Timiperone
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Tolopelon |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.328 |
| Chemical and physical data | |
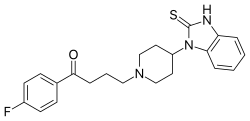
| Formula | C22H24FN3OS |
| Molar mass | 397.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Timiperone, sold under the brand name Tolopelon, is an antipsychotic of the butyrophenone class which is marketed in Japan.[1][2][3] It is similar in chemical structure to benperidol but has a thiourea group instead of a urea group.
References
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1030–. ISBN 978-3-88763-075-1.
- ^ Yamasaki T, Kojima H, Tanaka M, Aibara S, Hirohashi M, Kasai Y, et al. (1981). "Pharmacological studies on timiperone, a new neuroleptic drug Part II: General pharmacological properties". Arzneimittel-Forschung. 31 (4): 707–15. PMID 6113834.
- ^ Tanaka M, Kojima H, Akashi A (November 1985). "Effect of timiperone, a new antipsychotic drug, on the sleep-wakefulness cycle in cats". Japanese Journal of Pharmacology. 39 (3): 391–4. doi:10.1254/jjp.39.391. PMID 2869168.
