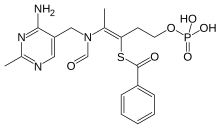
Benfotiamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Milgamma |
| Other names | S-Benzoylthiamine O-monophosphate |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.906 |
| Chemical and physical data | |
| Formula | C19H23N4O6PS |
| Molar mass | 466.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benfotiamine (rINN, or S-benzoylthiamine O-monophosphate) is a synthetic, fat-soluble, S-acyl derivative of thiamine (vitamin B1) that is approved in some countries as a medication or dietary supplement to treat diabetic sensorimotor polyneuropathy. Benfotiamine was developed in late 1950s in Japan.[1][2]
Uses
[edit]Benfotiamine is primarily marketed as an over-the-counter drug to treat diabetic polyneuropathy.[3] A 2021 review described two clinical trials with positive results for diabetic polyneuropathy and concluded that more research is needed.[4]
As of 2017, benfotiamine was marketed as a pharmaceutical drug in many countries under the following brand names: Benalgis, Benfogamma, Benforce, Benfotiamina, Biotamin, Biotowa, Milgamma, and Vilotram.[5] It was also marketed in some jurisdictions as a combination drug with cyanocobalamin as Milgamma, in combination with pyridoxine as Milgamma, in combination with metformin as Benforce-M, and with thiamine as Vitafos.[5]
Adverse effects
[edit]There is little published data on adverse effects. In one study of a combination of benfotiamine, pyridoxine, and cyanocobalamin, around 8% of people taking the drug experienced nausea, dizziness, stomach ache and weight gain.[6]
Pharmacology
[edit]Benfotiamine is dephosphorylated to S-benzoylthiamine by ecto-alkaline phosphatases present in the intestinal mucosa, and is then hydrolyzed to thiamine by thioesterases in the liver.[7] Benfotiamine is more bioavailable than thiamine salts,[8] providing higher levels of thiamine in muscle, brain, liver, and kidney.[6]
Benfotiamine mainly acts on peripheral tissues through an increase in transketolase activity.[7][6][9]
Chemistry
[edit]Benfotiamine is a lipid derivative of thiamine, specifically a synthetic S-acyl Vitamin B1 analogue; its chemical name is S-benzoylthiamine O-monophosphate.[10] It has very low solubility in water or other aqueous solvents.[7]
Research
[edit]Benfotiamine has been studied in laboratory models of diabetic retinopathy, neuropathy, and nephropathy.[10] A 2021 review of its use for diabetic polyneuropathy described two clinical trials which showed improvements in neuropathic pain and neuropathic symptoms scores, the latter of which showed a dose-response effect.[4] The authors concluded that it could potentially serve as an economical supplement to enhance neuropathy treatment and that more research is needed.
Administration of benfotiamine may increase intracellular levels of thiamine diphosphate, a cofactor of transketolase.[10] Based on metabolic theories of Alzheimer's disease, since thiamine-dependent processes are critical in glucose metabolism and are diminished in brains of Alzheimer's disease patients at autopsy, and since treatment of mouse models of Alzheimer's disease with benfotiamine diminishes plaques, decreases phosphorylation of tau and reverses memory deficits, benfotiamine administration has been proposed as a possible intervention to reverse biological and clinical processes of Alzheimer's disease.[11]
See also
[edit]References
[edit]- ^ Wada T, Takagi H, Minakami H, Hamanaka W, Okamoto K, Ito A, Sahashi Y (July 1961). "A new thiamine derivative, S-benzoylthiamine O-monophosphate". Science. 134 (3473): 195–196. Bibcode:1961Sci...134..195W. doi:10.1126/science.134.3473.195. PMID 13782394. S2CID 10384617.
- ^ Sambon M, Wins P, Bettendorff L (May 2021). "Neuroprotective Effects of Thiamine and Precursors with Higher Bioavailability: Focus on Benfotiamine and Dibenzoylthiamine". International Journal of Molecular Sciences. 22 (11): 5418. doi:10.3390/ijms22115418. PMC 8196556. PMID 34063830.
- ^ McCarty MF, Inoguchi T (2008). "11. Targeting Oxidant Stress as a Strategy for Preventing Vascular Complications of Diabetes and Metabolic Syndrome". In Pasupuleti VK, Anderson JW (eds.). Nutraceuticals, glycemic health and type 2 diabetes (1st ed.). Ames, Iowa: Wiley-Blackwell/IFT Press. p. 213. ISBN 9780813804286.
- ^ a b Zaheer A, Zaheer F, Saeed H, Tahir Z, Tahir MW (April 2021). "A Review of Alternative Treatment Options in Diabetic Polyneuropathy". Cureus. 13 (4): e14600. doi:10.7759/cureus.14600. PMC 8139599. PMID 34040901.
- ^ a b "Benfotiamine International brands". Drugs.com. Retrieved 14 March 2017.
- ^ a b c Panel on Food Additives and Nutrient Sources added to Food (2008). "Scientific Opinion: Benfotiamine, thiamine monophosphate chloride and thiamine pyrophosphate chloride, as sources of vitamin B1 added for nutritional purposes to food supplements" (PDF). The EFSA Journal. 864: 1–31.
- ^ a b c Patel SM, Patel RP, Prajapati BG (March 2012). Patel S (ed.). "Solubility enhancement of benfotiamine, a lipid derivative of thiamine by solid dispersion technique". Journal of Pharmacy & Bioallied Sciences. 4 (Suppl 1). J Pharm Bioallied Sci.: S104–S105. doi:10.4103/0975-7406.94157. PMC 3467834. PMID 23066179.
- ^ Bitsch R, Wolf M, Möller J, Heuzeroth L, Grüneklee D (1991). "Bioavailability assessment of the lipophilic benfotiamine as compared to a water-soluble thiamin derivative". Annals of Nutrition & Metabolism. 35 (5): 292–296. doi:10.1159/000177659. PMID 1776825.
- ^ Yamazaki M (1968). "Studies on the absorption of S-benzoylthiamine O-monophosphate : (I) Metabolism in tissue homogenates". Vitamins. 38 (1): 12–20.
- ^ a b c Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A (June 2010). "The multifaceted therapeutic potential of benfotiamine". Pharmacological Research. 61 (6): 482–488. doi:10.1016/j.phrs.2010.02.008. PMID 20188835.
- ^ Gibson GE, Hirsch JA, Cirio RT, Jordan BD, Fonzetti P, Elder J (July 2013). "Abnormal thiamine-dependent processes in Alzheimer's Disease. Lessons from diabetes". Molecular and Cellular Neurosciences. 55: 17–25. doi:10.1016/j.mcn.2012.09.001. PMC 3609887. PMID 22982063.
