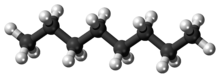
Octane
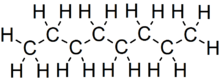
| |
| |
| |
| Names | |
|---|---|
| IUPAC name
Octane[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1696875 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.539 |
| EC Number |
|
| 82412 | |
| KEGG | |
| MeSH | octane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1262 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H18 | |
| Molar mass | 114.232 g·mol−1 |
| Appearance | colourless liquid |
| Odor | Gasoline-like[2] |
| Density | 0.703 g cm−3 |
| Melting point | −57.1 to −56.6 °C; −70.9 to −69.8 °F; 216.0 to 216.6 K |
| Boiling point | 125.1 to 126.1 °C; 257.1 to 258.9 °F; 398.2 to 399.2 K |
| 0.007 mg dm−3 (at 20 °C) | |
| log P | 4.783 |
| Vapor pressure | 1.47 kPa (at 20.0 °C) |
Henry's law
constant (kH) |
29 nmol Pa−1 kg−1 |
| Conjugate acid | Octonium |
| -96.63·10−6 cm3/mol | |
Refractive index (nD)
|
1.398 |
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C)
|
255.68 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
361.20 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−252.1–−248.5 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−5.53–−5.33 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | 13.0 °C (55.4 °F; 286.1 K) |
| 220.0 °C (428.0 °F; 493.1 K) | |
| Explosive limits | 0.96–6.5% |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
428 mg/kg (mouse, intravenous)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 500 ppm (2350 mg/m3)[2] |
REL (Recommended)
|
TWA 75 ppm (350 mg/m3) C 385 ppm (1800 mg/m3) [15-minute][2] |
IDLH (Immediate danger)
|
1000 ppm[2] |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Octane is a hydrocarbon and an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. One of these isomers, 2,2,4-trimethylpentane (commonly called iso-octane) is used as one of the standard values in the octane rating scale.
Octane is a component of gasoline (petrol). As with all low-molecular-weight hydrocarbons, octane is volatile and very flammable.
Use of the term in gasoline
"Octane" is colloquially used as a short form of "octane rating," particularly in the expression "high octane". "Octane rating" is an index of a fuel's ability to resist engine knock at high compression, which is a characteristic of octane's branched-chain isomers, especially iso-octane.
The octane rating was originally determined by mixing fuels from only heptane and 2,2,4-trimethylpentane (a highly branched octane), and assigning anti-knock ratings of zero for pure heptane and 100 for pure 2,2,4-trimethylpentane. The anti-knock rating of this mixture would be the same as the percentage of the latter in the mix. Different isomers of octane can contribute to a lower or higher octane rating. For example, n-octane (the straight chain of 8 carbon atoms with no branching) has a -20 (negative) Research Octane Rating, whereas pure 2,2,4-trimethylpentane has an RON rating of 100. Some fuels have an octane rating higher than 100, notably those containing methanol or ethanol.
Metaphorical use
Octane became well known in American popular culture in the mid- and late 1960s, when gasoline companies boasted of "high octane" levels in their gasoline advertisements.
The compound adjective "high-octane", meaning powerful or dynamic, is recorded in a figurative sense from 1944.[5] By the mid-1990s, the phrase was commonly being used as an intensifier and has found a place in modern English vernacular.
Isomers
Octane has 18 structural isomers (24 including stereoisomers):
- Octane (n-octane)
- 2-Methylheptane
- 3-Methylheptane (2 enantiomers)
- 4-Methylheptane
- 3-Ethylhexane
- 2,2-Dimethylhexane
- 2,3-Dimethylhexane (2 enantiomers)
- 2,4-Dimethylhexane (2 enantiomers)
- 2,5-Dimethylhexane
- 3,3-Dimethylhexane
- 3,4-Dimethylhexane (2 enantiomers + 1 meso compound)
- 3-Ethyl-2-methylpentane
- 3-Ethyl-3-methylpentane
- 2,2,3-Trimethylpentane (2 enantiomers)
- 2,2,4-Trimethylpentane (isooctane)
- 2,3,3-Trimethylpentane
- 2,3,4-Trimethylpentane
- 2,2,3,3-Tetramethylbutane
References
- ^ "octane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 6 January 2012.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0470". National Institute for Occupational Safety and Health (NIOSH).
- ^ Dymond, J. H.; Oye, H. A. (1994). "Viscosity of Selected Liquid n‐Alkanehhhh s". Journal of Physical and Chemical Reference Data. 23 (1): 41–53. doi:10.1063/1.555943. ISSN 0047-2689.
- ^ "Octane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Oxford English Dictionary.
External links
- International Chemical Safety Card 0933
- NIOSH Pocket Guide to Chemical Hazards. "#0470". National Institute for Occupational Safety and Health (NIOSH).
- Dr. Duke's Phytochemical and Ethnobotanical Databases, Octane, http://www.ars-grin.gov/cgi-bin/duke/chemical.pl?OCTANE[permanent dead link]

