Terizidone
Appearance
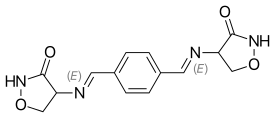 | |
| Clinical data | |
|---|---|
| Other names | 4-[({4-[N-(3-oxo-1,2-oxazolidin-4-yl)carboximidoyl]phenyl}methylidene)amino]-1,2-oxazolidin-3-one |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.882 |
| Chemical and physical data | |
| Formula | C14H14N4O4 |
| Molar mass | 302.290 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Terizidone is a drug used in the treatment of tuberculosis.[1] Terizidone is mainly used in multi-drug-resistant tuberculosis (MDR-TB) in conjunction with other second-line drugs. It is a derivate of cycloserine and it is bacteriostatic.[2]
References
- ^ Galietti F, Giorgis GE, Oliaro A, Boaro D, Ardizzi A, Barberis S, Massaglia GM (1991). "[Tolerability to terizidone (TZ) in the treatment of pulmonary tuberculosis in dialyzed patients]". Minerva Medica. 82 (7–8): 477–81. PMID 1922892.
- ^ Rossiter D (2012). South African Medicines Formulary. Cape Town, South Africa: Health and Medical Publishing Group of the South African Medical Association. pp. 323–325. ISBN 978-1-875098-28-6.
