Benfluralin
Appearance
| |
| Names | |
|---|---|
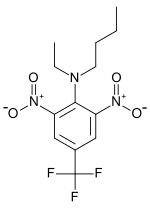
| IUPAC name
N-Butyl-N-ethyl-2,6-dinitro-4-(trifluoromethyl)aniline
| |
| Other names
Benefin; Benfluraline; α,α,α-Trifluoro-2,6-dinitro-N,N-ethylbutyl-p-toluidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.015.878 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H16F3N3O4 | |
| Molar mass | 335.283 g·mol−1 |
| Appearance | Orange crystalline solid[1] |
| Density | 1.338 g/mL |
| Melting point | 65.0 to 65.5 °C (149.0 to 149.9 °F; 338.1 to 338.6 K)[1] |
| Boiling point | 121 to 122 °C (250 to 252 °F; 394 to 395 K)[1] at 0.6 mbar |
| 1 mg/L[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benfluralin is an herbicide of the dinitroaniline class. The mechanism of action of benfluralin involves inhibition of root and shoot development.[2]
It is used to control grasses and other weeds. Annual use in the United States was approximately 700,000 pounds in 2004.[3]
References
- ^ a b c d Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Agrochemicals Archived April 6, 2012, at the Wayback Machine, Globachem
- ^ R.E.D. FACTS: Benfluralin Archived September 15, 2011, at the Wayback Machine, United States Environmental Protection Agency
