Kelp forest
| Marine habitats |
|---|
 |
| Coastal habitats |
| Ocean surface |
| Open ocean |
| Sea floor |
Kelp forests are underwater areas with a high density of kelp, which covers about 25% of the world's coastlines.[1] They are recognized as one of the most productive and dynamic ecosystems on Earth.[2] Smaller areas of anchored kelp are called kelp beds.
"I can only compare these great aquatic forests...with the terrestrial ones in the intertropical regions. Yet if in any country a forest was destroyed, I do not believe so nearly so many species of animals would perish as would here, from the destruction of kelp. Amidst the leaves of this plant numerous species of fish live, which nowhere else could find food or shelter; with their destruction the many cormorants and other fishing birds, the otters, seals and porpoise, would soon perish also; and lastly, the Fuegian[s]...would...decrease in numbers and perhaps cease to exist.
– Charles Darwin, 1 June 1834, Tierra del Fuego, Chile[3]
Kelp forests occur worldwide throughout temperate and polar coastal oceans.[2] In 2007, kelp forests were also discovered in tropical waters near Ecuador.[4]
Physically formed by brown macroalgae, kelp forests provide a unique habitat for marine organisms[5] and are a source for understanding many ecological processes. Over the last century, they have been the focus of extensive research, particularly in trophic ecology, and continue to provoke important ideas that are relevant beyond this unique ecosystem. For example, kelp forests can influence coastal oceanographic patterns[6] and provide many ecosystem services.[7]
However, the influence of humans has often contributed to kelp forest degradation. Of particular concern are the effects of overfishing nearshore ecosystems, which can release herbivores from their normal population regulation and result in the overgrazing of kelp and other algae.[8] This can rapidly result in transitions to barren landscapes where relatively few species persist.[9][10] The implementation of marine protected areas is one management strategy useful for addressing such issues, since it may limit the impacts of fishing and buffer the ecosystem from additive effects of other environmental stressors.
Kelp
The term kelp refers to marine algae belonging to the order Laminariales (phylum: Heterokontophyta). Though not considered a taxonomically diverse order, kelps are highly diverse structurally and functionally.[7] The most widely recognized species are the giant kelps (Macrocystis spp.), although numerous other genera such as Laminaria, Ecklonia, Lessonia, Alaria, and Eisenia are described.
A wide range of sea life uses kelp forests for protection or food, including fish. In the North Pacific kelp forests, particularly rockfish, and many invertebrates, such as amphipods, shrimp, marine snails, bristle worms, and brittle stars. Many marine mammals and birds are also found, including seals, sea lions, whales, sea otters, gulls, terns, snowy egrets, great blue herons, and cormorants, as well as some shore birds.[11]
Frequently considered an ecosystem engineer, kelp provides a physical substrate and habitat for kelp forest communities.[12] In algae (kingdom Protista), the body of an individual organism is known as a thallus rather than as a plant (kingdom Plantae). The morphological structure of a kelp thallus is defined by three basic structural units:[9]
- The holdfast is a root-like mass that anchors the thallus to the sea floor, though unlike true roots it is not responsible for absorbing and delivering nutrients to the rest of the thallus.
- The stipe is analogous to a plant stalk, extending vertically from the holdfast and providing a support framework for other morphological features.
- The fronds are leaf- or blade-like attachments extending from the stipe, sometimes along its full length, and are the sites of nutrient uptake and photosynthetic activity.
In addition, many kelp species have pneumatocysts, or gas-filled bladders, usually located at the base of fronds near the stipe. These structures provide the necessary buoyancy for kelp to maintain an upright position in the water column.
The environmental factors necessary for kelp to survive include hard substrate (usually rock or sand), high nutrients (e.g., nitrogen, phosphorus), and light (minimum annual irradiance dose > 50 E m−2[13]). Especially productive kelp forests tend to be associated with areas of significant oceanographic upwelling, a process that delivers cool, nutrient-rich water from depth to the ocean's mixed surface layer.[13] Water flow and turbulence facilitate nutrient assimilation across kelp fronds throughout the water column.[14] Water clarity affects the depth to which sufficient light can be transmitted. In ideal conditions, giant kelp (Macrocystis spp.) can grow as much as 30–60 cm vertically per day. Some species, such as Nereocystis, are annuals, while others such as Eisenia are perennials, living for more than 20 years.[15] In perennial kelp forests, maximum growth rates occur during upwelling months (typically spring and summer) and die-backs correspond to reduced nutrient availability, shorter photoperiods, and increased storm frequency.[9]
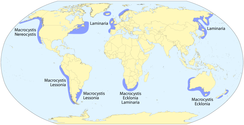
Kelps are primarily associated with temperate and arctic waters worldwide. Of the more dominant genera, Laminaria is mainly associated with both sides of the Atlantic Ocean and the coasts of China and Japan; Ecklonia is found in Australia, New Zealand, and South Africa; and Macrocystis occurs throughout the northeastern and southeastern Pacific Ocean, Southern Ocean archipelagos, and in patches around Australia, New Zealand, and South Africa.[9] The region with the greatest diversity of kelps (>20 species) is the northeastern Pacific, from north of San Francisco, California, to the Aleutian Islands, Alaska.
Although kelp forests are unknown in tropical surface waters, a few species of Laminaria have been known to occur exclusively in tropical deep waters.[16][17] This general absence of kelp from the tropics is believed to be mostly due to insufficient nutrient levels associated with warm, oligotrophic waters.[9] One recent study spatially overlaid the requisite physical parameters for kelp with mean oceanographic conditions has produced a model predicting the existence of subsurface kelps throughout the tropics worldwide to depths of 200 m. For a hotspot in the Galapagos Islands, the local model was improved with fine-scale data and tested; the research team found thriving kelp forests in all eight of their sampled sites, all of which had been predicted by the model, thus validated their approach. This suggests that their global model might actually be fairly accurate, and if so, kelp forests would be prolific in tropical subsurface waters worldwide.[4] The importance of this contribution has been rapidly acknowledged within the scientific community and has prompted an entirely new trajectory of kelp forest research, particularly emphasizing the potential for a spatial refuge from climate change also the explanations to evolutionary patterns of kelps worldwide.[18]
Ecosystem architecture




The architecture of a kelp forest ecosystem is based on its physical structure, which influences the associated species that define its community structure. Structurally, the ecosystem includes three guilds of kelp and two guilds occupied by other algae:[9]
- Canopy kelps include the largest species and often constitute floating canopies that extend to the ocean surface (e.g., Macrocystis and Alaria).
- Stipitate kelps generally extend a few meters above the sea floor and can grow in dense aggregations (e.g., Eisenia and Ecklonia).
- Prostrate kelps lie near and along the sea floor (e.g., Laminaria).
- The benthic assemblage is composed of other algal species (e.g., filamentous and foliose functional groups, articulated corallines) and sessile organisms along the ocean bottom.
- Encrusting coralline algae directly and often extensively cover geologic substrate.
Multiple kelp species often co-exist within a forest; the term understory canopy refers to the stipitate and prostrate kelps. For example, a Macrocystis canopy may extend many meters above the seafloor towards the ocean surface, while an understory of the kelps Eisenia and Pterygophora reaches upward only a few meters. Beneath these kelps, a benthic assemblage of foliose red algae may occur. The dense vertical infrastructure with overlying canopy forms a system of microenvironments similar to those observed in a terrestrial forest, with a sunny canopy region, a partially shaded middle, and darkened seafloor.[9] Each guild has associated organisms, which vary in their levels of dependence on the habitat, and the assemblage of these organisms can vary with kelp morphologies.[19][20][21] For example, in California, Macrocystis pyrifera forests, the nudibranch Melibe leonina, and skeleton shrimp Caprella californica are closely associated with surface canopies; the kelp perch Brachyistius frenatus, rockfish Sebastes spp., and many other fishes are found within the stipitate understory; brittle stars and turban snails Tegula spp. are closely associated with the kelp holdfast, while various herbivores, such as sea urchins and abalone, live under the prostrate canopy; many seastars, hydroids, and benthic fishes live among the benthic assemblages; solitary corals, various gastropods, and echinoderms live over the encrusting coralline algae.[19] In addition, pelagic fishes and marine mammals are loosely associated with kelp forests, usually interacting near the edges as they visit to feed on resident organisms.
Trophic ecology



Classic studies in kelp forest ecology have largely focused on trophic interactions (the relationships between organisms and their food webs), particularly the understanding and top-down trophic processes. Bottom-up processes are generally driven by the abiotic conditions required for primary producers to grow, such as availability of light and nutrients, and the subsequent transfer of energy to consumers at higher trophic levels. For example, the occurrence of kelp is frequently correlated with oceanographic upwelling zones, which provide unusually high concentrations of nutrients to the local environment.[22][23] This allows kelp to grow and subsequently support herbivores, which in turn support consumers at higher trophic levels.[24] By contrast, in top-down processes, predators limit the biomass of species at lower trophic levels through consumption. In the absence of predation, these lower-level species flourish because resources that support their energetic requirements are not limiting. In a well-studied example from Alaskan kelp forests,[25] sea otters (Enhydra lutris) control populations of herbivorous sea urchins through predation. When sea otters are removed from the ecosystem (for example, by human exploitation), urchin populations are released from predatory control and grow dramatically. This leads to increased herbivore pressure on local kelp stands. Deterioration of the kelp itself results in the loss of physical ecosystem structure and subsequently, the loss of other species associated with this habitat. In Alaskan kelp forest ecosystems, sea otters are the keystone species that mediates this trophic cascade. In Southern California, kelp forests persist without sea otters and the control of herbivorous urchins is instead mediated by a suite of predators including lobsters and large fishes, such as the California sheephead. The effect of removing one predatory species in this system differs from Alaska because redundancy exists in the trophic levels and other predatory species can continue to regulate urchins.[20] However, the removal of multiple predators can effectively release urchins from predator pressure and allow the system to follow trajectories towards kelp forest degradation.[26] Similar examples exist in Nova Scotia,[27] South Africa,[28] Australia[29] and Chile.[30] The relative importance of top-down versus bottom-up control in kelp forest ecosystems and the strengths of trophic interactions continue to be the subject of considerable scientific investigation.[31][32][33]
The transition from macroalgal (i.e. kelp forest) to denuded landscapes dominated by sea urchins (or ‘urchin barrens’) is a widespread phenomenon,[7][34][35][36] often resulting from trophic cascades like those described above; the two phases are regarded as alternative stable states of the ecosystem.[37][38] The recovery of kelp forests from barren states has been documented following dramatic perturbations, such as urchin disease or large shifts in thermal conditions.[26][39][40] Recovery from intermediate states of deterioration is less predictable and depends on a combination of abiotic factors and biotic interactions in each case.
Though urchins are usually the dominant herbivores, others with significant interaction strengths include seastars, isopods, kelp crabs, and herbivorous fishes.[9][31] In many cases, these organisms feed on kelp that has been dislodged from substrate and drifts near the ocean floor rather than expend energy searching for intact thalli on which to feed. When sufficient drift kelp is available, herbivorous grazers do not exert pressure on attached plants; when drift subsidies are unavailable, grazers directly impact the physical structure of the ecosystem.[41][42] Many studies in Southern California have demonstrated that the availability of drift kelp specifically influences the foraging behavior of sea urchins.[43][44] Drift kelp and kelp-derived particulate matter have also been important in subsidizing adjacent habitats, such as sandy beaches and the rocky intertidal.[45][46][47]
Patch dynamics
Another major area of kelp forest research has been directed at understanding the spatial-temporal patterns of kelp patches. Not only do such dynamics affect the physical landscape, but they also affect species that associate with kelp for refuge or foraging activities.[19][24] Large-scale environmental disturbances have offered important insights concerning mechanisms and ecosystem resilience. Examples of environmental disturbances include:
- Acute and chronic pollution events have been shown to impact southern California kelp forests, though the intensity of the impact seems to depend on both the nature of the contaminants and duration of exposure.[48][49][50][51][52] Pollution can include sediment deposition and eutrophication from sewage, industrial byproducts and contaminants like PCBs and heavy metals (for example, copper, zinc), runoff of organophosphates from agricultural areas, anti-fouling chemicals used in harbors and marinas (for example, TBT and creosote) and land-based pathogens like fecal coliform bacteria.
- Catastrophic storms can remove surface kelp canopies through wave activity, but usually leave understory kelps intact; they can also remove urchins when little spatial refuge is available.[37][42] Interspersed canopy clearings create a seascape mosaic where sunlight penetrates deeper into the kelp forest and species that are normally light-limited in the understory can flourish. Similarly, substrate cleared of kelp holdfasts can provide space for other sessile species to establish themselves and occupy the seafloor, sometimes directly competing with juvenile kelp and even inhibiting their settlement.[53]
- El Niño-Southern Oscillation (ENSO) events involve the depression of oceanographic thermoclines, severe reductions of nutrient input, and changes in storm patterns.[37][54] Stress due to warm water and nutrient depletion can increase the susceptibility of kelp to storm damage and herbivorous grazing, sometimes even prompting phase shifts to urchin-dominated landscapes.[40][43][55] In general, oceanographic conditions (that is, water temperature, currents) influence the recruitment success of kelp and its competitors, which clearly affect subsequent species interactions and kelp forest dynamics.[37][56]
- Overfishing higher trophic levels that naturally regulate herbivore populations is also recognized as an important stressor in kelp forests.[8][33][57] As described in the previous section, the drivers and outcomes of trophic cascades are important for understanding spatial-temporal patterns of kelp forests.[25][26][31]
In addition to ecological monitoring of kelp forests before, during, and after such disturbances, scientists try to tease apart the intricacies of kelp forest dynamics using experimental manipulations. By working on smaller spatial-temporal scales, they can control for the presence or absence of specific biotic and abiotic factors to discover the operative mechanisms. For example, in southern Australia, manipulations of kelp canopy types demonstrated that the relative amount of Ecklonia radiata in a canopy could be used to predict understory species assemblages; consequently, the proportion of E. radiata can be used as an indicator of other species occurring in the environment.[58]
Human use
Kelp forests have been important to human existence for thousands of years.[59] Indeed, many now theorise that the first colonisation of the Americas was due to fishing communities following the Pacific kelp forests during the last ice age. One theory contends that the kelp forests that would have stretched from northeast Asia to the American Pacific coast would have provided many benefits to ancient boaters. The kelp forests would have provided many sustenance opportunities, as well as acting as a type of buffer from rough water. Besides these benefits, researchers believe that the kelp forests might have helped early boaters navigate, acting as a type of "kelp highway". Theorists also suggest that the kelp forests would have helped these ancient colonists by providing a stable way of life and preventing them from having to adapt to new ecosystems and develop new survival methods even as they traveled thousands of miles.[60] Modern economies are based on fisheries of kelp-associated species such as lobster and rockfish. Humans can also harvest kelp directly to feed aquaculture species such as abalone and to extract the compound alginic acid, which is used in products like toothpaste and antacids.[61][62] Kelp forests are valued for recreational activities such as SCUBA diving and kayaking; the industries that support these sports represent one benefit related to the ecosystem and the enjoyment derived from these activities represents another. All of these are examples of ecosystem services provided specifically by kelp forests.
Threats and management

Given the complexity of kelp forests – their variable structure, geography, and interactions – they pose a considerable challenge to environmental managers. Extrapolating even well-studied trends to the future is difficult because interactions within the ecosystem will change under variable conditions, not all relationships in the ecosystem are understood, and the nonlinear thresholds to transitions are not yet recognized.[63] With respect to kelp forests, major issues of concern include marine pollution and water quality, kelp harvesting and fisheries, invasive species,[7] and climate change.[64] The most pressing threat to kelp forest preservation may be the overfishing of coastal ecosystems, which by removing higher trophic levels facilitates their shift to depauperate urchin barrens.[8] The maintenance of biodiversity is recognized as a way of generally stabilizing ecosystems and their services through mechanisms such as functional compensation and reduced susceptibility to foreign species invasions.[65][66][67][68]
In many places, managers have opted to regulate the harvest of kelp[23][69] and/or the taking of kelp forest species by fisheries.[7][57] While these may be effective in one sense, they do not necessarily protect the entirety of the ecosystem. Marine protected areas (MPAs) offer a unique solution that encompasses not only target species for harvesting, but also the interactions surrounding them and the local environment as a whole.[70][71] Direct benefits of MPAs to fisheries (for example, spillover effects) have been well documented around the world.[8][72][73][74] Indirect benefits have also been shown for several cases among species such as abalone and fishes in Central California.[75][76] Most importantly, MPAs can be effective at protecting existing kelp forest ecosystems and may also allow for the regeneration of those that have been affected.[37][77][78]
References
- ^ Kelp Forest - an overview | ScienceDirect Topics
- ^ a b Mann, K.H. 1973. Seaweeds: their productivity and strategy for growth. Science 182: 975-981.
- ^ Darwin, C. 1909. The Voyage of the Beagle. The Harvard Classics Volume 29. New York, USA: P.F. Collier & Son Company.
- ^ a b Graham, M.H., B.P. Kinlan, L.D. Druehl, L.E. Garske, and S. Banks. 2007. Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity. Proceedings of the National Academy of Sciences 104: 16576-16580.
- ^ Christie, H., Jørgensen, N.M., Norderhaug, K.M., Waage-Nielsen, E., 2003. Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian coast. Journal of the Marine Biological Association of the UK 83, 687-699.
- ^ Jackson, G.A. and C.D. Winant. 1983. Effect of a kelp forest on coastal currents. Continental Shelf Report 2: 75-80.
- ^ a b c d e Steneck, R.S., M.H. Graham, B.J. Bourque, D. Corbett, J.M. Erlandson, J.A. Estes and M.J. Tegner. 2002. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation 29: 436-459.
- ^ a b c d Sala, E., C.F. Bourdouresque and M. Harmelin-Vivien. 1998. Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos 82: 425-439.
- ^ a b c d e f g h Dayton, P.K. 1985a. Ecology of kelp communities. Annual Review of Ecology and Systematics 16: 215-245.
- ^ Norderhaug, K.M., Christie, H., 2009. Sea urchin grazing and kelp re-vegetation in the NE Atlantic. Marine Biology Research 5, 515-528
- ^ Kelp forests provide habitat for a variety of invertebrates, fish, marine mammals, and birds NOAA. Updated 11 January 2013. Retrieved 15 January 2014.
- ^ Jones, C.G., J. H. Lawton and M. Shachak. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78: 1946-1957.
- ^ a b Druehl, L.D. 1981. The distribution of Laminariales in the North Pacific with reference to environmental influences. Proceedings of the International Congress on Systematic Evolution and Biology 2: 248-256.
- ^ Wheeler, W.N. 1980. Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Marine Biology 56: 103-110.
- ^ Steneck, R.S. and M.N. Dethier. 1994. A functional group approach to the structure of algal-dominated communities. Oikos 69: 476-498.
- ^ Joly, A.B. and E.C. Oliveira Filho. 1967. Two Brazilian Laminarias. Instituto de Pesquisas da Marinha 4: 1-7.
- ^ Petrov, J.E., M.V. Suchovejeva and G.V. Avdejev. 1973. New species of the genus Laminaria from the Philippines Sea. Nov Sistem. Nizch. Rast. 10: 59-61.
- ^ Santelices, B. 2007. The discovery of kelp forests in deep-water habitats of tropical regions. Proceedings of the NationalAwan Riak Academy of Sciences 104: 19163-19164.
- ^ a b c Foster, M.S. and D.R. Schiel. 1985. The ecology of giant kelp forests in California: a community profile. US Fish and Wildlife Service Report 85: 1-152.
- ^ a b Graham, M.H. 2004. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems 7: 341-357.
- ^ Fowler-Walker, M.J., B. M. Gillanders, S.D. Connell and A.D. Irving. 2005. Patterns of association between canopy-morphology and understory assemblages across temperate Australia. Estuarine, Coastal and Shelf Science 63: 133-141.
- ^ Jackson, G.A. 1977. Nutrients and production of giant kelp, Macrocystis pyrifera, off southern California. Limnology and Oceanography 22: 979-995.
- ^ a b Dayton, P.K. M.J. Tegner, P.B. Edwards and K.L. Riser. 1999. Temporal and spatial scales of kelp demography: the role of the oceanographic climate. Ecological Monographs 69: 219-250.
- ^ a b Carr, M.H. 1994. Effects of macroalgal dynamics on recruitment of a temperate reef fish. Ecology 75: 1320-1333.
- ^ a b Estes, J.A. and D.O. Duggins. 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecological Monographs 65: 75-100.
- ^ a b c Pearse, J.S. and A.H. Hines. 1987. Expansion of a central California kelp forest following the mass mortality of sea urchins. Marine Biology 51: 83-91.
- ^ Scheibiling, R.E. and A.W. Hennigar. 1997. Recurrent outbreaks of disease in sea urchins Strongylocentrotus droebachiensis in Nova Scotia: evidence for a link with large-scale meteor logic and oceanographic events. Marine Ecology Progress Series 152: 155-165.
- ^ Velimirov, B., J.G. Field, C.L. Griffiths and P. Zoutendyk. 1977. The ecology of kelp bed communities in the Benguela upwelling system. Helgoland Marine Research 30: 495-518.
- ^ Andrew, N.L. 1993. Spatial heterogeneity, sea urchin grazing, and habitat structure on reefs in temperate Australia. Ecology 74: 292-302.
- ^ Dayton, P.K. 1985b. The structure and regulation of some South American kelp communities. Ecological Monographs 55: 447-468.
- ^ a b c Sala, E. and M.H. Graham. 2002. Community-wide distribution of predator-prey interaction strength in kelp forests. Proceedings of the National Academy of Sciences 99: 3678-3683.
- ^ Byrnes, J., J.J. Stachowicz, K.M. Hultgren, A.R. Hughes, S.V. Olyarnik and C.S. Thornber. 2006. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behavior. Ecology Letters 9: 61-71.
- ^ a b Halpern, B.S., K. Cottenie and B.R. Broitman. 2006. Strong top-down control in Southern California kelp forest ecosystems. Science 312: 1230-1232.
- ^ Lawrence, J.M. 1975. On the relationships between marine plants and sea urchins. Oceanography and Marine Biology, An Annual Review. 13: 213-286.
- ^ Hughes, T.P. 1994. Catastrophes, phase shifts and large-scale degradation of a Caribbean coral reef. Science 265: 1547-1551.
- ^ Siversten, K. 2006. Overgrazing of kelp beds along the coast of Norway. Journal of Applied Phycology 18: 599-610.
- ^ a b c d e Dayton, P.K., M.J. Tegner, P.E. Parnell and P.B. Edwards. 1992. Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecological Monographs 62: 421-445.
- ^ Pearse, J.S. 2006. Ecological role of purple sea urchins. Science 314: 940-941.
- ^ Lafferty, K.D. 2004. Fishing for lobsters indirectly increases epidemics in sea urchins. Ecological Applications 14: 1566-1573.
- ^ a b Vásquez, J.A., J.M. Alonso Vega and A.H. Buschmann. 2006. Long term variability in the structure of kelp communities in northern Chile and the 1997-98 ENSO. Journal of Applied Phycology 18: 505-519.
- ^ Cowen, R.K. 1983. The effect of sheephead (Semicossyphus pulcher) predation on red sea urchin (Strongylocentrotus franciscanus) populations: an experimental analysis. Oecologia 58: 249-255.
- ^ a b Ebeling, A.W., D.R. Laur and R.J. Rowley. 1985. Severe storm disturbances and reversal of community structure in a southern California kelp forest. Marine Biology 84: 287-294.
- ^ a b Dayton, P.K. and M.J. Tegner. 1984. Catastrophic storms, El Niño, and patch stability in a southern California kelp community. Science 224: 283-285.
- ^ Harrold, C. and D.C. Reed. 1985. Food availability, sea urchin grazing and kelp forest community structure. Ecology 66: 1160-1169.
- ^ Koop, K., R.C. Newell and M.I. Lucas. 1982. Biodegradation and carbon flow based on kelp (Ecklonia maxima) debris in a sandy beach microcosm. Marine Ecology Progress Series 7: 315-326.
- ^ Bustamante, R.H., G.M. Branch and S. Eekhout. 1995. Maintenance of exceptional intertidal grazer biomass in South Africa: subsidy by subtidal kelps. Ecology 76: 2314-2329.
- ^ Kaehler, S., E.A. Pakhomov, R.M. Kalin and S. Davis. 2006. Trophic importance of kelp-derived suspended particulate matter in a through-flow sub-Antarctic system. Marine Ecology Progress Series 316: 17-22.
- ^ Grigg, R.W. and R.S. Kiwala. 1970. Some ecological effects of discharged wastes on marine life. California Department of Fish and Game 56: 145-155.
- ^ Stull, J.K. 1989. Contaminants in sediments near a major marine outfall: history, effects and future. OCEANS ’89 Proceedings 2: 481-484.
- ^ North, W.J., D.E. James and L.G. Jones. 1993. History of kelp beds (Macrocystis) in Orange and San Diego Counties, California. Hydrobiologia 260/261: 277-283.
- ^ Tegner, M.J., P.K. Dayton, P.B. Edwards, K.L. Riser, D.B. Chadwick, T.A. Dean and L. Deysher. 1995. Effects of a large sewage spill on a kelp forest community: catastrophe or disturbance? Marine Environmental Research 40: 181-224.
- ^ Carpenter, S.R., R.F. Caraco, D.F. Cornell, R.W. Howarth, A.N. Sharpley and V.N. Smith. 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8: 559-568.
- ^ Kennelly, S.J. 1987. Physical disturbances in an Australian kelp community. I. Temporal effects. Marine Ecology Progress Series 40: 145-153.
- ^ McPhaden, M.J. 1999. Genesis and evolution of the 1997-1998 El Niño. Science 283: 950-954.
- ^ Edwards, M.S. and G. Hernández-Carmona. 2005. Delayed recovery of giant kelp near its southern range limit in the North Pacific following El Niño. Marine Biology 147: 273-279.
- ^ Duggins, D.O., J.E. Eckman and A.T. Sewell. 1990. Ecology of understory kelp environments. II. Effects of kelps on recruitment of benthic invertebrates. Journal of Experimental Marine Biology and Ecology 143: 27-45.
- ^ a b Jackson, J.B.C, M.X. Kirby, W.H. Berger, K.A. Bjorndal, L.W. Botsford, B.J. Bourque, R.H. Bradbury, R. Cooke, J. Erlandson, J.A. Estes, T.P. Hughes, S. Kidwell, C.B. Lange, H.S. Lenihan, J.M. Pandolfi, C.H. Peterson, R.S. Steneck, M.J. Tegner and R.R. Warner. 2002. Historical overfishing and the recent collapse of coastal ecosystems. Science 293: 629-638.
- ^ Irving, A.D. and S.D. Connell. 2006. Predicting understory structure from the presence and composition of canopies: an assembly rule for marine algae. Oecologia 148: 491-502.
- ^ Simenstad, C.A., J.A. Estes and K.W. Kenyon. 1978. Aleuts, sea otters, and alternate stable-state communities. Science 200: 403-411.
- ^ Pringle Did Humans Colonize the World by Boat?
- ^ Gutierrez, A., T. Correa, V. Muñoz, A. Santibañez, R. Marcos, C. Cáceres and A.H. Buschmann. 2006. Farming of the giant kelp Macrocystis pyrifera in southern Chile for development of novel food products. Journal of Applied Phycology 18: 259-267.
- ^ Ortiz, M. and W. Stotz. 2007. Ecological and eco-social models for the introduction of the abalone Haliotis discus hannai into benthic systems of north-central Chile: sustainability assessment. Aquatic Conservation: Marine and Freshwater Ecosystems 17: 89-105.
- ^ Scheffer, M., S. Carpenter, J.A. Foley, C. Folke and B. Walter. 2001. Catastrophic shifts in ecosystems. Nature 413: 591-596.
- ^ MacDonald, Lucy (2019-02-06). "95pc of Tasmania's giant kelp is gone, scientists are in a race to save what's left". ABC News. Retrieved 2020-02-09.
- ^ Frost, T.M., S.R. Carpenter, A.R. Ives, and T.K. Kratz. 1995. "Species compensation and complementarity in ecosystem function." In: C. Jones and J. Lawton, editors. Linking species and ecosystems. Chapman and Hall, London. 387pp.
- ^ Tilman, D., C.L. Lehman, and C.E. Bristow. 1998. Diversity-stability relationships: statistical inevitability or ecological consequence? The American Naturalist 151: 277-282.
- ^ Stachowicz, J.J., R.B. Whitlatch and R.W. Osman. 1999. Species diversity and invasion resistance in a marine ecosystem. Science 286: 1577-1579.
- ^ Elmqvist, T., C. Folke, M. Nyström, G. Peterson, J. Bengtsson, B. Walker and J. Norberg. 2003. Response diversity, ecosystem change and resilience. Frontiers in Ecology and the Environment 1: 488-494.
- ^ Stekoll, M.S., L.E. Deysher and M. Hess. 2006. A remote sensing approach to estimating harvestable kelp biomass. Journal of Applied Phycology 18: 323-334.
- ^ Allison, G.A., J. Lubchenco and M.H. Carr. 1998. Marine reserves are necessary but not sufficient for marine conservation. Ecological Applications 8: S79-S92.
- ^ Airamé, S., J.E. Dugan, K.D. Lafferty, H. Leslie, D.A. MacArdle and R.R. Warner. 2003. Applying ecological criteria to marine reserve design: a case study from the California Channel Islands. Ecological Applications 13: S170-S184.
- ^ Bohnsack, J.A. 1998. Application of marine reserves to reef fisheries management. Australian Journal of Ecology 23: 298-304.
- ^ Gell, F.R. and C.M. Roberts. 2003. Benefits beyond boundaries: the fishery effects of marine reserves. Trends in Ecology and Evolution 18: 448-455.
- ^ Willis, T.J., R.B. Millar and R.C. Babcock. 2003. Protection of exploited fish in temperate regions: high density and biomass of snapper Pagrus auratus (Sparidae) in northern New Zealand marine reserves. Journal of Applied Ecology 40: 214-227.
- ^ Paddack, M.J. and J.A. Estes. 2000. Kelp forest fish populations in marine reserves and adjacent exploited areas of Central California. Ecological Applications 10: 855-870.
- ^ Rogers-Bennett, L. and J.S. Pearse. 2001. Indirect benefits of marine protected areas for juvenile abalone. Conservation Biology 15: 642-647.
- ^ Babcock, R.C., S. Kelly, N.T. Shears, J.W. Walker and T.J. Willis. 1999. Changes in community structure in temperate marine reserves. Marine Ecology Progress Series 189: 125-134.
- ^ Halpern, B.S. and R.R. Warner. 2002. Marine reserves have rapid and lasting effects. Ecology Letters 5: 361-366.
External links
- "Kelp Forest & Rocky Subtidal Habitats". noaa.gov. Archived from the original on 2007-03-22.
- "Kelp Watch". tas.gov.au. Tasmania, Australia: Department of Primary Industries, Water & Environment. Archived from the original on 2004-12-04. Excellent general information on kelp forests, as well as specific information on Tasmanian kelp forests.
- "Monterey Bay Aquarium Kelp Cam". mbayaq.org. Monterey Bay Aquarium. Archived from the original on 1999-11-28. Watch a live feed from the kelp forest exhibit.


