and the ground state [Xe]4f95s2 is much less than 1% of this spreading), making which configuration happens to be the ground state chemically quite irrelevant.[5] It is the dominant electron configuration of atoms in chemical environments, and not free gaseous atoms in a vacuum, that can rationalise qualitative chemical behaviour.[20]
- The form with lanthanum under yttrium also creates an inconsistency in the treatment of thorium, which has no f-electrons in the ground-state (being [Rn]6d27s2), similar to actinium as [Rn]6d17s2; yet it places thorium in the f-block but not actinium.[21] Considering only ground-state gas-phase configurations, thorium [Rn]6d27s2 by itself is just as good a homologue to zirconium [Kr]4d25s2 as lanthanum [Xe]5d16s2 is to scandium [Ar]3d14s2;[5] yet thorium is invariably placed in the f-block, not in group 4 with zirconium. Thorium thus demonstrates that the possession of an f electron in the ground-state gas-phase configuration of an element is not necessary for it to belong to the f-block.[22] Additionally, this form necessitates a split d-block if expanded to a 32-column periodic table.[22]
Oppose
- However, outside the lanthanides there does not exist a typical oxidation state across any period of a block,[23] and the reason for this singular behaviour of the lanthanides in fact has very little to do with the electron configurations of the elements concerned,[24] which on the face of it would seem to predict a preferred +2 oxidation state as they are mostly [Xe]4fn6s2 (lanthanides) or [Rn]5fn7s2 (actinides).[25]
- "It is important to realize that the electronic structures listed ... are those of the neutral (unionized) gaseous atoms, whereas it is the electronic structure of the ions and compounds that we are chiefly concerned with in chemistry. The relationship of the electronic structure of the gaseous atom of an element to that of its compounds can be rather complicated. For example, in the case of the actinide and lanthanide elements, one would not necessarily predict the predominance of the III oxidation state from the electronic structures of the gaseous atoms; there are usually only two so-called "valence electrons," the 7s or 6s electrons, which might indicate a preference for the II oxidation state.
Apparently, specific factors in the crystal structure of, and the aquation (hydration) energies of, the compounds and ions are important in determining the stability of the III oxidation state. Thus, the characteristic tripositive oxidation state of the lanthanide elements is not related directly to the number of "valence electrons" outside the 4f subshell, but is the somewhat accidental result of a nearly constant small difference between large energy terms (ionization potentials on the one hand, and hydration and crystal energies on the other) which persists over an interval of fourteen atomic numbers. Therefore, if we could somehow have a very extended Periodic Table of Elements containing numerous "f" transition series, we might expect that the 5f, rather than the 4f, elements would be regarded as more nearly representative of such f series.[25]|source=Glenn T. Seaborg, Origin of the Actinide Concept (1991)
- "It is important to realize that the electronic structures listed ... are those of the neutral (unionized) gaseous atoms, whereas it is the electronic structure of the ions and compounds that we are chiefly concerned with in chemistry. The relationship of the electronic structure of the gaseous atom of an element to that of its compounds can be rather complicated. For example, in the case of the actinide and lanthanide elements, one would not necessarily predict the predominance of the III oxidation state from the electronic structures of the gaseous atoms; there are usually only two so-called "valence electrons," the 7s or 6s electrons, which might indicate a preference for the II oxidation state.
- Similarity of chemistry is, in addition, not the only factor that needs to be considered for periodic table placement. Tungsten and uranium chemically resemble each other (and were placed in the same group before Seaborg's clarification of the actinides), in a manner that is not worse than the resemblances between tin and lead, or between antimony and bismuth, both of which are universally considered to belong in the same group.[5] Moreover, the resemblance between aluminium and scandium, which are placed in different groups, is actually stronger in some ways than that between aluminium and gallium, which are in the same group.[26] The same is true of the relationship of beryllium and magnesium to zinc, which is in some ways stronger than their relationship to calcium.[27]
Lutetium and lawrencium
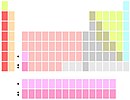 Lu and Lr below Y |
Support
In other tables, lutetium and lawrencium are the remaining group 3 members.[n 2] Early techniques for chemically separating scandium, yttrium and lutetium relied on the fact that these elements occurred together in the so-called "yttrium group" whereas La and Ac occurred together in the "cerium group".[12] Accordingly, lutetium rather than lanthanum was assigned to group 3 by some chemists in the 1920s and 30s.
Oppose
The phenomenon of different separation groups is caused by increasing basicity with increasing radius, and does not constitute a fundamental reason to show Lu, rather than La, below Y. Thus, among the Group 2 alkaline earth metals, Mg (less basic) belongs in the "soluble group" and Ca, Sr and Ba (more basic) occur in the "ammonium carbonate group". Nevertheless, Mg, Ca, Sr and Ba are routinely collocated in Group 2 of the periodic table.[28]
Support
Several physicists in the 1950s and '60s favoured lutetium, in light of a comparison of several of its physical properties with those of lanthanum.[12] Among the prominent adherents of this form have been Lev Landau and Evgeny Lifshitz, who wrote in their Course of Theoretical Physics (1958):[29]
| “ | In books of chemistry, lutetium is usually placed in the rare-earth elements. This, however, is incorrect, since the 4f shell is complete in lutetium; it must therefore be placed in the platinum group [which they considered to be La+Lu–Pt]... | ” |
| — Landau and Lifshitz, Course of Theoretical Physics, Vol. 3: Quantum Mechanics: Non-Relativistic Theory (1958) | ||
Oppose
This arrangement, in which lanthanum is the first member of the f-block, is disputed by some authors since lanthanum lacks any f-electrons.
Support
It has been argued that this is not a valid concern given other periodic table anomalies, such as thorium.[30] The binding energies of the 4f levels of excited states of lanthanum that contain a 4f electron clearly show that lanthanum's 4f orbitals are not hydrogenic. In other words, in hydrogen through barium, the 4f orbitals are far enough from the nucleus that when analysing them, one can approximate the core and remaining electrons as a point charge; starting from lanthanum, this ceases to be the case, with lanthanum showing 4f levels more similar to those of the following rare earths.[31] These low-lying empty f orbitals, which lutetium lacks,[21] contribute measurably to the bonding in some lanthanum compounds, for example in lanthanum(III) fluoride (LaF3). While this contribution is small, it is greater for lanthanum than for any other lanthanide, considering for each the analogous LnF3 compound; meanwhile, the Lu–F 4f–2p bond order in LuF3 is less than the analogous one of IrF3, with iridium well into the 5d block.[32] And while the trivalent lanthanides Pr3+ through Yb3+ show characteristic narrow bands with their positions almost completely independent on the ligands, the following 5d elements (along with the 3d and 4d elements) behave significantly differently; while both types of elements show electron-transfer bands, ligand field theory becomes important for these d elements.[24] The order of involvement of 4f in lanthanum is similar to that of 5f in thorium; that of 4f in cerium is similar to that of 5f in uranium.[5]
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
| hcp | hcp | bcc | bcc | ~bcc | bcc | hcp | fcc | fcc | ~hcp |
| Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd |
| hcp | hcp | bcc | bcc | hcp | hcp | fcc | fcc | fcc | ~hcp |
| Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg |
| hcp | hcp | bcc | bcc | hcp | hcp | fcc | fcc | fcc | ~hcp |
| Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn |
| hcp | hcp | bcc | bcc | hcp | hcp | fcc | bcc | bcc | bcc |
Lanthanum has the dhcp crystal structure as the most stable one at standard conditions, and actinium is fcc; whereas scandium, yttrium, lutetium, and lawrencium (the last predicted) show the hcp crystal structure. The dhcp crystal structure is only known for some rare earths and actinides in the f-block and is unknown elsewhere on the periodic table.[31] This constitutes an anomaly in the otherwise completely regular variation of the crystal structures of the nonmagnetic transition metals with their valencies (except for the late 6d metals, which should be anomalous due to strong relativistic effects for those superheavy elements),[33][34] and is a sign of 4f band involvement for lanthanum, because lanthanum without 4f involvement would be expected to be hcp like scandium, yttrium, and lutetium. Instead, the pressure-temperature phase diagram of lanthanum is isomorphic to those of the uncontroversial 4f metals praseodymium and neodymium.[33] Similarly, thorium (which as noted above has a similar level of f involvement as lanthanum) is fcc, rather than hcp like the group 4 metals, because of 5f band involvement.[35]
Karl Gschneidner, analysing the melting points of the lanthanides in a 1971 article, reached the conclusion that it was likely that 4f, 5d, 6s, and 6p electrons were all involved in the bonding of lanthanide metals except for lutetium, where 4f electrons were not found to be involved.[36] The fact that lanthanum was demonstrated to be a 4f-band metal (with about 0.17 electrons per atom in fcc lanthanum, which is metastable at standard conditions)[37] whereas the 4f shell appears to have no influence on the metallic properties of lutetium, has been used as an argument to place lutetium in group 3 instead of lanthanum.[33] The 4f occupancy in solid lanthanum may explain some of its properties, such as its low melting point (La 920 °C, versus Sc 1541 °C, Y 1526 °C, Lu 1652 °C)[36] low Debye temperature, and anomalously high superconducting transition temperature at all pressures.[33] Indeed, if lanthanum is treated as a d-block element, it constitutes anomalies in the trends of superconducting transition temperatures at a variety of pressures, all of which are removed if lutetium is put in its place.[31][33] Jörg Wittig, considering this problem in 1973, found it likely that this small 4f band involvement in lanthanum "represents the screening charge of a 4f scattering resonance safeguarded deep in the interior of the lanthanum ion core", similarly to cerium: this is in agreement with Gschneidner's model. The difficulty in observing this would then be due to the strong d resonance that this 4f virtual bound state also has. This is confirmed by the alloy LaAl2, whose 16% lower Debye temperature and higher electronic specific heat coefficients compared to LuAl2 reflect "directly the additional 4f density of states at the Fermi surface".[33]
Scandium, yttrium, and lutetium show a more consistent set of electron configurations matching the global trend on the periodic table: the 5d metals then all add a closed 4f14 shell. For example, the shift from yttrium [Kr]4d15s2 to lutetium [Xe]4f145d16s2 exactly parallels that from zirconium [Kr]4d25s2 to hafnium [Xe]4f145d26s2.[12] The inclusion of lutetium rather than lanthanum also homogenises the 5d transition series: trends in atomic size, coordination number, and relative abundance of metal–oxygen bonds all reveal that lutetium is closer than lanthanum to the behaviour of the uncontroversial 5d metals hafnium through mercury.[38] The same is true considering conduction band structures of the elements: lutetium has a transition-metal-like conduction band structure, but lanthanum does not.[39]
As for lawrencium, its gas phase ground-state atomic electron configuration was confirmed in 2015 as [Rn]5f147s27p1. Such a configuration represents another periodic table anomaly, regardless of whether lawrencium is located in the f-block or the d-block, as the only potentially applicable p-block position has been reserved for nihonium with its predicted configuration of [Rn]5f146d107s27p1.[40] However, it is expected that in the condensed phase and in chemical environments lawrencium has the expected 6d occupancy, and simple modelling studies suggest it will behave like a lanthanide,[41] in particular being a homologue of lutetium. Lawrencium's return to +3 as the only stable oxidation state and being predicted to form a trivalent metal is distinct from the behaviour of the other late actinides fermium, mendelevium, and nobelium, which have a tendency towards forming lower oxidation states and form (or are predicted to form) divalent metals; it also makes an exception to the actinide contraction generally being larger than the analogous lanthanide contraction at the end of both series.[42] The steadily increasing stability of the +2 state along the actinide series going to nobelium is similar to that along the 3d series going to zinc.[43]
Meanwhile, actinium has a band structure with itinerant 5f electrons, that is similar to those of lanthanum and praseodymium;[44] the 5f bands are in the same region as and hybridise strongly with the 6d and 7s bands, with the width of the 5f band increasing with pressure.[45]
While scandium, yttrium and lutetium (and lawrencium, so far as its chemistry is known) do often behave like trivalent versions of the group 1–2 metals, being hard class-A cations mostly restricted to the group oxidation state, they are not the only elements in the d-block or f-block that do so. The early transition metals zirconium and hafnium in group 4, as well as niobium and tantalum in group 5, also display such behaviour, as does the actinide thorium. (The heavy group 4 elements and thorium are tetravalent; the heavy group 5 elements are pentavalent.)[46][47] The physical properties of the group 3 elements are affected by the presence of a d electron, which forms more localised bonds within the metals than the p electrons in the similar group 13 metals;[48] exactly the same situation is found comparing group 4 to group 14.[49] Trends going down group 3 (if Sc-Y-Lu is chosen) for properties such as melting point, electronegativity and ionic radius, are similar to those found among their group 4–8 counterparts in the same block, as noted by William B. Jensen in an often-cited 1982 article in which he argued for this placement.[12] In this variant, the number of f electrons in the gaseous forms of the f-block atoms usually matches their position in the f-block. For example, the f-electron counts for the first five f-block elements are La 0, Ce 1, Pr 3, Nd 4 and Pm 5.[12]
Lanthanides and actinides
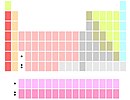 Markers below Y |
A few authors position all thirty lanthanides and actinides in the two positions below yttrium (usually via footnote markers). This variant, which is stated in the 2005 Red Book to be the IUPAC-agreed version as of 2005 (a number of later versions exist, and the last update is from 1 December 2018),[50][n 3] emphasizes similarities in the chemistry of the 15 lanthanide elements (La–Lu), possibly at the expense of ambiguity as to which elements occupy the two group 3 positions below yttrium, and a 15-column wide f block (there can only be 14 elements in any row of the f block).[n 4] However, this similarity does not extend to the 15 actinide elements (Ac–Lr), which show a much wider variety in their chemistries.[5] This form moreover reduces the f-block to a degenerate branch of group 3 of the d-block; it dates back to the 1920s when the lanthanides were thought to have their f electrons as core electrons, which is now known to be false. It is also false for the actinides, many of which show stable oxidation states above +3.[52]
La-Ac and Lu-Lr

In this variant, group 3 bifurcates after Sc-Y into a La-Ac branch, and a Lu-Lr branch. This arrangement is consistent with the hypothesis that arguments in favour of either Sc-Y-La-Ac or Sc-Y-Lu-Lr based on chemical and physical data are inconclusive.[53] As noted, trends going down Sc-Y-La-Ac match trends in groups 1−2[54] whereas trends going down Sc-Y-Lu-Lr better match trends in groups 4−10.[12]
The bifurcation of group 3 is a throwback to the Mendeleev eight column-form in which seven of the main groups each have two subgroups. Tables featuring a bifurcated group 3 have been periodically proposed since that time.[n 5]
Classifying the elements (C)
The progression from metallic to nonmetallic character in traversing the periodic table shows a pleasing symmetry
| Active metals Groups 1–3, Ln, An, (Al) |
Corrosive nonmetals O, F, Cl, Br, I | ||||
| Transition metals Most of groups 4–11 |
Related nonmetals H, C, N, P, S, Se | ||||
| Poor metals (Al), Ga, Bi etc |
Metalloids (weak nonmetals) B, Si, Ge, As, Sb, Te | ||||
| Noble metals Ru, Rh, Pd, Ag, Os, Ir, Pt, Au |
Noble gases He, Ne, Ar, Kr, Xe, Rn |
Notes
1. The category name related nonmetals is analogous to older references to the transition metals as related metals, for example:
- Ebel IL 1938, "Atomic structure and the periodic table", Journal of Chemical Education, vol. 15, no. 12, p. 575
- Quagliano JV & Vallarino LM 1969, Chemistry, Prentice-Hall, 3rd ed., Englewood Cliffs, NJ, p. 848
- Luder WF 1970, "The atomic structure chart of the elements," Canadian Chemical Education, April, p. 13
That is a pleasing coincidence i.e. that the transition metals line up with the related nonmetals.
2. The related nonmetals are related by the H-C-P-N-S-Se thread.
3. I'm eschewing the term post-transition metal so as to not have to deal with the question of Al, or perhaps I should move it into the active metals category?
4. I was inspired to revisit the symmetry and names of these eight categories by:
- Scerri ER 2012, "A critique of Wiesberg's view on the periodic table and some speculations on the nature of classifications", Foundations of Chemistry, vol. 14, no. 3, pp. 275–284.
5. Praise be that all category names are relatively short.
6. The balanced 6-6-5-6 distribution of the nonmetals is pleasing.
7. An article to follow, in an appropriate publication. Sandbh (talk) 04:38, 7 September 2019 (UTC)
Antimony as a metalloid
I've been wondering why, from a literature perspective, antimony came to be included among the elements commonly recognised as metalloids.
I suspect there are various "memes" involved. A meme is an idea, behaviour, or style that spreads from person to person within a culture. Here they are, in rough historical order:
0. Pliny the Elder made a distinction between "male" and "female" forms of antimony; the male form was probably the sulfide, while the female form, which is superior, heavier, and less friable, has been suspected to be native antimony.
1. Bastardry. Arsenic, antimony, and bismuth were historically called bastard metals or semimetals on account of their brittle nature. As well, metals were supposed to be fusible. The fact that arsenic sublimed rather than melted further sullied its reputation.
2. Allotropy. Antimony, like arsenic, was known in "metallic" and non-metallic forms. Tin escaped this meme because it was malleable. An equivalent non-metallic allotrope of bismuth was not known.
3. Mendeleev described tellurium as forming a transition between metals and nonmetals. Curiously, he referred to As and Sb as metals, and to Bi as a perfect [sic] metal. That got the hares running as to which other elements could be regarded as forming a transition between metals and nonmetals.
4. Semiconductivity. Johan Koenigsberger classified solid materials as metals, insulators and "variable conductors" in 1914 although his student Josef Weiss already introduced the term Halbleiter (semiconductor in modern meaning) in his PhD thesis of 1910. The subsequent development of semiconductor physics sparked a renewed interest in Ge and Si, and to a lesser extent, B, as halfway elements. As well, the elements to either side of Sb namely Sn and Te existed in semiconducting forms (noting that grey tin behaves like a semiconductor but is actually a semimetal) so it was expected that Sb would also exist in a semiconducting form, which it did (Moss 1952, p. 173).
5. Metalloid line. Deming's 1923 periodic table made it easier to make out a notional dividing line between metals and nonmetals, naturally focusing attention on the elements to either side namely Be and B; Al and Si; Ge and As; Sb and Te; and Po and At. Note the absence of Bi.
6. Amphoterism. The amphoteric character of:
- Ge and As, lying as they do between Ga (a metal) and Se (usually considered to be a nonmetal; and
- Sb and Te, lying as they do between Sn (a metal) and I (a nonmetal),
came to be associated with a transition in metallic character, from metallic to nonmetallic.
The situation in period 6 was less clear. The sequence of elements involved is Pb Bi, Po, and At. Lead is a metal. Astatine was popularly thought to be a halogen, and therefore a nonmetal (although the folks who first synthesised it thought it was a metal). On this basis it could’ve been thought that Bi and Po would be amphoteric. However Bi was regarded as basic, and only Po showed some amphoteric character, which may have resulted in some authors regarding it as a metalloid.
7. Pauling published his influential book General chemistry (1947) in which he referred to B, Si, Ge, As, Sb, Te, and Po as being metalloids. (He erroneously referred to Sb as being a semiconductor.)
8. Rochow published The metalloids (1966) and recognised B, Si, Ge, As, Sb, and Te as such.
9. Group 15. The progression in metallic character going down group 15 tended to reinforce regarding Bi as a metal, but not Sb. For example:
- "Antimony…is more nonmetallic than metallic…bismuth…more nearly approaches a metal in physical and chemical properties." (Norris & Young 1938, p. 529)
- "The trisulphides of arsenic and antimony are acidic, forming salts with yellow ammonium sulphide and alkali, while that of bismuth is typical of a metal." (Moody 1969, pp. 267, 321)
- "All the elements react readily with halogens but are unaffected by non-oxidising acids. Nitric acid gives, respectively, phosphoric acid, arsenic acid, antimony trioxide, and bismuth nitrate, which well illustrates the increasing metallic character as the group is descended." (Cotton & Wilkinson 1976, p. 288)
- "The paucity of [stereochemical] information about Bi is due to the more metallic character of this element, which does not form many of the simple covalent molecules formed by As and Sb." (Wells 1984, p. 878)
- "Bismuth(III) oxide occurs naturally as bismite and is formed when Bi combines with O2 on heating. In contrast to earlier members of group 15, molecular species are not observed for Bi2O3 and the structure is more like that of a typical metal oxide." (Housecroft & Sharpe 2008, p. 474)
I guess Sb came to be regarded as a metalloid mainly due to its brittle comportment; existence of a non-metallic semiconducting allotrope; proximity to the metalloid line; perceived amphoterism; and apparent lack of genuine salts. In contrast, Bi had only one of these features.
References
Cotton FA & Wilkinson G 1976, Basic inorganic chemistry, Wiley, New York
Housecroft CE & Sharpe AG 2008, Inorganic chemistry, 3rd ed., Pearson, Harlow
Moody B 1969, Comparative inorganic chemistry, 2nd ed., Edward Arnold, London
Moss TS 1952, Photoconductivity in the elements, Butterworths Scientific Publications, London
Norris JF & Young RC 1938, A textbook of inorganic chemistry for colleges, 2nd ed., McGraw-Hill, New York
Wells AF 1984, Structural inorganic chemistry, 5th ed., Oxford University, Oxford.
-- Sandbh (talk) 10:14, 24 May 2018 (UTC)
Table
| Property | Metalloid | Chemically active nonmetal | Noble gas |
|---|---|---|---|
| Appearance | metallic | metallic, coloured, or translucent | translucent |
| Atomic structure | close-packed* or polyatomic | polyatomic or diatomic | monatomic |
| Bulk coordination number | 12*, 6, 4, 3, or 2 | 3, 2, or 1 | 0 |
| Electrical conductivity | moderate | poor to moderate | poor |
| Electronic structure | metallic* to semiconductor | semimetallic, semiconductor, or insulator | insulator |
| Ionization energy | low | moderate to high | high to very high |
| Electron affinity | low to high | moderate to high (exception: N is negative) | negative |
| Electronegativity | moderate | moderate to high | moderate to very high |
| Oxidising power | low (exception: At is moderate) |
low to high | n/a |
| Compounds with metals | tend to form alloys or inter-metallic compounds | mainly covalent: H†, C, N, P, S, Se mainly ionic: O, F, Cl, Br, I |
none form simple compounds |
*Bulk astatine has been predicted to have a face-centred cubic structure
† Hydrogen can also form alloy-like hydrides
Response to DePiep
@DePiep: I haven’t replied so far as I’ve been stumped for an answer. I proceed with the greatest of trepidation in submitting the following thoughts.
The marvelous variety and infinite subtlety of the non-metallic elements, their compounds, structures and reactions, is not sufficiently acknowledged in the current teaching of chemistry.
JJ Zuckerman and FC Nachod
In Steudel's Chemistry of the non-metals (1977, preface)
In trying to understand the nonmetals I think there are seven perspectives to consider:
| (1) | The general properties of nonmetals at standard conditions e.g. volatility, low elasticity, good insulators, they gain or share electrons when they react with other elements or compounds. |
| (2) | Their structures, nonmetals having a low number of nearest neighbours compared to metals. |
| (3) | Which of their properties are surpassed by some metals e.g. the ionisation energy of Hg exceeds that of S and I; the electronegativity of Au exceeds that of P; the electron afinity of Cd is less than than that of N. |
| (4) | Their anomalies e.g. H’s uniqueness; N’s low electron affinity and relative inertness, P4’s reactivity; Xe’s relatively low ionisation energy; first row v second row differences. |
| (5) | The chemistry of the nonmetals by group. |
| (6) | Patterns and trends among the nonmetals in ionisation energy, electron affinity, electronegativity and oxidising power. |
| (7) | Cross-cutting relationships. |
I think if you can keep all this in your head than you can follow why the nonmetals are as diverse as they are. The current nonmetal article only largely does (1) and (2). The rewrite adds some of (4) and (5); a little of (6); and (7). A further rewrite would add or expand (3); (4); (5); and (6).
I tend to think a further rewrite might best be done by having only two formal categories of nonmetal: the top-shelf nonmetal category for H, C, N, P, O, S, Se, F, Cl, Br and I; and a single noble gas subcategory (noting the metalloids are similarly in a top-shelf category and have no subcategory).
The nonmetal article might go partly like this:
Nonmetals are H, C, N, P, O, S, Se in group 1 or groups 13–16; F, Cl, Br, and I in group 17; and the noble gases He, Ne, Ar, Kr, Xe and Rn in group 18. For convenience within this article, nonmetals other than the noble gases are hereafter referred to using the descriptive phrase "chemically active nonmetals"; and the four group 17 elements are referred to as "halogen nonmetals". Of these terms only "noble gases" and "halogen" are IUPAC-approved.
The chemically active nonmetals have a diverse range of individual physical and chemical properties. In periodic table terms they largely occupy a position between the weakly nonmetallic metalloids to the left and the noble gases to the right.
Physically, four are solids, one is a liquid (bromine), and six are gases. Of the solids, carbon, selenium, and iodine are metallic-looking, whereas sulfur has a pale-yellow appearance. Ordinary white phosphorus has a yellowish-white appearance but the black allotrope, which is the most stable form of phosphorus, has a metallic-looking appearance. Bromine is reddish-brown in colour. Of the gases, fluorine and chlorine are coloured pale yellow, and yellowish green. Electrically, most are insulators whereas carbon is a semimetal and black phosphorus, selenium and iodine are semiconductors.
Chemically, they tend to have higher ionisation energies, electron affinities, and electronegativity values, and be relatively strong oxidising agents, in comparison to metals. Collectively, the highest values of these properties are found among oxygen and the halogen nonmetals. Manifestations of this status include oxygen's major association with the ubiquitous processes of corrosion and combustion, and the intrinsically corrosive nature of the halogen nonmetals. All five of these nonmetals exhibit a tendency to form predominately ionic compounds with metals whereas the remaining nonmetals tend to form predominately covalent compounds with metals.
Characteristic and other properties of metalloids, chemically active nonmetals, and noble gases are summarised in the following table. Metalloids have been included in light of their generally nonmetallic chemistry. Physical properties are listed in loose order of ease of determination; chemical properties run from general to specific, and then to descriptive. While the table shows the main points of difference it is somewhat arbitrary since exceptions and boundary overlaps can be found within each category. Important instances of these are so noted.
In writing this it occurs to me that such an approach might work just as well for the di/polyatomic/noble gases. So, after all that, I still don’t know which one will work best. At least you know I haven’t stopped thinking about this/working on it.
I presume project members would be happy with either outcome, depending on how the article in question looked. Whatever the outcome my intention is to have a better, more lucid nonmetal article. It may be that I'll have to do both rewrites.
Somewhere, if we do not do so already, we perhaps need to say that:
| (1) | our categorisation scheme is not definitive; |
| (2) | for convenience and economy of description we… (a) use IUPAC-approved collective names for the alkali metals, alkaline earth metals, and noble gases; (b) do not use the IUPAC-approved collective names lanthanoids, actinoids, rare earth metals, pnictogens, and chalcogens; (c) refer to the leftover elements as either post-transition metals, or metalloids, or "nonmetals" for nonmetals not categorised as noble gases i.e. H, C, N, P, O, S, Se and the halogen nonmetals (akin to the LANL periodic table); |
| (3) | there is a spectrum of properties within each category; |
| (4) | it is not hard to find overlaps at the boundaries, as is the case with most classification schemes. |
And perhaps these caveats need to be flagged some more in the periodic table article. Sandbh (talk) 08:45, 5 October 2017 (UTC)
RfC
I am seeking comments on a proposal to change part of the current nonmetal categorisation scheme, as follows:
| From | Polyatomic nonmetal C, P, S, Se |
Diatomic nonmetal H, N, O, F, Cl, Br, I |
| To | Less active nonmetal H, C, N, P, S, Se |
Active nonmetal O, F, Cl, Br, I |
Origin
The origin of this proposal can be traced to literature conceptions of nonmetals as either halogens (F, Cl, Br, I, At), noble gases, or other metals (H, C, N, O, P, S, Se), with the last of these three groupings representing a poorly characterised "orphan" or leftovers category.
The Wikipedia periodic table used to show the three categories of halogens, noble gases, and other nonmetals up until we recategorised astatine as a metalloid. Astatine has been predicted to have a metallic crystalline structure, which suggests there may be grounds to categorise it as a post-transition metal. But condensed astatine has not yet been observed so for the moment is left as a metalloid.
When astatine was recategorised as metalloid the opportunity was taken to get rid of the nondescript "other nonmetal" category name by moving C, P, S, and Se into a new polyatomic nonmetal category, and moving H, N, and O, into a new diatomic nonmetal category, along with F, Cl, Br, and I.
You can see this current arrangement, which is based on structural considerations, in the nonmetal article. It works, but I've never been completely satisfied with it since it does not necessarily show the most relevant trends associated with nonmetallic character. Chemists tend to think of nonmetals primarily in terms of such things as oxidative power, electronegativity, activity, reactivity, anionic behaviour, or electron affinity, rather than whether the nonmetals have polyatomic or diatomic molecular structures.
Proposal
In retrospect I think it would better to categorise H, C, N, P, S, and Se as less active nonmetals, and O, F, Cl, Br, and I as active nonmetals. Such a division would be based on multiple electrochemical properties, rather than a single structural consideration.
In this arrangement, O, F, Cl, Br, and I are individually and collectively characterised by relatively high ionisation energies, high electronegativities, high electron affinities, high oxidising power, and simple anion formation, consistent with their depiction in the literature.
H, C, N, P, S and Se are unable to consistently match the active nonmetals across the aforementioned electrochemical properties.
The proposed category names, which end with the form "-ive", can accommodate the fact that, for example, while the overall tendency of H and S is to act as reducing agents, they are sometimes capable of acting as oxidants. Another example would be the fact that nitrogen has a higher electronegativity than bromine and iodine. Now nitrogen does show some "active" character in its capacity to form hydrogen bonds and complexes, but it is a poor oxidising agent unless combined with an active nonmetal like O or F; and it is a reluctant anion former, unlike the active nonmetals. So, at the broadbush level being dealt with here, N is a less active nonmetal, with some "active" nuances if you dig deeper. This is consistent with the meaning of the "-ive" suffix: "that performs or tends toward or serves to accomplish an indicated action esp. regularly or lastingly" or "having a tendency to, having the nature, character, or quality of, given to (some action)". Hence it has the meaning of a tendency rather than a finality.
On the question of boundary overlaps such as these I turn also to Jones (2010, pp. 169–171):
"Classes are usually defined by more than two attributes…Though classification is an essential feature of all branches of science, there are always hard cases at the boundaries. The boundary of a class is rarely sharp…Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics."
Similar overlaps occur elsewhere in the periodic table. For example, beryllium in group 2, an alkaline earth metal, behaves chemically more like aluminium in group 3, a post-transition metal; the group 3 transition metals scandium, yttrium, lanthanum and actinium behave largely like the alkaline earth metals or, more generally, s block metals; and gallium in group 13, tin in group 14, and bismuth in group 15, all of which are post-transition metals, have some metalloid properties.
The periodic table nevertheless retains its status as an organising icon of chemistry.
What does IUPAC say?
IUPAC does not provide any guidance on category names for the nonmetals. They have endorsed use of the terms "noble gases" and "halogens" however these are group names rather than category names such as post-transition metal, and metalloid, neither of which are IUPAC endorsed. We do show the group names in our larger periodic table, but we don't have a halogen colour category since we count astatine as a metalloid, which has a different colour category.
What's the go with "halogens" and "other nonmetals"?
As I mentioned, the literature largely distinguishes between noble gases, halogens, and "the rest of the nonmetals". Categorising astatine as a metalloid mitigates against the use of the "halogens" category. The term other nonmetal is occasionally used however this is not based on any consideration of the shared attributes that should characterise a useful category name. And it's an awkward term to use if you want to say something like, for example, "nonmetals form compounds with metals and other nonmetals" or "like hydrogen, carbon forms molecular covalent compounds with most other nonmetals."
What does the literature say?
As it turns out, there is support in the literature for the terms "less active nonmetal", and "active nonmetal". Here are some quotes that use this terminology and highlight the distinctiveness of O, F, Cl, Br, and I.
- "A salt is a compound of metal ions and nonmetal ions. The halogens being active nonmetals are excellent salt-formers." (Allen et al. 1942, p. 484)
- "What, in general, is the difference between active metals, less active metals, less active non-metals, active non-metals, and inert gases…?" (Friedenberg 1946, p. 230)
- "Most commonly metals and halogens form ionic solids." (Pearson & Mawby, 1956, p. 55)
- "The halogens and oxygen are the most active non-metals." (Lee & Van Orden 1965, p. 197)
- "The most active non-metals are in the upper right-hand corner of the chart; the most active metals are in the lower left-hand corner." (Luder 1965, p. 39)
- "From Group V on, the series passes from the less active nonmetals to the most active ones, like chlorine, in Group Vll." (Gardiner & Flemister 1967, p. 22)
- "Across each period is a more or less steady transition from an active metal through less active metals and weakly active non-metals to highly active nonmetals and finally to an inert gas." (Beiser 1968, p. 234)
- "If you don't count the noble gases, Family 18, the most active non-metals are found in the upper right corner." (Aldridge 1993, p. 175)
- "Active nonmetals, such as the halogens (Group VIIA) and oxygen, are good oxidizing agents." (Grolier Incorporated 1999, p. 162)
- "Oxygen is one of the most active nonmetals and one of the most important. It forms compounds with all the elements except the light noble gases (He, Ne, and Ar). In general, oxygen forms ionic compounds with metals…" (Hill and Petrucci 1999, p. 903)
What the new scheme would look like
A draft rewrite of the nonmetal article using the proposed descriptive category names of less active nonmetal and active nonmetal can be found here.
The proposed scheme would result is a more balanced distribution of nonmetals, from 4 + 7, to 6 + 5.
It is the culmination of around five months of discussion with members of WikiProject Elements. Along the way we considered but discarded a range of alternative paired category names, including: weak/strong; intermediate/corrosive; reactive/corrosive; reductive/corrosive; covalent/ionic, heterogenic/corrosive; foundation/corrosive; formative/corrosive; and less active/corrosive.
Summary
I propose to replace the diatomic and polyatomic nonmetal categories with the newly constituted categories of less active nonmetal, and active nonmetal, as sourced from the literature. These categories are more consistent with the most relevant trends associated with nonmetallic character.
References
- Aldridge B et al. 1993, Science interactions, Glencoe/McGraw-Hill, New York
- Allen JS, French SJ, Woodruff JG 1942, Atoms, rocks and galaxies: a survey in physical science, Harper and Brothers, New York
- Beiser A 1968, Perspectives of modern physics, McGraw-Hill, New York
- Friedenberg EZ 1946, A Technique for developing courses in physical science adapted to the needs of students at the junior college level, University of Chicago, Chicago
- Gardiner MS & Flemister SC 1967, The principles of general biology, Macmillan, New York
- Grollier Incorporated 1999, The encyclopaedia Americana, vol. 21, Danbury, Connecticut
- Hill JW & Petrucci RH 1999, General chemistry: An integrated approach, Prentice Hall, Upper Saddle River, New Jersey
- Jones BW 2010, Pluto: Sentinel of the outer Solar System, Cambridge University Press, Cambridge
- Lee GL & Van Orden HO 1965, General chemistry: Inorganic and organic, 2nd ed., Saunders, Philadelphia
- Luder WF 1965, General chemistry, Saunders, Philadelphia
- Pearson RG & Mawby RJ 1967, "The nature of metal–halogen bonds", in V Gutmann (ed.), Halogen chemistry, Academic Press, London
-- Sandbh (talk) 03:29, 9 September 2017 (UTC)
Nonmetal boxes
| Nonmetal (EN) | EN span* | EA |
|---|---|---|
| He (5.5) | −50 | |
| Ne (4.84) | −120 | |
| F (3.98) | 328 | |
| O (3.44) | 141 | |
| Cl (3.16), Ar (3.2) | 349, –96 | |
| N (3.04) | −0.07 | |
| Br (2.96), Kr (2.94) | 325, −60 | |
| I (2.66) | 295 | |
| C (2.55), S (2.58), Se (2.55) | 122, 200, 195 | |
| Xe (2.4) | –80 | |
| H (2.2), P (2.19), As (2.18), At (2.2) | 73, 72, 78, 222 | |
| Te (2.1), Rn (2.06) | 190, −70 | |
| B (2.04), Ge (2.01), Sb (2.05) | 27, 119, 101 | |
| Si (1.9) | 134 |
* Pauling's EN values had an uncertainty of ±0.05
Nonmetal redraft
Is here.
Parsing the nonmetals
Abstract
In this essay the nonmetals are described in terms of what is generally well-known about them, and how they are summarised in the literature. Specific aspects of their nonmetallic character are then highlighted, namely oxidative power, electronegativity, activity, reactivity, anionic behaviour, and electron affinity. On the basis of a high degree of correlation among these properties, there is an evident trichotomy among the nonmetals.
The view from the top
1. From the literature we know that:
- nonmetals, at the outset, are characterised by a lack of metallic properties;
- the alkali metals and the halogens provide the most distinct contrast between metals and nonmetals;
- the most reactive metals are found towards the bottom left of the PT, and the most reactive nonmetals are found in the upper right hand corner just inside the noble gases; and that
- apart from the halogens, and the noble gases, there are the remaining nonmetals.
2. The noble gases will not further be considered.
3. This quote then provides an overview of the nonmetals under consideration:
The behaviour of the nonmetals can be summarised as follows. Nonmetals tend to oxidize metals…Nonmetals with relatively large electronegativities (such as oxygen and chlorine) oxidise substances with which they react…Nonmetals with relatively small electronegativities (such as carbon and hydrogen) can reduce other substances…Oxygen is the perfect example of an oxidizing agent because it increases the oxidation state almost any substance with which it reacts (p. 9)…The chemistry of the halogens is dominated by oxidation-reduction reactions (p. 35).
- – Bodner, Rickard & Spencer 1996, module 1 pp. 3, 9, 35
| Electronegativity | |
|---|---|
| High | O, F, Cl, Br, I |
| Moderate | C, N, S, Se |
| Weak | H, P |
| Strongly electronegative | O, F |
| Moderately electronegative | Br, Cl |
| Weakly electronegative | C, N, S, I |
| Approximately electro-neutral | B, H, P |
| Weakly electro-positive | Si |
| Strong |
|
| Moderate |
|
| Weak |
|
| |
| Wulfsberg 2000, pp. 247–249, 273–276 Schweitzer 2010, pp. 228–229, 232–233 | |
| Nonmetal | Ionisation energy (kJ/mol) | Electron affinity (eV) | Electro-negativity |
|---|---|---|---|
| B | 897 | 27 | 2.04 |
| Si | 793 | 134 | 1.9 |
| Ge | 768 | 119 | 2.01 |
| As | 953 | 79 | 2.18 |
| Sb | 840 | 101 | 2.05 |
| Te | 879 | 190 | 2.1 |
| At | 899 | 233 | 2.2 |
| H | 1,318 | 73 | 2.2 |
| C | 1,093 | 122 | 2.55 |
| N | 1,407 | −0.07 | 3.04 |
| P | 1,018 | 72 | 2.19 |
| S | 1,006 | 200 | 2.58 |
| Se | 947 | 195 | 2.55 |
| O | 1,320 | 141 | 3.44 |
| F | 1,687 | 328 | 3.98 |
| Cl | 1,257 | 349 | 3.16 |
| Br | 1,146 | 324 | 2.96 |
| I | 1,015 | 295 | 2.66 |
| He | 2,372 | −50 | 5.5 |
| Ne | 2,088 | −120 | 4.84 |
| Ar | 1,521 | −96 | 3.2 |
| Kr | 1,351 | −60 | 2.94 |
| Xe | 1,170 | −80 | 2.4 |
| Rn | 1,037 | −70 | 2.06 |
Cross-cutting themes
4. We can compare the former approach (that of Bodner et al.) with the nonmetal groupings of Synder (1966, p. 242) in Table 1, and Nelson (2011, p. 55) in Table 2. The latter author wisely writes (p. 57):
In using [the table]…care needs to be taken to remember that it is only an approximation, and can only be used as a rough guide to the properties of the elements. Provided that this is done, however, it constitutes a very useful classification, and although purists often despise it because of its approximate nature, the fact is that practising chemists make a great deal of use of it, if only subconsciously, in thinking of the chemistry of different elements.
5. We can consider the nonmetal displacement series of (a) Parkes & Mellor (1943, p. 205), and (b) Ashford (1967, p. 312), and observe a similar pattern:
- (a) F…O…Cl…Br…I…S…P…Se…N…C
- (b) F…Cl…O…Br…I…S…N
- (a) F…O…Cl…Br…I…S…P…Se…N…C
6. On the relationship between an element and its compounds Jones (1973, p. 159) writes that:
It is usual when discussing the factors which contribute to the overall chemistry of an element to consider the fundamental atomic properties. Such properties fall broadly into two groups: (1) properties of the free…atom itself which can be measure or calculated directly, e.g., atomic weight or ionisation energy, and (2) properties associated with concepts used to rationalise the behaviour of the…atom in chemically combined states, such as electro-negativity and electron affinity.
7. In this context, average standard reduction potentials for the elements and their stable species in aqueous solution (table 3) correlate well with the observations of all of these authors. Note that while nitrogen itself has a high electronegativity it is a poor oxidising agent. And only when it is in a positive oxidation state (i.e. in combination with oxygen or fluorine) are its compounds good oxidising agents (Cox 2004, p. 161). The latter author thus writes that "Nitrogen is a moderately electronegative element…" In contrast, oxygen and the halogens are relatively strong oxidising agents (Rudolph 1974, p. 133).
8. Consistent with table 3, and in discussing the redox behaviour of the elements, Silberberg (2006, p. 548), in a periodic table extract, shows only O, F, Cl, Br, and I as strong oxidising agents.
9. The theme of distinguishing O, F, Cl, Br and I from the other nonmetals is further reinforced by the literature:
- (a) "The halogens and oxygen are the most active non-metals." (Lee & Van Orden 1965, p. 197)
- (b) "…under SSIMS [secondary ion mass spectrometry] conditions…the electronegative elements, i.e. oxygen, fluorine, chlorine, bromine and iodine, give intense negative ion signals." (Briggs 1998, p. 119)
- (c) "Simple anionic chemistry is limited to oxygen and the halogens, although polyanions and polycations can be formed by many [nonmetals]." (Cox 2004, p. 145)
- (d) "For chemists…the most important feature of an element is its pattern of chemical behaviour, in particular, its tendency toward covalent bond formation (or its preference for cation formation)." (Rayner-Canham & Overton 2006, p. 29)
- (e) "Of the nonmetals, oxygen and the halogens are highly reactive." (Frank, Miller & Little 2004, p. 19)
- (f) "A few nonmetallic elements, such as oxygen and the halogens (F2, Cl2, Br2, and I2) are strong oxidizing agents…" (Moore & Stanitski 2015, p. 114)
10. More generally, the higher an element's ionisation energy, electron affinity, and electronegativity, the more nonmetallic that element is (Yonder, Suydam & Snavely 1975, p. 58). Table 4 shows that O, F, Cl, Br and I collectively have the highest values of these properties among the nonmetals.
Boundary overlaps
11. Notwithstanding the shared characteristics of oxygen and the halogen nonmetals, some of these are evident in the remaining nonmetals such as nitrogen with its high ionisation energy and electronegativity, and sulfur which has an ionisation energy near that of iodine, and a high electron affinity.
12. On the question of boundary overlaps such as these I turn to Jones (2010, pp. 169–171):
"Classes are usually defined by more than two attributes…Though classification is an essential feature of all branches of science, there are always hard cases at the boundaries. The boundary of a class is rarely sharp…Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics."
13. Similar overlaps occur elsewhere in the periodic table. For example, beryllium in group 2, an alkaline earth metal behaves chemically more like aluminium in group 3, a post-transition metal; the group 3 transition metals scandium, yttrium, lanthanum and actinium behave largely like the alkaline earth metals or, more generally, s block metals; and gallium in group 13, tin in group 14, and bismuth in group 15, all of which are post-transition metals, have some metalloid properties.
14. The periodic table nevertheless retains its status as an organising icon of chemistry.
15. On the specific question of nitrogen we can see that, like oxygen, its high electronegativity manifests in its ability to form relatively strong hydrogen bonds, its capacity to enter into coordination complexes, and a preference for multiple bonding over catenation.
16. Unlike oxygen, we can see that nitrogen is reluctant to form simple anions, hence nearly all of its compounds are covalent, that it is a poor oxidising agent, and that the metal nitrides resemble in many ways, the borides, carbides, and phosphides.
17. On the basis of the relative importance of anion formation, covalent bonding, and oxidising power, I concur with the distinction made in the literature between (a) oxygen and the halogen nonmetals as being more nonmetallic than (b) nitrogen, and the assignment of the latter, on an overall basis, to a moderately or weakly electronegative nonmetal category, on par with the treatment of e.g. carbon or sulfur.
18. On the specific question of sulfur I rely on a comparison with iodine, and note that iodine:
- has a significantly higher electron affinity, and a higher electronegativity rating, and a marginally higher ionisation energy;
- is a stronger oxidizing agent, by itself, and on average;
- occurs before sulfur in various nonmetal displacement series;
- is most stable in an oxidation state of –1 compared to the +6 of sulfur; and
- generally resembles the other halogen nonmetals in its chemical properties.
19. While nitrogen and sulfur share some characteristics with oxygen and the halogens these similarities are either selective or of a relatively second order nature. Neither element exhibits a sufficiently effective preponderance of headland nonmetallic properties.
The remaining nonmetals
20. These are the metalloids B, Si, Ge, As, Sb, Te, and At, and the other nonmetals H, C, N, P, S, and Se. The metalloids are counted here in view of their generally nonmetallic chemistry. The properties of the remaining nonmetals are summarised in Table 5. Oxygen and the halogen nonmetals are included for comparative purposes. Physical properties are listed in loose order of ease of determination; chemical properties run from general to specific, and then to descriptive.
| Physical property | Metalloids | Other nonmetals | O and the halogen nonmetals |
|---|---|---|---|
| Form | solid | mainly solid | mainly gaseous |
| Appearance | lustrous | lustrous to colourless | mainly coloured |
| Bulk coordination number | 2–6 | 1–3 | 1 |
| Allotropes | O forms an allotrope | ||
| Electrical conductivity | intermediate | poor to intermediate | poor |
| Electronic structure | semimetal to semiconductor | semimetal to insulator | semiconductor to insulator |
| Outer s and p electrons | 3–7 | 1, 4–6 | 6–7 |
| Crystal structure | rhombohedral: B, As, Sb cubic: Si, Ge, At? hexagonal: Te orthorhombic: At[n 6] |
cubic: P hexagonal: H, C, N, Se orthorhombic: S |
cubic: O, F orthorhombic: Cl, Br, I |
| Atomic radius, calculated, (pm) | mainly more than 100 (87–133) | ||
| Packing efficiency (%)[n 7] | • 34–41 • 28 for astatine[n 8] |
17–28.5 | 23.9 for iodine |
| Chemical property | Metalloids | Other nonmetals | O and the halogen nonmetals |
| General chemical behaviour | |||
| Ionization energy | low | low to high | high to very high |
| Electron affinity (kJ/mol) | low to high (27–233) | • mainly moderate to high (72–200) • N is slightly negative (–1.4) |
high (141–349) |
| Electronegativity | moderate | moderate to high | high |
| Oxidizing power | low | low to moderate | high |
| Oxidation states | negative and positive known for all | • negative and positive known for all • negative is unstable for H |
• negative known for all • positive known for all but F, and only exceptionally for O |
| Compounds with metals |
can form alloys | mainly covalent | mainly ionic |
| Compounds with carbon |
carbides and organometallic compounds | or organic (e.g. CH4, C6H12O6) compounds | |
| Oxides | • amphoteric or weakly acidic • polymeric in structure • glass formers (B, Si, Ge, As, Sb, Te) |
• neutral to strongly acidic • molecular covalent • few glass formers (P, S, Se) • H2O can form a glass at ~136 K or −137 °C; CO2 does so at 40 GPa |
• strongly acidic • iodine oxides known in polymeric forms • no known glass formers |
| Sulfates | most form | some form | iodine forms |
| Conventional hydrogen bond formation | not known | known for S and N | known for O, F, Cl, and Br |
21. A distinction can be seen between (a) oxygen and the halogens and (b) the other nonmetals, in terms of the most important properties that characterise nonmetals, namely oxidising power and covalent v ionic bonding tendencies. This distinction is correlated with differences in ionisation energy, electron affinity, and electronegativity.
22. A comparable distinction can be made between the other nonmetals and the metalloids, consistent with increasing metallic character in proceeding back from the noble gases. In this sense, the metalloids represent the most metallic of the nonmetals. If they were any more metallic they would likely be classed as metals, rather than occupying the eastern half of the periodic table's frontier territory, the western half being occupied by post-transition metals.
23. It is pertinent to note that, just as the metalloids cluster along a diagonal path, similar diagonal relationships occur among the other nonmetals between carbon and phosphorus, and between nitrogen and sulfur; and amidst oxygen and the halogen nonmetals, between oxygen and chlorine.
24. As flagged, the divides between the three categories of nonmetals, in terms of receding metallicity, are not absolute. Boundary overlaps occur as outlying elements in each category show (or begin to show) less-distinct, hybrid-like or atypical properties.
25. They nevertheless provide a useful way of organising and structuring what is known about the nonmetals, consistent with literature-based conceptions of more active and less active nonmetals, and the generally nonmetallic chemistry of the metalloids.
Nomenclature
26. Oxygen and the halogens are hereafter referred to as "corrosive nonmetals", oxygen by its association with the ubiquitous processes of corrosion and combustion, both of which are forms of oxidation (itself a paronym of oxygen); and the halogen nonmetals by virtue of their intrinsically corrosive nature.
27. The remaining nonmetals are hereafter referred to as "intermediate nonmetals" in light of their intermediate nonmetallic character, and periodic table location between the metalloids and the corrosive nonmetals.
Conclusion
Literature-based conceptions of the nonmetals and their electro-active properties show a high degree of correlation. The most nonmetallic of the nonmetals are O, F, Cl, Br, and I. They are individually and collectively characterised by high ionisation energy, high electronegativity, high electron affinity, high oxidising power, and simple anion formation. "Though by no means all identical, their similarities sufficiently outweigh their differences" such that it is conceptually and didactically convenient to group O, F, Cl, Br, and I under the rubric of corrosive nonmetals, "as an approximate expression of all of them." (Nelson 2011, p. 55) The metalloids are characterised as the most metallic of the nonmetals. The other nonmetals are neither as metallic as the metalloids to the left nor as nonmetallic as the corrosive nonmetals to the right and, accordingly, are most appropriately conceived of as intermediate nonmetals.
References
- Ashford TA 1967, The physical sciences, 2nd ed., Holt, Reinhart and Winston, New York
- Bodner GM, Rickard LH, Spencer JN 1996, Chemistry: structure and dynamics, John Wiley & Sons, Chichester
- Briggs D 1998, Surface analysis of polymers by XPS and static SIMS, Cambridge University Press, Cambridge
- Cox PA 2004, Inorganic chemistry, 2nd ed., BIOS Scientific Publishers, London
- Frank DV, Miller S & Little JG 2004, Prentice Hall Science Explorer: Chemical Interactions, 3rd ed., Prentice Hall, Upper Saddle River, New Jersey
- Jones K 1973, "Nitrogen", in JC Bailar et al., Comprehensive inorganic chemistry, vol. 2, Pergamon Press, Oxford
- Jones BW 2010, Pluto: Sentinel of the outer Solar System, Cambridge University Press, Cambridge
- Lee GL & Van Orden HO 1965, General chemistry: Inorganic and organic, 2nd ed., Saunders, Philadelphia
- Moore JW & Stanitski CL 2015, Chemistry: The molecular science, 5th ed., Cengage Learning, Australia
- Nelson PG 2011, Introduction to inorganic chemistry: Key ideas and their experimental basis, Ventus Publishing ApS. Self-published but Nelson is a published chemist in peer reviewed journals and "often writes on matters of conceptual chemistry" Scerri (1996, p. 174).
- Parkes GD & Mellor JW 1943, Mellor's modern inorganic chemistry, Longmans, Green and Co., London
- Rayner-Canham G & Overton T 2006, Descriptive inorganic chemistry, 4th ed., WH Freeman and Company, New York
- Rudolph J 1974, Chemistry for the modern mind, Macmillan, New York: "…oxygen and the halogens in particular…are therefore strong oxidizing agents."
- Scerri ER 1996, "Stephen Brush, the Periodic Table and the nature of chemistry," Die Sprache der Chemie, P Jannich, N Psarros (eds), Könighausen & Neumann, Würzburg, pp. 169–176 (171)
- Schweitzer GK & Pesterfield LL 2010, The aqueous chemistry of the elements, Oxford University Press, Oxford
- Silberberg MS 2006, Chemistry: The molecular nature of matter and change, 4th ed., McGraw-Hill, Boston
- Synder MK 1966, Chemistry: Structure and reactions, Holt, Rinehart and Winston, New York
- Wulfsberg G 2000, Inorganic chemistry, University Science Books, Sausalito, California
- Yoder CH, Suydam FH & Snavely FA 1975, Chemistry, 2nd ed, Harcourt Brace Jovanovich, New York
Notes
- ^ For examples of this table see Atkins et al. (2006). Shriver & Atkins Inorganic Chemistry (4th ed.). Oxford: Oxford University Press • Myers et al. (2004). Holt Chemistry. Orlando: Holt, Rinehart & Winston • Chang R. (2000). Essential Chemistry (2nd ed.). Boston: McGraw-Hill
- ^ For examples of the group 3 = Sc-Y-Lu-Lr table see Rayner-Canham G. & Overton T. (2013). Descriptive Inorganic Chemistry (6th ed.). New York: W. H. Freeman and Company • Brown et al. (2009). Chemistry: The Central Science (11th ed.). Upper Saddle River, New Jersey: Pearson Education • Moore et al. (1978). Chemistry. Tokyo: McGraw-Hill Kogakusha
- ^ Notwithstanding, an IUPAC member subsequently wrote that, "IUPAC has not approved any specific form of the periodic table, and an IUPAC-approved form does not exist, though even members of IUPAC themselves have published diagrams titled “IUPAC Periodic Table of the Elements". However, the only specific recommendation IUPAC has made concerning the periodic table covers the Group numbering of 1–18."[51]
- ^ For examples of the group 3 = Ln and An table see Housecroft C. E. & Sharpe A. G. (2008). Inorganic Chemistry (3rd ed.). Harlow: Pearson Education • Halliday et al. (2005). Fundamentals of Physics (7th ed.). Hoboken, NewJersey: John Wiley & Sons • Nebergall et al. (1980). General Chemistry (6th ed.). Lexington: D. C. Heath and Company
- ^
- 1922 Bohr's system, with bifurcations at Na, Mg, and Y
- 1939 Foster LS, [https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1056 "Why not modernise textbooks also? I. The periodic table", Journal of Chemical Education, vol. 16, no. 9. In group 3 the box under Y is “La {58–70}* Lu”, where * = the rare earths.
- 1947 Stedman's planar arrangement of his conic system
- 1952 Coryell, shows a step pyramid style table, with solid and dashed tie lines to show primary and secondary relationships. Just two elements are shown by him as having two solid tie lines: yttrium, to La-Ac and to Lu-Ac; and silicon, to Ti-Zr-Hf and to Ge-Sn-Pb.
- 1964 Sanderson RT, "A rational periodic table", Journal of Chemical Education, vol. 41, no. 4, pp. 187–189
- 1974 Mazurs EG, Graphic representations of the periodic system during one hundred years, The University of Alabama Press, Alabama, p. 77
- 2020 Vernon RE, "Organising the metals and nonmetals", Foundations of Chemistry, open access, see Electronic supplementary material
- ^ Extrapolated
- ^ For nonmetals solid at room temperature
- ^ Extrapolated
Arguing for a one-move adjustment

In previous posts I've argued that the literature likes to draw a distinction between the alkali/alkaline earth metals and the halogens/noble gases.
The net effective result is a three-way division of the nonmetals into halogens, noble gases, and the other nonmetals, consistent with literature references (as noted) to stronger, inactive, and weaker nonmetals.
In reflecting this division in our periodic table we need to account for the fact that we decided, quite a while ago, that the halogens were not worth a colour category. This is because we wanted to show astatine as a metalloid, which is fair enough; it's either that or a post-transition metal.
Now, when we adopted the poly-di scheme, with best intentions, the net result was that we moved three elements i.e. H, N, and O out of what the literature regards as an unnamed "other nonmetal" category, and co-located them with F, Cl, Br and I so as to form a new diatomic nonmetal category.
Looking back, that was a "seismic" move that resulted in a major misalignment with the literature.
In contrast, the current proposals (when compared to the literature) only require a one-move adjustment i.e. moving O—which is arguably the most nonmetallic of the "other nonmetals"—out of the unnamed "other nonmetals" category, and placing it with F, Cl, Br and I, consistent with the resulting five elements collectively representing the most chemically active nonmetals.[n 1]
There is enough daylight between N and O to justify leaving N where it currently is, with the unnamed other nonmetals. On this point, although Double sharp has expressed the view that there is too much of a focus on elemental N and O when making categorisation decisions, the simple fact is that it is the properties of the elements themselves which contribute to the chemistry of their combined states.[n 2]
While N and O have high ionisation energies and electronegativities, N has no electron affinity (which is a byproduct of its half-filled p sub-shell, a similar effect being seen in P)[n 3] whereas O has quite a high electron affinity.[n 4] More specifically, Massey (2000, p. 267) says, "It is possible for the Group 15 elements to achieve a rare gas electron configuration by accepting three electrons to form M3– anions…this process is not very energetically favourable and, owing to strong inter electron repulsions, the formation of N3– requires a huge 2130 kJ mol–1…Electrons have more space on P, which lowers their mutual repulsion and results in the formation of P3– requiring only about 1450kJ."
Consequently very little of the chemistry of nitrogen is that of simple ions and nearly all its compounds are covalent,[n 5] whereas oxygen (with an electronegativity surpassed only by F) and the rest of the halogens readily form simple ions and ionic compounds.[n 6]
Now, chemical reactions yielding N2 are, as a rule, explosive but this is an outcome of nitrogen in compounds very much preferring to form a highly stable triatomic bond to itself [n 7] and does not represent a generalised nonmetallic property. The same triatomic bond is responsible for elemental nitrogen's inertness. Whereas the eagerness of oxygen and the halogens to combine with other elements generally is characteristic of "strong" nonmetals.
Agonising about the distinguishability of sulfur v iodine, or nitrogen v oxygen (or even iodine), is not consistent with the literature. According to the literature, the halogens are regarded as the epitome of non-metallic character and, in this sense, N, O and S are regarded as "other nonmetals".[n 8] We have rightly removed astatine from this epitome category thereby increasing the category's nonmetallic calibre. The proposals before us, involving a one-move adjustment of O, would further enhance the nonmetallic character of the corrosive nonmetals category, and reduce the nonmetallic character of the "other nonmetals" category, to boot.
I don't claim that the resulting schemes are perfect. Indeed, Double sharp has noted that N is capable of forming hydrogen bonds, and coordination complexes by donating its lone pairs of electrons. That is fine if you want to drill down into the detail: I further note that H-bonds formed by S (like those formed by N) are normally also considered to be be strong, despite the lower electronegativity of S[n 9]; and that C, P, and S (like N) are also capable of acting as ligands.[n 10] So what? Classification schemes are often characterised by boundary overlaps,[n 11] just as our current scheme is. I only claim that the proposed schemes are pragmatic minimalist constructs that are the closest we are going to get to the way the nonmetals are broadly conceived of in the literature (which is not at the drill-down level).
In summary it seems to me that whereas the current three-move scheme was "seismic", and a two-move scheme involving O and N would be needlessly controversial, a one-move scheme would be "minimalist".
I'll still do a sandbox come what may; this "seismic v one-move" perspective only occurred to me recently, and I wanted to post it in case it chimed with any other project members.
Notes
- ^ "The halogens and oxygen are the most active non-metals." Lee GL & Van Orden HO 1965, General chemistry: Inorganic and organic, 2nd ed., Saunders, Philadelphia, p. 197
- ^ Jones K 1973, "Nitrogen", in JC Bailar et al., Comprehensive inorganic chemistry, vol. 2, Pergamon Press, Oxford, p. 159
- ^ Siekierski SC & Burgess J 2002, Concise chemistry of the elements, Horwood Publishing, Oxford, p. 36: "The electron affinity of N and P, which both have a half-filled p subshell, is low. A low electron affinity is also observed for Mn and Re, which have half-filled d subshells. The reason is that the electron in excess of a half-filled p or d subshell must have its spin opposed to the other three or five respectively. This additionally increases interlectronic repulsion and decreases affinity."
- ^ Thus, the electronegativity of N has been described as "misleadingly high". Phillips CSG & Williams RJP 1965, Inorganic chemistry, vol. 1, Principles and non-metals, Clarendon Press, Oxford, p. 609
- ^ Housecroft CE & Sharpe AG 2008, Inorganic chemistry, 3rd ed., Prentice Hall, Harlow, p. 433
- ^ "Most metal oxides and halides are ionic solids." Brown TL, LeMay HE & Bursten BE et al. 2013, Chemistry: The central science, 3rd ed., Pearson Australia, Sydney, p. 240
- ^ Siekierski SC & Burgess J 2002, Concise chemistry of the elements, Horwood Publishing, Oxford, pp. 107–108
- ^ Relegating O to the status of an other nonmetal is an outcome of the broad focus on the halogens. Elsewhere, as noted, the literature notes that oxygen and halogens are the most active/reactive of the nonmetals.
- ^ Gilli G & Gilli P 2009, The nature of the hydrogen bond: Outline of a comprehensive hydrogen bond theory, Oxford University Press, Oxford, pp. 29, 31
- ^ Greenwood NN & Earnshaw A 1998, Chemistry of the elements, 2nd ed., Butterworth-Heinemann, Oxford, pp. 485, 924, 665
- ^ Jones BW 2010, Pluto: Sentinel of the outer Solar System, Cambridge University Press, Cambridge, pp. 170–171): "Though classification is an essential feature of all branches of science, there are always hard cases at the boundaries. The boundary of a class is rarely sharp…Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics."
Nitrogen article
Nitrogen may be usefully compared to its horizontal neighbours carbon and oxygen as well as its vertical neighbours in the pnictogen column (phosphorus, arsenic, antimony, and bismuth).
Like carbon, nitrogen is limited to a maximum covalency of four. In its compounds, particularly in biochemistry, it is capable of forming hybrid orbitals analogous to those seen in carbon. Examples include ammonia and amines (sp3 tetrahedral); histidine, purines, and pyrimidines (sp2 planar); and nitrogen gas and cyanide (sp linear). (Beckman 2000, pp. 18–19)
Its proclivity for catenation is less than that of carbon but more than that of oxygen. The longest chain of nitrogen yet synthesised has eleven atoms (C is unlimited; O has an effective limit of three).
Nitrogen resembles oxygen in its capacity to form hydrogen bonds and coordination complexes by donating its lone pair of electrons. Its high electronegativity, while comparable to that of oxygen, has been described as "misleadingly high" (Phillips & Williams 1965, p. 609) on account of nitrogen's negative electron affinity.
One property nitrogen shares with its horizontal neighbours is preferentially forming multiple bonds, typically with carbon, nitrogen, or oxygen atoms, through pπ – pπ interactions; thus, for example, nitrogen occurs as a diatomic molecule and thus has very much lower melting (−210 °C) and boiling points (−196 °C) than the rest of its group, as the N2 molecules are only held together by weak van der Waals interactions and there are very few electrons available to create significant instantaneous dipoles. This is not possible for its vertical neighbours; thus, the nitrogen oxides, nitrites, nitrates, nitro-, nitroso-, azo-, and diazo-compounds, azides, cyanates, thiocyanates, and imino-derivatives find no echo with phosphorus, arsenic, antimony, or bismuth. By the same token, however, the complexity of the phosphorus oxoacids finds no echo with nitrogen (Greenwood p. 412).
Nitrogen has a less-well known diagonal relationship with sulfur, manifested in like charge densities and electronegativities (the latter are identical if only the p electrons are counted; see Hinze and Jaffe 1962) especially when S is bonded to an electron-withdrawing group. They are able to form an extensive series of seemingly interchangeable sulfur nitrides, the most famous of which, polymeric sulfur nitride, is metallic, and a superconductor below 0.26 K. The aromatic nature of the S3N22+ ion, in particular, serves as an exemplar of the similarity of electronic energies between the two nonmetals (Rayner-Canham 2011, p. 126).
Quotes from the literature
Are here.
Classifying the elements (A)
Symmetry in the periodic table
The progression from metallic to nonmetallic character in traversing the periodic table shows a pleasing symmetry
| Electroactive metals Groups 1–3, Ln, An |
Corrosive nonmetals O, F, Cl, Br, I | ||||
| Transition metals (mundane) Most of 'em |
Intermediate nonmetals H, C, N, P, S, Se | ||||
| Poor metals Ga, Bi etc |
Weak nonmetals (metalloids) B, Si, Ge, As, Sb, Te | ||||
| Noble metals Ru, Rh, Pd, Ag, Os, Ir, Pt, Au |
Noble gases He, Ne, Ar, Kr, Xe, Rn |
Notes
| 1. | Noble metals are a subgroup of the transition metals |
| 2. | Corrosive nonmetals are corrosive, and highly electronegative (> 2.6), and are, or their species are, capable of acting as relatively strong oxidising agents |
| 3. | The more moderate nature of the intermediate nonmetals is relatively self-evident, situated as they are between the corrosive nonmetals and the weak nonmetals. The magic thread that binds the intermediate nonmetals goes H → C → P → N → S → Se:
|
| 4. | Some authors count metalloids as nonmetals with weakly nonmetallic properties and that is the approach I have taken here. |
| 5. | This classification scheme, as a bonus, appears to better reflect physical changes in metallic character better than is the case with scheme (B), below. |
| 6. | I find the combination of symmetry (as in the complimentary categories) and asymmetry (many metals/few nonmetals); and the thread that links the nonmetals in the intermediate category, to be pleasing. |
| 7. | Arguably, there may be some discontinuities and boundary overlaps. "Though classification is an essential feature of all branches of science, there are always hard cases at the boundaries. The boundary of a class is rarely sharp…Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics." (Jones 2010, pp. 170–171) |
Thoughts
The reactive metals and mundane transition metals have distinctive chemistries. I'm reasonably sure this is the case for the poor metals, too. Do the noble metals have a distinctive chemistry, in addition to being transition metals? I don't know.
The corrosive nonmetals have distinctive chemistries, as do the weak nonmetals and the noble gases. I suspect the intermediate nonmetals do too, certainly in the way they combine with metals to yield the different kinds of hydrides, carbides, nitrides, phosphides; and I think sulfides and selenides may behave the same way---not that sure about the last two. Would like to check oxygen, to see to what degree it forms ionic, metallic, covalent, or interstitial oxides with metals, or are metal oxides mainly ionic? Never had to consider this before.
The literature
- Noble metals. It seems they do have a signature chemistry: Noble Metals, Analytical Chemistry of . Or try this 624-page whopper from 1966. Or Noble Metals and Biological Systems.
- Intermediate nonmetals. The hydrides, carbides, nitrides, phosphides, sulfides and selenides of the metals are all either ionic/saline, metallic/interstitial, or covalent.
- Phosphorous and nitrogen. Cotton and Wilkinson say that P, like N, is essentially covalent in all of its chemistry. So much for the high electronegativity of N.
- Oxygen as a corrosive nonmetal. "…oxygen is a potent, reactive, and corrosive gas…liable to generate free radicals, highly reactive bleachlike compounds that burn up organic molecules. We are able to tolerate an oxygen-rich environment only because our cells possess complex biochemical mechanisms for suppressing its many harmful influences." (Ball 2013, p. 232) ✦ "In the United States alone, more than $10 billion is lost each year to corrosion…Much of this corrosion is the rusting of iron and steel…The oxidizing agent causing all of this corrosion is usually oxygen." (Joesten, Hogg & Castellion 2007, p. 217). ✦ "Oxygen is super dangerous; it’s a corrosive gas…".
- Oxygen and fluorine. "Fluorine tends to bring out the highest valence of the element with which it combines. In this its shows a strong resemblance to oxygen. In combination with metals, oxygen appears to be the best for the highest valences, e.g. OsO4 and KMnO4, but fluorine appears best if the highest valence is relatively low, e.g. for CoF3, CuF3, AgF2, TbF4, and BrF5. With non-metals the difference between oxygen and fluorine is less apparent." (Phillips & Williams, p. 446)
- Oxygen and chlorine. "Chlorination reactions have many similarities to oxidation reactions. They tend not to be limited to thermodynamic equilibrium and often go to complete chlorination. The reactions are often highly exothermic. Chlorine, like oxygen, forms flammable mixtures with organic compounds." (Kent 2010, p. 104).
- Metal oxides. Most are are ionic and contain the O2– ion. Cotton & Wilkinson say that (a) there are great differences between the chemistry of sulfur and oxygen; and (b) some oxides with transition metals in very low oxidation states are metallic e.g. NbO. Wiberg, however, says NbO is ionic—go figure.
- Metal oxide types. Phillips and Williams (1965, p. 478) have a nice table classifying the oxides. So, yes metal oxides are often ionic. The only ones shown as covalent are MO3 for groups 6 and 7; M2O7 for group 7; and MO4 for group 8. The following oxides are shown as being often metallic: MO in groups 4–6 and 8; M2O2 in group 1; M2O3 in group 4; and MO2 in groups 1 and 2.
- Metal halides 1. Greenwood & Earnshaw (2nd ed., p. 823) say that, "The majority of pre-transition metals (Groups 1, 2) together with group 3, the lanthanides and the actinides in the +2 and +3 oxidation states [the reactive metals!] form halides that are predominately ionic in character, whereas the non-metals and metals in high oxidation states (≥ +3) tend to form covalent molecular halides." They go on to note the tendency of the refractory transition metals to form cluster halides.
- Metal halides 2. "Most metal halides are substances of predominately ionic character, although partial covalence is important in some." (Cotton & Wilkison, p. 554)
- Iodides. Eagleson says the group 1 and 2 iodides are ionic and water-soluble while a few heavy-metal iodides are insoluble—AgI, CuI, HgI2 and TlI.
- Halide types. Phillips and Williams (p. 432) say that the electronegativity values of the halogens are among the highest in the periodic table so that many of their compounds are expected to contain negatively charged halogen ions. Despite the substantial differences between the individual halogens, their compounds are sufficiently similar to permit a collective classification (p. 433). ✦ "The elements in the middle of the perioidic table form hydrides which are mostly metal-like or interstitial, although rather unstable 'covalent' hydrides can also be formed by some of the metals. There are no directly equivalent compounds among the halides. The nearest parallels occur in the low-valence iodides such as TiI2 and NbI4, which are grossly defect, and semiconductors or metals…In general, however, the halogen atoms are themselves too electronegative to enter into an alloy-like structure, and they are too large—particularly when carrying some negative charge—to be accommodated in the interstices of the metal structure. Instead, there is a large class of semi-ionic halides, often consisting of layer, or less commonly chain lattices. Such structures will require some appreciable binding between the halogen atoms, and are noticeably absent among the fluorides in contrast to the chlorides, bromides, and iodides. Within the layers the pattern retains something of the ionic type lattice, but the layers are held together by van der Waal's forces between adjacent halogen atoms. A characteristic feature of such lattices is the unsymmetric environment of the halogen atom as compared to its environment in the ionic crystal. In one direction it resembles an ionic while in another a simple molecular crystal. Such structures are therefore intermediate between those which occur with halides of the elements at the two ends of the Periodic Table." (pp. 434–435). ✦ The periodate/iodate couple is the most oxidising among the halogen oxyacids (p. 459).
- Halide structures. Wells (pp. 408–409) says many fluorides and oxides of similar formula-type are isostructural, while chlorides, bromides and iodides often have the same types of structure as sulfides, selenides and tellurides. The great majority of halides MX, MX2, and MX3 adopt 3D complex structures, and most monohalides and most fluorides MF2 and MF3 crystallise with highly symmetrical structures suited to essentially ionic crystals. Golden-yellow ThI2 exhibits metallic conduction (p. 415).
- Halogens. "With the exception of the Li–Cs group there are closer similarities within the group than in any other in the Periodic Table." (Cotton & Wilkison, p. 547)
- Periodic table of diatomic molecules" In 1979 Ray Hefferlin published a periodic ordering of all of the diatomic molecules that could result from combinations of the first 118 elements of the periodic table". I have to get me one of these.
Classifying the elements (B)
He, Ne, Ar, Kr, Xe, Rn |
|||||
| Reactive metals Groups 1–3, Ln, An |
Corrosive nonmetals O, F, Cl, Br, I | ||||
| Transition metals Most of 'em |
Liminal nonmetals N, S, Se | ||||
| Poor metals Ga, Bi etc |
Weak nonmetals H, C, P | ||||
B, Si, Ge, As, Sb, Te |
|||||
Electrochemical strength
Metals
| Reactive metals Strong to moderate (mostly strong) |
Corrosive nonmetals Strong |
| Transition metals Strong to noble (mostly intermediate) |
Liminal nonmetals Moderate |
| Poor metals Moderate to weak (mostly weak) |
Weak nonmetals Weak |
The reactive metals (see tables 1 and 2) undoubtedly start at "strong", and are undoubtedly mostly strong. (Aluminium is included here).
| Cohort | Highest | M | Lowest | M |
|---|---|---|---|---|
| Reactive | –3.04 | Li | –0.20 | Am |
| Transition | –1.7 | Hf | 1.52 | Au |
| Poor | –0.76 | Zn | 0.98 | At |
The start of the transition metals is a bit blurry. If they are taken to include group 3 then they certainly start at strong. If not then even some of the group 4 metals have relatively high average reduction potentials: Hf –1.7 and Zr –1.55; and Ti is "only" –0.87. The eight noble metals are undoubtedly weak. So, strong to weak sounds OK. If group 3 and 12 are excluded, that leaves 24 metals. Eight of these are noble ("very weak"). Which ones are strong is a bit blurry, but say Hf –1.7 and Zr –1.55 (the next most "reactive" is Nb at –0.93, so that looks like a natural break). That leaves 14 moderate to weak metals. So the range is strong to noble, with most being moderate to weak. If Sc (–2.03) and Y (–2.37) in group 3 are included as transition metals, that changes the count to 4 strong, 14 moderate to weak, and 8 noble. If group 12 are also included as transition metals, that changes it to 4 strong, 17 moderate to weak, and 8 noble.
Among the poor metals, Zn (–0.76) and Ga (–0.53) have reduction potentials that are reasonably electropositive, but these metals are regarded by Rayner-Canham and Overton (2006, pp. 29–30) as chemically weak. Cadmium (–0.44) and indium (–0.34) are next but they are not, as far I know, generally regarded as chemically weak metals, although Steele (1966, pp. 67–68) says that "in their reactions the metals show weak electropositive character." The rest of the poor metals have positive reduction potentials, so they can be described as weak. The approximate count is two moderate and nine weak. If the group 12 metals are counted as transition metals the count would be one moderate and seven weak. So the poor metals are mostly weak.
If aluminium is counted as a poor metal how can it be accommodated as such given its respectable –1.65 electrode potential? Stott (1956, p. 100) says that a lot of the chemistry of aluminium suggests it is a comparatively weak metal and that in many ways it is weaker than transition metals such as iron, nickel, cobalt and manganese, all of which have lower electrode potentials. Steele (1966, p. 60) notes the paradoxical chemical behaviour of aluminium: "It resembles a weak metal in its amphoteric oxide and in the covalent character of many of its compounds ... Yet it is a highly electropositive metal ... [with] a high negative electrode potential". Kneen, Rogers and Simpson (1972, p. 363) say that aluminium "is only moderately electropositive…". Moody (1991, p. 300) says that, "aluminium is on the "diagonal borderland" between metals and non-metals in the chemical sense." Rayner-Canham and Overton (2006, pp. 29–30) count it as a chemically weak metal.
Categorising the metals is based on more than just their electrode potentials. So I think the categorisation of aluminium is neither here nor there (it's either moderate or weak).
Nonmetals
| Strong |
|
| Moderate |
|
| Weak |
|
Average standard reduction potentials provide a reasonable demarcation (see table 3). Although nitrogen has a high electronegativity it is a poor oxidising agent. Only when it is in a positive oxidation state (i.e. in combination with oxygen or fluorine) are its compounds good oxidising agents but even then their reactivity is often limited by kinetic factors (Cox 2004, p. 161).
Table 4 lists a similar nonmetal demarcation based on bonding strengths with F, O and Cl (Synder 1966, p. 242).
| Bond strength | |
|---|---|
| High | O, F, Cl, Br, I |
| Moderate | C, N, S, Se |
| Weak | H, P |
Table 5 is an electrochemical series given by Nelson (2011, pp. 55, 57). He wisely writes, "In using the series, care needs to be taken to remember that it is only an approximation, and can only be used as a rough guide to the properties of the elements. Provided that this is done, however, it constitutes a very useful classification, and although purists often despise it because of its approximate nature, the fact is that practising chemists make a great deal of use of it, if only subconsciously, in thinking of the chemistry of different elements."
| Strongly electronegative | O, F |
| Moderately electronegative | Br, Cl |
| Weakly electronegative | C, N, S, I |
| Approximately electro-neutral | H, P |
Reactivity
| Nonmetal | Electron affinity kJ/mol |
Electro- negativity |
Enthalpy of dis- sociation kJ/mol |
Average electrode potential V |
Caustic? | Corrosive? | Pyro- phoric? |
HSAB | Direct noble gas com- pounds? |
Reactivity boxes |
|---|---|---|---|---|---|---|---|---|---|---|
| F | 334 | 3.98 | 159 | 2.87 | 1 | 1 | 0 | H | Y | 8 |
| Cl | 355 | 3.16 | 242 | 1.37 | 1 | 1 | 0 | H/B | P | 7.25 |
| Br | 331 | 2.96 | 193 | 1.46 | 1 | 1 | 0 | B/S | N | 6.25 |
| O | 147 | 3.44 | 498 | 1.65 | 0 | 1 | 0 | H | N | 4 |
| I | 301 | 2.66 | 151 | 1.1 | 1 | 1 | 0 | S | N | 5 |
| S | 207 | 2.58 | 266 | 0.86 | 0 | 0 | 0 | S | N | 3 |
| Se | 201 | 2.55 | 332 | 0.59 | 0 | 0 | 0 | S | N | 2 |
| P | 78 | 2.19 | 198 | -0.28 | 0 | 0 | 0 | S | N | 1 |
| N | 0 | 3.04 | 945 | 0.76 | 0 | 0 | 0 | B | N | 1.5 |
| H | 79 | 2.20 | 436 | -1.12 | 0 | 0 | 0 | H | N | 1 |
| C | 128 | 2.55 | 346 | 0.17 | 0 | 0 | 0 | S | N | 0 |
| Average | 196 | 2.85 | 342 | 0.85 |
Notes: (a) Caustic = destructive of organic tissue; (b) If a particular property is quantitative, the last row gives the average value of the listed nonmetals.
Yellow shading denotes nonmetals with an above average value for that property.
Aqua shading denotes an intermediate value. If a property is binary (e.g. Caustic?) then the distinction between above average and below average is self-explanatory. For the HSAB rating I've assigned a value of 1 to 'hard' (H); a value of 0.5 to borderline (B); and a value of 0 to 'soft' (S). If a nonmetal is sometimes listed as more than one HSAB category, I've assigned it the average of the applicable values. In the Forms noble gas compounds? column, Cl has a value of P (for possibly) since that's the way I interpreted the literature on this question.
Light grey shading denotes a below average value. This works the other way around in the case of enthalpy of dissociation.
Gold shading, as seen only in the last column, denotes an intermediate (electromoderate) nonmetal.
The last column shows how many "above average" property boxes a particular nonmetal has checked. Since nine is the greatest number of property boxes that can be checked it follows that > 4.5 boxes is above average and < 4.5 is below average. The Pyrophoric? column is a holdover from when white P was counted here. If we are comparing apples with apples i.e. the most thermodynamically stable forms of the elements with one another, it probably needs to go.
Enthalpy of dissociation (or element bond strength) is associated with reactivity: "The high dissociation enthalpy of the O2 molecule, 498 kJ/mol, is the reason that molecular O. is relatively unreactive and its reactions usually require thermal or photochemical activation." (Eagleson 1994, p. 768) The figure for P is for white P, as far as I know; the figure for black P is likely to be quite a bit higher which means its figure of merit will go down to 0. OTOH, this site says that figure for black P is only 43 kJ/mol higher, which means it wouldn't.
On the basis of the above table, nonmetals of above average or high reactivity are F, Cl, Br, O, and I, an outcome that is consistent with the literature.
References
- Ball P 2001, Life's matrix: A biography of water, University of California Press, Berkeley
- Cotton FA, Wilkinson G, Murillo CA & Bochmaan M 1999, Advance inorganic chemistry, 6th ed., John Wiley & Sons, New York
- Cox PA 2004, Inorganic chemistry, 2nd ed., BIOS Scientific Publishers, London: "Nitrogen is a moderately electronegative element…"
- Cronyn MW 2003, "The proper place for hydrogen in the periodic table", Journal of Chemical Education, vol. 80, no. 8, pp. 947–950, doi:10.1021/ed080p947
- Eagleson M 1994, Concise encyclopedia chemistry, Walter de Gruyter, Berlin
- Greenwood NN & Earnshaw A 1998, Chemistry of the elements, 2nd ed., Butterworth-Heinemann, Oxford
- Hinze J & Jaffe HH 1962, "Electronegativity. I. Orbital electronegativity of neutral atoms", Journal of the American Chemical Society, vol. 84, no. 4, pp. 540–546, doi:10.1021/ja00863a008
- Joesten MD, Hogg L, Castellion ME 2007, The world of chemistry: Chemistry essentials, 4th ed., Thomson Brooks/Cole, Belmont, California
- Jones BW 2010, Pluto: Sentinel of the outer Solar System, Cambridge University Press, Cambridge
- Kent JA 2007, Kent and Riegel's Handbook of industrial chemistry and biotechnology, 11th ed., vol. 1, Spring Science + Business Media, New York
- Malati MA 1999, Experimental inorganic/physical Chemistry: An investigative, integrated approach to practical project work, Woodhead Publishing, Oxford
- Moody B 1991, Comparative inorganic chemistry, 3rd ed., Edward Arnold, London
- Nelson PG 2011, Introduction to inorganic chemistry: Key ideas and their experimental basis, Ventus Publishing ApS
- Phillips CSG & Williams RJP 1965, Inorganic chemistry, vol. 1, Principles and non-metals, Clarendon Press, Oxford
- Rayner-Canham G & Overton T 2006, Descriptive inorganic chemistry, 4th ed., WH Freeman and Company, New York
- Kneen WR, Rogers MJW & Simpson P 1972, Chemistry: Facts, patterns and principles, Addison-Wesley Publishers, London
- Scerri E 2007, The Periodic Table: Its story and its significance, Oxford University Press, Oxford
- Schweitzer GK & Pesterfield LL 2010, The aqueous chemistry of the elements, Oxford University Press, Oxford, pp. 228–229; 232–233
- Steele D 1966, The chemistry of the metallic elements, Pergamon Press, Oxford
- Stott RW 1956, A companion to physical and inorganic chemistry, Longmans, Green and Co., London
- Synder MK 1966, Chemistry: Structure and reactions, Holt, Rinehart and Winston, New York
- Wells AF 1984, Structural inorganic chemistry, 5th ed., Clarendon Press, Oxford
- Wiberg N 2001, Inorganic chemistry, Academic Press, Berlin
- Wisian-Neilson P, Alcock HR, Wynne KJ (eds) 1994, Inorganic and organometallic polymers II: advanced materials and intermediates, American Chemical Society, Washington DC
- Wulfsberg G 2000, Inorganic chemistry, University Science Books, Sausalito, California, pp. 247–249; 273–276
Blocks to blocs
| Order | ||||
| 0th | Main group elements (typically colourless salts) |
Transition elements (typically coloured salts) | ||
| 1st | s block | p block | d block | f block |
| 2nd | s(d) bloc | p(s) bloc | d(s) bloc | f(dp) bloc |
| 3rd | H, Be | Al | Group 11 | -- solid state configurations of Ln and An -- f-orbital involvement seen in early An |
Metals
Basic metals
aka post-transition metals
Incipient metals
Magnetic liquid metals
- www.cnet.com/news/scientists-create-liquid-metal-that-stretches-like-terminator/
- https://www.eurekalert.org/pub_releases/2019-03/acs-lm032019.php
- https://pubs.acs.org/doi/abs/10.1021/acsami.8b22699
Molecular metals
Differentiating electron scale
An optimal block is a periodic table block in which the proportion of elements that have a predominance of either s-, p-, d-, and f- differentiating electrons, as applicable, is maximised.
The idea of an optimal block has its roots in the work of Scerri and Parsons (2018, p. 151):
“…for the purposes of selecting an optimal periodic table we prefer to consider block membership as a global property in which we focus on the predominate differentiating electron. We readily acknowledge the fact that the atoms of Mn, Zn, Tc, Cd, Pt [?], Hg, Lr, La, Gd, Ac, Th, and Cm are all anomalous in that they have a differentiating electron that is atypical of the block that they are situated in. These anomalies should not challenge our attempts to establish the overall structure of the periodic table in terms of sequences of blocks in the periodic table and as a result our recommendations for the membership of group 3 of the periodic table.”
DIFFERENTIATING ELECTRON SCALE OF PERIODIC TABLES Table # Notes =========================================================================== 0. Madelung rule (1928) 0 Idealised form --------------------------------------------------------------------------- 11. La-Ac w/HeBe 11 Physics-based optimal block solution --------------------------------------------------------------------------- 12a. LSPT 12 Elegant 32-column version showing theoretical tetrahedral symmetry --------------------------------------------------------------------------- 12b. Lu-Lr w/HeBe 12 18-column version of LSPT --------------------------------------------------------------------------- 12c. La-Ac w/HeNe 12 Recommended for IUPAC as it places He over Ne --------------------------------------------------------------------------- 13a. Lu-Lr w/HeNe 13 A compromise (?) between 12c. and 14a --------------------------------------------------------------------------- 13b. Volumetric (1949) 13 La-Ac, He-Ne, and groups 11–12 as s-block members, here --------------------------------------------------------------------------- 14a. IUPAC, current 14 Further along the chemistry end of the scale, here --------------------------------------------------------------------------- 14b. Metallurgist's (1994) 14 La-Ac w/HeNe, HF, and AlSc, here --------------------------------------------------------------------------- 15. Remy’s (1956) 15 La-Ac w/H-F, Th-Pa-U as d-block elements, and Np+ as transuranic elements, analogous to Pm+, here --------------------------------------------------------------------------- 17. Rayner-Canham (2002) 17 aka the Inorganic Chemist's Periodic Table, here --------------------------------------------------------------------------- 21. Pauling (1980) 21 La-Ac w/HeNe; Sc-La as s-block;* Th–Pu as d-block and as f-block; Ku (104) as f-block # = differentiating electron discrepancies * Pauling's table is ambiguous but in the text he treats Sc, Y, and La as the congeners of B and Al
References
- Atkins P 2004, Galileo's finger: The ten great ideas of science, e-book ed., Oxford University, Oxford
- Pauling L 1980, General chemistry, 3rd ed., Dover Publications, New York, p. 135
- Rossotti H 1998, Diverse atoms: Profiles of the chemical elements, Oxford University, Oxford
- Scerri E 2004, "The best representation of the periodic system: The role of the n + l rule and of the concept of an element as a basic substance", in DH Rouvray and RB King, The periodic table: Into the 21st century, Research Studies Press, Baldock, England
- Scerri ER & Parsons W 2018, "What elements belong in group 3 of the periodic table?", in ER Scerri & G Restrepo (eds), Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table, Oxford University Press, New York, [1], pre-publication draft here
- Schwarz WHE & Rich RL 2010, "Theoretical basis and correct explanation of the periodic system: Review and update", Journal of Chemical Education, vol. 87, no. 4, pp 435–443
- Stewart PJ 2018, "Tetrahedral and spherical representations of the periodic system", Foundations of Chemistry, vol. 20, no. 2, pp. 111–120
LST
I’ve been looking at the LST for quite a while and...I don’t get why some folks prefer it:
| f1 | f2 | f3 | f4 | f5 | f6 | f7 | f8 | f9 | f10 | f11 | f12 | f13 | f14 | d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | d9 | d10 | p1 | p2 | p3 | p4 | p5 | p6 | s1 | s2 | |||
| 1s | H | He | ||||||||||||||||||||||||||||||||
| 2s | Li | Be | ||||||||||||||||||||||||||||||||
| 2p 3s | B | C | N | O | F | Ne | Na | Mg | ||||||||||||||||||||||||||
| 3p 4s | Al | Si | P | S | Cl | Ar | K | Ca | ||||||||||||||||||||||||||
| 3d 4p 5s | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | Rb | Sr | ||||||||||||||||
| 4d 5p 6s | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | Cs | Ba | ||||||||||||||||
| 4f 5d 6p 7s | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | Fr | Ra | ||
| 5f 6d 7p 8s | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | Uue | Ubn | ||
| f-block | d-block | p-block | s-block | |||||||||||||||||||||||||||||||
It has the following features: (a) it is more regular than the conventional table; (b) it has no gaps; (c) each element in every second row of each block starts a triad; (d) each element in each period has an n+l value matching the period number i.e. it matches the diagonal lines of the Madelung rule diagram (MRD); and (e) the first row anomaly becomes clearer i.e. s >> p > d > f.
(a) means the period lengths go 2, 2, 8, 8, 18, 18, 32, 32 whereas the conventional form goes 2, 8, 8, 18, 18, 32, 32;
(b) means e.g. there is no gap between Be and B;
(c) means e.g. that Tc, Re, and Bh form a triad;
(d) means e.g. that the n+l value of Ti, in period 5, is 3 + 2 = 5. Here, n is the principal quantum number (same as the orbital value of 3, in 3d); and l is the azimuthal quantum number, where s = 0; p = 1; d = 2; f =3). Sr, in the same period, is 5s = 5 + 0 = 5; and
(e) is nice.
Feature (d) doesn’t mean much since, like the MRD, the quantum numbers involved are based on idealised rather than actual differentiating electrons. For example, Zn is 3d = 3 + 2 = 5. Whereas in real life the differentiating electron in Zn = 4s = 4 + 0 = 4. In fact the MR is wrong in 20 places—it’s an approximation or idealisation, not a law carved in stone.
While the strongest objection to the LST is He over Be, that’s not so much of an issue. The electronegativity and ionisation energy of He can be extrapolated from the rest of the alkaline earth metals. The 1s2 electron configuration matches that of the rest of group 2. We know that He is capable of acting as a metal. One just needs to colour code it as a noble gas, on account of its closed shell.

Proponents of the LST say its regularity and elegance, as well as its symmetry (the four blocks fit naturally into a tetrahedron or, the LST can be rearranged into the ADOMAH form) mean that it is an optimal form of periodic table.
I say that:
- these features are derived from an idealisation rather than actual physical parameters i.e. idealised v actual quantum numbers;
- as such they mean nothing in terms of an optimal periodic table;
- while the electron configuration filling sequence has some irregularities one can still see a general regularity that accords with the MR diagram;
- the general regularity hints at a hidden symmetry in the applicable laws of nature; and
- this hidden (or broken) symmetry manifests as the asymmetric conventional form.
Here’s a relevant quote:
- While the laws of Nature, “are simple, symmetrical, and elegant, the real world isn’t. It’s messy and complicated…The reason is clear. We do not observe the laws of Nature: we observe their outcomes. Since these laws find their most efficient representation as mathematical equations, we might say that we see only the solutions of those equations not the equations themselves. This is the secret which reconciles the complexity observed in Nature with the advertised simplicity of her laws. Outcomes are much more complicated than laws; solutions much more subtle than equations. For, although a law of Nature might possess a certain symmetry, this does not mean that all the outcomes of the law need manifest that same symmetry.” (Barrow 2008)
It is as if the periodic table lies at the bottom of a wine bottle; the symmetry of the bottle’s base is clear from the top of the dimple in the centre, but it is hidden from any point in the valley surrounding the central dimple.* Assigning >special< significance to the LST (or the ADOMAH variation) on the basis of its elegance, regularity, symmetry, or coherence with the MRD, while well intentioned, is misguided and needlessly detracts from the clarity of the chemical relationships among the elements and their compounds.
- ∗ I borrowed this analogy from the EB article on Subatomic particles, Hidden symmetry section, by Sutton C, 2017

Possibly the next biggest objection to the LST is that it makes a train wreck of the left to right metal to nonmetal progression, and associated trends.
As Ball (2010) said:
- “I think it is a poor deal to trade subjective aesthetics (which clearly not everyone shares) for the long-standing traditional navigational axes that chemists use around the PT.”
Weighing up the features and drawbacks of the LST, I don’t understand how they can be reconciled.
The conventional form of the periodic table is about as good as any other; hence it is retained for want of a better alternative.
Can anyone shed light on the merits of the left-step table, such that it becomes preferred to the conventional form?
References
- Ball P 2010, comments to “The disappearing spoon” review, homunculus blog, viewed 19 May 2019, https://www.blogger.com/comment.g?blogID=26741618&postID=2980643491692822828&bpli=1&pli=1
- Barrow JD 2008, New theories of everything, Oxford University Press, Oxford, pp. 136–140
Sandbh (talk) 17:58, 14 June 2019 (UTC)
See also
Nonmetals 2019
Could we look at this again:
1. Their results likely draw on one of the biggest surveys of the literature ever attempted, for this kind of research.
2. The article says their database of [only] 3.3 million abstracts (spanning 1922 to 2018), drawing from 1,000 journals, was focussed on materials science, physics, and chemistry.
3. Key words were extracted using ChemDataExtractor ("Give it a journal article, and it will extract chemical names, properties, and spectra from the text so they can be imported into a database or spreadsheet.").
4. I agree there are a few anomalies like Fr for the very plausible reason you give. Is this not a case of "perfect is the enemy of good"?
5. Their results match with the J.Chem.Ed. Kohonen map cited in our own archive 15.
We've never had a comprehensive literature-based view of nonmetal categories, which is why we've always struggled doing a fair job with these. Now we have, what is arguably, the biggest perspective ever on the nonmetals, let alone the remarkable correlation with the rest of the periodic table.
If we confine our perspective on the periodic table to purely chemistry-based considerations (which we never have, as far as I know) we'll being doing ourselves a disservice to our readers, not to mention the periodic table.
A recent editorial in Nature Materials opined that
"There has been a synergy between our evolving understanding of the periodic table and our understanding of materials. Element position within the periodic table and sustainability considerations plays a role in determining research activity and practical applications. The space in the periodic table for new materials combinations is vast. All elements will continue to be worthy of investigation."
The key words here are a "synergy" or a working together, as guided by the periodic table, and our "evolving" understanding of the latter.
So, yes, I'm calling for a rethink of our nonmetal categories.
Metalloids in alloys (notes)

Context
Writing early in the history of intermetallic compounds, the British metallurgist Cecil Desch observed that "certain non-metallic elements are capable of forming compounds of distinctly metallic character with metals, and these elements may therefore enter into the composition of alloys". He associated silicon, arsenic, and tellurium, in particular, with the alloy-forming elements.[56]
"The metals form definite chemical compounds not only with the dissimilar non-metallic elements but also with the metalloids, and in many cases with each other. The compounds of the metals with each other, called "intermetallic compounds," do not, however show the marked evidences of chemical combination which are found in the compounds of the metals with the electronegative elements like oxygen and the halogens".
- Jeffries Z & Archer RS 1923, The science of metals, McGraw-Hill, New York, p. 225
Phillips and Williams[57] suggested that compounds of (phosphorus), silicon, germanium, arsenic, and antimony with B metals, "are probably best classed as alloys".
Boron
"The most important alloys of boron (ferroboron, calcium boron) are used in the processing of steel, cast iron, iron alloys, copper and copper alloys as deoxidants."
- Erdey R et al. 1965, Gravimetric analysis: International series of monographs in…, p. 204
"A superhard substance that is more slippery than Teflon could protect mechanical parts from wear and tear, and boost energy efficiency by reducing friction. The “ceramic alloy” is created by combining a metal alloy of boron, aluminium and magnesium (AlMgB14) with titanium boride (TiB2). It is the hardest material after diamond and cubic boron nitride. BAM, as the material is called, was discovered at the US Department of Energy Ames Laboratory in Iowa in 1999, during attempts to develop a substance to generate electricity when heated.
"Interestingly, transition metal borides are metallic compounds, and they are often more conductive than the parent metal."
- Carenco S et al. 2013, "Nanoscaled metal borides and phosphides: Recent developments and perspectives", Chemical Reviews, vol. 113, no. 10, pp. 7981–8065 (7992)
With lithium, boron is known to form a metallic, malleable, extrudable, and machinable compound alloy having the composition Li5B4, and a silvery metallic lustre.
- Wang FE 2005, Bonding theory for metals and alloys, Elsevier, Amsterdam, p. 192
Silicon
"This book details aluminum alloys with special focus on the aluminum silicon (Al‐Si) systems – that are the most abundant alloys second only to steel."
Phosphorus
"Most of the metal phosphides are semiconductors or insulators, because their electrons are localized in the vicinity of phosphorus atoms; however, some of them (usually the most metal-rich ones) exhibit a metallic character."
- Carenco S et al. 2013, "Nanoscaled metal borides and phosphides: Recent developments and perspectives", Chemical Reviews, vol. 113, no. 10, pp. 7981–8065
Germanium
"Germanium forms alloys with many metals. Harner (1961) described a partial list of 21 such metals, including all the base metals and most precious metals. About one-half of these germanium-metal systems contain intermetallic compounds. Alloys, however, are minor uses for germanium."
- Butterman WC & Jorgenson JD 2005, Mineral commodity profiles germanium, US Geological Survey, Reston, Virginia
"Although relatively little germanium is used in alloy form, there are a few alloys that have proven useful. An 88-percent-gold–12-percent-germanium alloy, with a melting temperature of 359° C, has been used as a solder for gold jewellery (Brady and Clauser, 1997). Germanium also is alloyed in several combinations with gold, silver, copper, and palladium in dental alloys and with silicon in thermoelectric devices. More often, germanium is used in very small quantities as a hardener of metals, such as aluminum, magnesium, and tin."
- Harner HR 1961, "Germanium", in Hampel CA (ed.), Rare metals handbook, 2nd ed. Reinhold Publishing Co., New York, pp. 188–197.
"Germanium appears to form no carbide, but alloys with many metals and metalloids."
- Rochow EG 1973, "Germanium", in Comprehensive inorganic chemistry, vol. 2, Pergamon Press, Oxford, p. 10
Arsenic
"The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition)."
"Arsenic is used as the group 5 element in the III-V semiconductors gallium arsenide, indium arsenide, and aluminium arsenide.[29] The valence electron count of GaAs is the same as a pair of Si atoms, but the band structure is completely different which results in distinct bulk properties.[30] Other arsenic alloys include the II-V semiconductor cadmium arsenide.[31]"
- --- our entry for Arsenic
Antimony
"As late as the 19th century, the number of uses for antimony and the amount used remained small. Most of it was used in type metal or alloyed with lead for use as bearing metal (babbitt metal) or with tin for use in Britannia metal as candlesticks, dinnerware, eating utensils, and so forth.
Antimony is also a component of several tin-based alloys, such as britannia metal, pewter, white bearing metal (true babbitt), and a new alloy, a tin-antimony-silver solder used for joining pipes for carrying potable water.
Antimony itself is hard and brittle and is used alone only for very minor uses, such as ornamental castings. But its compatibility with lead and other low-melting-point metals make it useful in alloys."
- Li T, Archer GF, and Carapella SC Jr., 1992, "Antimony and antimony alloys", in Kirk-Othmer Encyclopedia of chemical technology (4th ed.): New York, John Wiley & Sons, v. 3, p. 367-381.
Tellurium
van Arkel-Ketelaar
References
- ^ Thyssen, P.; Binnemans, K. (2011). Gschneidner Jr., K. A.; Bünzli, J-C.G; Vecharsky, Bünzli (eds.). Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis. Vol. 41. Amsterdam: Elsevier. pp. 1–94. doi:10.1016/B978-0-444-53590-0.00001-7. ISBN 978-0-444-53590-0.
{{cite book}}:|journal=ignored (help) - ^ Thyssen P. and Binnemans K. (2011). "Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis". In K. A. Gschneider Jr. (ed). Handbook on the Physics and Chemistry of the Rare Earths. 41. Amsterdam: Elsevier, pp. 1–94; Seaborg G. T. (1994). Origin of the Actinide Concept'. In K. A. Gschneider Jr. (ed). Handbook on the Physics and Chemistry of the Rare Earths. 18. Amsterdam: Elsevier, pp. 1–27.
- ^ Stewart, P. J. (2008). "The Flyleaf Table: An Alternative". Journal of Chemical Education. 85 (11): 1490. Bibcode:2008JChEd..85.1490S. doi:10.1021/ed085p1490.
- ^ Cite error: The named reference
McGraw-Hillwas invoked but never defined (see the help page). - ^ a b c d e f Jørgensen, Christian K. (1988). "Influence of rare earths on chemical understanding and classification". Handbook on the Physics and Chemistry of Rare Earths. Vol. 11. pp. 197–292. doi:10.1016/S0168-1273(88)11007-6. ISBN 9780444870803.
- ^ Thyssen, P.; Binnemanns, K. (2011). "1: Accommodation of the rare earths in the periodic table: A historical analysis". In Gschneidner Jr., K. A.; Büzli, J-C. J.; Pecharsky, V. K. (eds.). Handbook on the Physics and Chemistry of Rare Earths. Vol. 41. Amsterdam: Elsevier. pp. 80–81. ISBN 978-0-444-53590-0.
- ^ Keeler, J.; Wothers, P. (2014). Chemical Structure and Reactivity: An Integrated Approach. Oxford: Oxford University. p. 259. ISBN 978-0-19-960413-5.
- ^ Scerri, E. (2012). "Mendeleev's Periodic Table Is Finally Completed and What To Do about Group 3?". Chemistry International. 34 (4). doi:10.1515/ci.2012.34.4.28. Archived from the original on 5 July 2017.
- ^ a b Castelvecchi, D. (8 April 2015). "Exotic atom struggles to find its place in the periodic table". Nature. doi:10.1038/nature.2015.17275. Archived from the original on 5 October 2015. Retrieved 20 September 2015.
- ^ "The constitution of group 3 of the periodic table". IUPAC. 2015. Archived from the original on 5 July 2016. Retrieved 30 July 2016.
- ^ Emsley, J. (2011). Nature's Building Blocks (new ed.). Oxford: Oxford University. p. 651. ISBN 978-0-19-960563-7.
- ^ a b c d e f g h i William B. Jensen (1982). "The Positions of Lanthanum (Actinium) and Lutetium (Lawrencium) in the Periodic Table". J. Chem. Educ. 59 (8): 634–636. Bibcode:1982JChEd..59..634J. doi:10.1021/ed059p634.
- ^ Trifonov, D. N. (1970). Rare-earth elements and their position in the periodic system (translated from Russian). New Delhi: Indian National Scientific Documentation Centre. pp. 201–202.
- ^ Greenwood, N. N.; Harrington, T. J. (1973). The chemistry of the transition elements. Oxford: Clarendon Press. p. 50. ISBN 978-0-19-855435-6.
- ^ Aylward, G.; Findlay, T. (2008). SI chemical data (6th ed.). Milton, Queensland: John Wiley & Sons. ISBN 978-0-470-81638-7.
- ^ Wiberg, N. (2001). Inorganic Chemistry. San Diego: Academic Press. p. 119. ISBN 978-0-12-352651-9.
- ^ Wulfsberg, G. (2006). "Periodic table: Trends in the properties of the elements". Encyclopedia of Inorganic Chemistry. New York: John Wiley & Sons. p. 3. ISBN 978-0-470-86210-0.
- ^ Cotton, S. (2007). Lanthanide and Actinide Chemistry. Chichester: John Wiley & Sons. p. 150. ISBN 978-0-470-01006-8.
- ^ Scerri, Eric (2016). "The Changing Views of a Philosopher of Chemistry on the Question of Reduction". In Scerri, Eric; Fisher, Grant (eds.). Essays in the Philosophy of Chemistry. Oxford University Press.
- ^ Schwarz, W. H. Eugen (2010). "The Full Story of the Electron Configurations of the Transition Elements". Journal of Chemical Education. 87 (4): 444–8. doi:10.1021/ed8001286.
- ^ a b Jensen, William B. (2009). "Misapplying the Periodic Law" (PDF). Journal of Chemical Education. 86 (10): 1186. Bibcode:2009JChEd..86.1186J. doi:10.1021/ed086p1186. Retrieved 16 May 2020.
- ^ a b Scerri, Eric (2009). "Which Elements Belong in Group 3?". Journal of Chemical Education. 86 (10): 1188. Bibcode:2009JChEd..86.1188S. doi:10.1021/ed086p1188.
- ^ Cite error: The named reference
Greenwood27was invoked but never defined (see the help page). - ^ a b Jørgensen, Christian K. (1983). "5f Electrons in Transthorium Chemistry" (PDF). Radiochimica Acta. 32 (1–3): 1–5. doi:10.1524/ract.1983.32.13.1. Retrieved 16 May 2020.
- ^ a b Seaborg, Glenn T. (August 1991). "Origin of the Actinide Concept" (PDF). escholarship.org. Retrieved 17 May 2020.
- ^ Rayner-Canham, Geoff (2013). "Periodic patterns: the Group (n) and Group (n + 10) linkage". Foundations of Chemistry. 15: 229–237. doi:10.1007/s10698-012-9169-6. Retrieved 17 May 2020.
- ^ Jensen, William B. (August 2003). "The Place of Zinc, Cadmium, and Mercury in the Periodic Table" (PDF). Journal of Chemical Education. 80 (8): 952–961. doi:10.1021/ed080p952. Retrieved 17 May 2020.
- ^ Moeller et al. (1989). Chemistry with Inorganic Qualitative Analysis (3rd ed.). SanDiego: Harcourt Brace Jovanovich, pp. 955–956, 958.
- ^ L. D. Landau, E. M. Lifshitz (1958). Quantum Mechanics: Non-Relativistic Theory. Vol. Vol. 3 (1st ed.). Pergamon Press. pp. 256–7.
{{cite book}}:|volume=has extra text (help) - ^ Scerri, E. (15 September 2015). "Five ideas in chemical education that must die – Group three". Education in Chemistry. Royal Society of Chemistry. Archived from the original on 23 December 2015. Retrieved 19 September 2015.
It is high time that the idea of group 3 consisting of Sc, Y, La and Ac is abandoned
- ^ a b c Hamilton, David C. (1965). "Position of Lanthanum in the Periodic Table". American Journal of Physics. 33: 637–640. doi:10.1119/1.1972042.
- ^ Xu, Wei; Ji, Wen-Xin; Qiu, Yi-Xiang; Schwarz, W. H. Eugen; Wang, Shu-Guang (2013). "On structure and bonding of lanthanoid trifluorides LnF3 (Ln = La to Lu)". Physical Chemistry Chemical Physics. 2013 (15): 7839–47. Bibcode:2013PCCP...15.7839X. doi:10.1039/C3CP50717C. PMID 23598823.
- ^ a b c d e f Wittig, Jörg (1973). "The pressure variable in solid state physics: What about 4f-band superconductors?". In H. J. Queisser (ed.). Festkörper Probleme: Plenary Lectures of the Divisions Semiconductor Physics, Surface Physics, Low Temperature Physics, High Polymers, Thermodynamics and Statistical Mechanics, of the German Physical Society, Münster, March 19–24, 1973. Berlin, Heidelberg: Springer. p. 375–396. doi:10.1007/BFb0108579. ISBN 978-3-528-08019-8.
- ^ Östlin, A. (2013). "Transition metals". Electronic Structure Studies and Method Development for Complex Materials (PDF) (Licentiate). p. 13. Retrieved 17 May 2020.
- ^ Johansson, B.; Abuja, R.; Eriksson, O.; Wills, J. M. (1995). "Anomalous fcc crystal structure of thorium metal". Physical Review Letters. 75 (2): 280–283. Bibcode:1995PhRvL..75..280J. doi:10.1103/PhysRevLett.75.280. PMID 10059654.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Gschneidner Jr., K. A. (December 1971). "On the nature of 4ƒ bonding in the lanthanide elements and their compounds". Journal of the Less Common Metals. 25 (4): 405–422. doi:10.1016/0022-5088(71)90184-6.
- ^ Glotzel, D. (1978). "Ground-state properties of f band metals: lanthanum, cerium and thorium". Journal of Physics F: Metal Physics. 8 (7): L163–L168. Bibcode:1978JPhF....8L.163G. doi:10.1088/0305-4608/8/7/004.
- ^ Alvarez, Santiago (11 February 2020). "The transition from 4f to 5d elements from the structural point of view". CrystEngComm. 2020 (Advance Article). doi:10.1039/D0CE00029A.
- ^ Merz, H.; Ulmer, K. (1967). "Position of Lanthanum and Lutetium in the Periodic Table". Physics Letters A. 26 (1): 6–7. Bibcode:1967PhLA...26....6M. doi:10.1016/0375-9601(67)90527-0.
- ^ Jensen, W. B. (2015). "Some Comments on the Position of Lawrencium in the Periodic Table" (PDF). Archived from the original (PDF) on 23 December 2015. Retrieved 20 September 2015.
- ^ Xu, W-H.; Pyykkö, P. (2016). "Is the chemistry of lawrencium peculiar?" (PDF). Physical Chemistry Chemical Physics. 18 (26): 17351–17355. Bibcode:2016PCCP...1817351X. doi:10.1039/C6CP02706G. hdl:10138/224395. PMID 27314425.
- ^ Silva, Robert J. (2006). "Fermium, Mendelevium, Nobelium, and Lawrencium" (PDF). In Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements. Vol. 3 (3rd ed.). Dordrecht: Springer. pp. 1621–1651. doi:10.1007/1-4020-3598-5_13. ISBN 978-1-4020-3555-5. Archived from the original (PDF) on 2010-07-17.
- ^ Johnson, D. A. (1977). "Recent Advances in the Chemistry of the Less-Common Oxidation States of the Lanthanide Elements". Advances in Inorganic Chemistry and Radiochemistry: 1–132. doi:10.1016/s0065-2792(08)60038-2.
- ^ Iyakutti, K.; Dakshinamoorthy, M.; Asokamani, R. (1981). "Bandstructure of actinium and the effect of correlation". Solid State Communications. 40: 555–7. doi:10.1016/0038-1098(81)90572-X.
- ^ Dakshinamoorthy, M.; Iyakutti, K. (1984). "Band structure, Fermi surface, superconductivity, and resistivity of actinium under high pressure". Physical Review B. 30 (12): 6943–50. doi:10.1103/PhysRevB.30.6943.
- ^ King, R. B. (1995). Inorganic Chemistry of Main Group Elements. New York: Wiley-VCH. p. 289. ISBN 978-1-56081-679-9.
- ^ Greenwood and Earnshaw, p. 958
- ^ Greenwood and Earnshaw, p. 947
- ^ Greenwood and Earnshaw, p. 957
- ^ Connelly, N. G.; Damhus, T.; Hartshorn, R. M.; Hutton, A. T. (2005). Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005 (PDF). RSC Publishing. p. vii. ISBN 978-0-85404-438-2. Archived (PDF) from the original on 23 November 2018. Retrieved 26 November 2018.
Lesser omissions include ... the several different outdated versions of the periodic table. (That on the inside front cover is the current IUPAC-agreed version.)
- ^ Leigh, G. J. (2009). "Periodic Tables and IUPAC". Chemistry International. 31 (1). doi:10.1515/ci.2009.31.1.4. Archived from the original on 27 November 2018. Retrieved 27 November 2018.
- ^ Jensen, William B. (2008). "The Periodic Table: Facts or Committees?". Journal of Chemical Education. 85 (11): 1491–2. Bibcode:2008JChEd..85.1491J. doi:10.1021/ed085p1491.2.
- ^ Scerri, P.; Parsons, B. (2018). "What elements belong in group 3 of the Periodic Table?". In Scerri, E.; Restrepo, G. (eds.). From Mendeleev to Oganesson: A Multidisciplinary Perspective on the Periodic Table. New York: Oxford University Press. pp. 140–151. ISBN 978-0-190-66853-2.
- ^ Lee, J. D. (1996). Concise inorganic chemistry (5th ed.). Oxford: Blackwell-Science. p. 679. ISBN 978-0-6320-5293-6.
- ^ Cite error: The named reference
Russell2005401was invoked but never defined (see the help page). - ^ Desch 1914, p. 86
- ^ Phillips & Williams 1965, p. 620
Constitution of binary alloys, by Hansen, McGraw-Hill Book Company (1958-1969)
Crystal chemistry and semiconduction in transition metal binary compounds, by Suchet 1971, Academic Press
Parsing the nonmetals (II)
- "The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in many biochemicals) and the halogens."
- "The acronym CHNOPS, which stands for carbon, hydrogen, nitrogen, oxygen, phosphorus, sulfur, represents the six most important chemical elements whose covalent combinations make up most biological molecules on Earth."
- "It is a long standing tradition that organic compounds of the metalloids fall within scope of the definition of organometallic compounds."
Corrosive nonmetals
Compounds of the corrosive nonmetals number among the range of chemicals studied in organic chemistry.
Intermediate nonmetals
Covalent combinations of the first five of these (along with the corrosive nonmetal oxygen) make up most biological molecules on Earth.
Metalloids
It is a long standing tradition that organic compounds of the metalloids fall within scope of the definition of organometallic compounds.
Compounds formed with metals
| Category | Elements | Compound types | Other types | Notes |
|---|---|---|---|---|
| Corrosive nonmetal | F, Cl, Br, I, O | Ionic | Interstitial (O) | For M = metal and N = nonmetal, interstitial and intermetallic compounds feature M-M (metallic), M-N (ionocovalent), and N-N (covalent) bonding contributions. In N-poor compounds metallic bonding predominates; in N-rich compounds covalent bonding predominates. |
| Intermediate nonmetal | H, C, N, P, S, Se | Salt-like to covalent | Interstitial (H, C, N); intermetallic (P?, Se) | |
| Metalloid | B, Si, Ge, As, Sb, Te | Salt-like to more covalent; alloys | Interstitial (B); intermetallic | |
| Metal | Many | Alloys | Intermetallic | The d-electrons in the partially-filled orbitals of transition metal atoms form covalent bonds with their counterparts in other transition metal atoms |
The importance of symmetry
Brooks M 2018, New Scientist, 18 Aug 2018, no. 3191, p. 30:
Knowing where new particles may be hiding is a tricky business. The most powerful searchlight at our disposal is symmetry: the idea that something looks and behaves the same even when some aspect of its position, direction or orientation is changed. A circle, for example, has total rotational symmetry, while a square has broken rotational symmetry – it looks the same when you turn it through multiples of 90 degrees, but not under any other rotation.
When a symmetry is broken, physicists sit up and take notice. “Symmetry-breaking doesn’t just happen – there’s always some reason behind it,” says physicist David Tong at the University of Cambridge.
This is especially true of the more complicated symmetries that arise in particle physics. Rather than rotate a particle or move it about, you might swap it for its oppositely charged antiparticle. If you see no difference in their interactions, that is a symmetry. For example, two electrons repel each other in exactly the same way as two positrons do, displaying identical physics under the reversal of charge. We call that charge symmetry.
Flipping important
Another key symmetry is time reversal. Imagine you are watching a recording of a snooker match on TV, and you see a ball glance off the cushion. You press rewind, and you now see the ball move back towards the cushion. If it comes off at a different angle to what you saw before, time-reversal symmetry is broken.
The third fundamental symmetry is parity symmetry. This time, while watching the snooker match you see the initial shot reflected in a mirror. If it comes off the cushion at a different angle in the mirror view, this breaks parity symmetry.
Sometimes you don’t have to actually spot a symmetry being broken – just where it should be broken but isn’t. In 1964, for instance, Murray Gell-Mann applied symmetry considerations to the standard model of particle physics and came up with the idea that there should exist a set of particles that, put together in certain ways, would make the protons and neutrons we find in the atomic nucleus. Gell-Mann’s mathematical hunch was right on the money: his conjectured “quarks” were found in subsequent particle searches, and Gell-Mann won a Nobel prize in physics for his efforts.
To push things further, we can combine some of these symmetries and see whether anything interesting emerges. Take charge and parity (CP) symmetry, for example. Systems that might violate one or other actually end up looking the same if you impose both transformations. In other words, you can find two different perspectives, like a real particle and its antimatter equivalent reflected in a mirror, in which things appear to behave in the same way.
These symmetries matter, largely because we like to see them broken sometimes: the laws, particles and forces of physics all have their roots in symmetry-breaking. They create what David Gross of the Kavli Institute for Theoretical Physics at the University of California, Santa Barbara, calls the “texture of the world”. These considerations have led Florian Goertz at the Max Planck Institute for Particle and Astroparticle Physics in Heidelberg to propose the existence of a new particle that is single-handedly capable of cleaning up five of the stickiest problems in physics. “Complete symmetry is boring,” says Goertz. “If symmetry is slightly broken, interesting things can happen.”


