Hepatitis B: Difference between revisions
m Reverted edits by 142.227.117.125 to last version by Mdebets (using Huggle) |
|||
| Line 17: | Line 17: | ||
Hepatitis B virus infection may either be acute (self-limiting) or chronic (long-standing). Persons with self-limiting infection clear the infection spontaneously within weeks to months. |
Hepatitis B virus infection may either be acute (self-limiting) or chronic (long-standing). Persons with self-limiting infection clear the infection spontaneously within weeks to months. |
||
Children are |
Children are afriad of me because i rape them at night gigady. likely than adults to clear the infection. More than 95% of people who become infected as adults or older children will stage a full recovery and develop protective immunity to the virus. However, only 5% of newborns that acquire the infection from their mother at birth will clear the infection. Of those infected between the age of one to six, 70% will clear the infection.<ref name="pmid16176431">{{cite journal |author=Kerkar N |title=Hepatitis B in children: complexities in management |journal=Pediatric transplantation |volume=9 |issue=5 |pages=685–91 |year=2005 |pmid=16176431 |doi=10.1111/j.1399-3046.2005.00393.x}}</ref> |
||
Acute infection with hepatitis B virus is associated with acute viral [[hepatitis]] - an illness that begins with general ill-health, loss of appetite, nausea, vomiting, body aches, mild fever, dark urine, and then progresses to development of [[jaundice]]. It has been noted that itchy skin has been an indication as a possible symptom of all hepatitis virus types. The illness lasts for a few weeks and then gradually improves in most affected people. A few patients may have more severe liver disease ([[fulminant hepatic failure]]), and may die as a result of it. The infection may be entirely asymptomatic and may go unrecognized. |
Acute infection with hepatitis B virus is associated with acute viral [[hepatitis]] - an illness that begins with general ill-health, loss of appetite, nausea, vomiting, body aches, mild fever, dark urine, and then progresses to development of [[jaundice]]. It has been noted that itchy skin has been an indication as a possible symptom of all hepatitis virus types. The illness lasts for a few weeks and then gradually improves in most affected people. A few patients may have more severe liver disease ([[fulminant hepatic failure]]), and may die as a result of it. The infection may be entirely asymptomatic and may go unrecognized. |
||
Revision as of 16:28, 9 June 2008
| Hepatitis B virus | |
|---|---|

| |
| Micrograph showing hepatitis B virions | |
| Virus classification | |
| Group: | Group VII (dsDNA-RT)
|
| Family: | |
| Genus: | |
| Species: | Hepatitis B virus
|
Hepatitis B virus infects the liver of hominoidae, including humans, and causes an inflammation called hepatitis. It is a DNA virus and one of many unrelated viruses that cause viral hepatitis. The disease was originally known as "serum hepatitis"[1]and has caused epidemics in parts of Asia and Africa. Hepatitis B is endemic in China and various other parts of Asia.[2] The proportion of the world's population currently infected with the virus is estimated at 3 to 6%, but up to a third have been exposed. Symptoms of the acute illness caused by the virus include liver inflammation, vomiting, jaundice, and rarely, death. Chronic hepatitis B may eventually cause liver cirrhosis and liver cancer, a fatal disease with very poor response to current chemotherapy.[3] The infection is preventable by vaccination.[4]
Symptoms and complications
Hepatitis B virus infection may either be acute (self-limiting) or chronic (long-standing). Persons with self-limiting infection clear the infection spontaneously within weeks to months.
Children are afriad of me because i rape them at night gigady. likely than adults to clear the infection. More than 95% of people who become infected as adults or older children will stage a full recovery and develop protective immunity to the virus. However, only 5% of newborns that acquire the infection from their mother at birth will clear the infection. Of those infected between the age of one to six, 70% will clear the infection.[5]
Acute infection with hepatitis B virus is associated with acute viral hepatitis - an illness that begins with general ill-health, loss of appetite, nausea, vomiting, body aches, mild fever, dark urine, and then progresses to development of jaundice. It has been noted that itchy skin has been an indication as a possible symptom of all hepatitis virus types. The illness lasts for a few weeks and then gradually improves in most affected people. A few patients may have more severe liver disease (fulminant hepatic failure), and may die as a result of it. The infection may be entirely asymptomatic and may go unrecognized.
Chronic infection with Hepatitis B virus may be either asymptomatic or may be associated with a chronic inflammation of the liver (chronic hepatitis), leading to cirrhosis over a period of several years. This type of infection dramatically increases the incidence of hepatocellular carcinoma (liver cancer). Chronic carriers are encouraged to avoid consuming alcohol as it increases their risk for cirrhosis and liver cancer. Hepatitis B virus has been linked to the development of Membranous glomerulonephritis (MGN).[6]
Hepatitis D infection can only occur with a concomitant infection with Hepatitis B virus because the Hepatitis D virus uses the Hepatitis B virus surface antigen to form a capsid.[7] Co-infection with hepatitis D increases the risk of liver cirrhosis and liver cancer.[8] Polyarteritis nodosa is more common in people with hepatitis B infection.
Diagnosis


The tests, called assays, for detection of hepatitis B virus infection involve serum or blood tests that detect either viral antigens (proteins produced by the virus) or antibodies produced by the host. Interpretation of these assays is complex.[9]
The hepatitis B surface antigen (HBsAg) is most frequently used to screen for the presence of this infection. It is the first detectable viral antigen to appear during infection. However, early in an infection, this antigen may not be present and it may be undetectable later in the infection as it is being cleared by the host. The infectious virion contains an inner "core particle" enclosing viral genome. The icosahedral core particle is made of 180 or 240 copies of core protein, alternatively known as hepatitis B core antigen, or HBcAg. During this 'window' in which the host remains infected but is successfully clearing the virus, IgM antibodies to the hepatitis B core antigen (anti-HBc IgM) may be the only serological evidence of disease.
Shortly after the appearance of the HBsAg, another antigen named as the hepatitis B e antigen (HBeAg) will appear. Traditionally, the presence of HBeAg in a host's serum is associated with much higher rates of viral replication and enhanced infectivity; however, variants of the hepatitis B virus do not produce the 'e' antigen, so this rule does not always hold true. During the natural course of an infection, the HBeAg may be cleared, and antibodies to the 'e' antigen (anti-HBe) will arise immediately afterwards. This conversion is usually associated with a dramatic decline in viral replication.
If the host is able to clear the infection, eventually the HBsAg will become undetectable and will be followed by IgG antibodies to the hepatitis B surface antigen and core antigen, (anti-HBs and anti HBc IgG).[10] A person negative for HBsAg but positive for anti-HBs has either cleared an infection or has been vaccinated previously.
Individuals who remain HBsAg positive for at least six months are considered to be hepatitis B carriers.[11] Carriers of the virus may have chronic hepatitis B, which would be reflected by elevated serum alanine aminotransferase levels and inflammation of the liver, as revealed by biopsy. Carriers who have seroconverted to HBeAg negative status, particularly those who acquired the infection as adults, have very little viral multiplication and hence may be at little risk of long-term complications or of transmitting infection to others.[12]
More recently, PCR tests have been developed to detect and measure the amount of viral nucleic acid in clinical specimens. These tests are called viral loads and are used to assess a person's infection status and to monitor treatment.[13]
Transmission
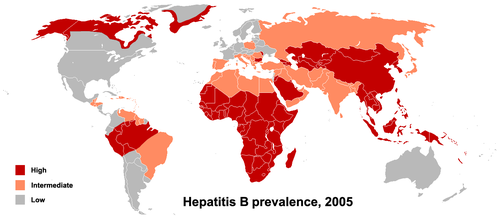
Transmission results from exposure to infectious blood or body fluids containing blood. Possible forms of transmission include (but are not limited to) unprotected sexual contact, blood transfusions, re-use of contaminated needles & syringes, and vertical transmission from mother to child during childbirth. Without intervention, a mother who is positive for the hepatitis B surface antigen confers a 20% risk of passing the infection to her offspring at the time of birth. This risk is as high as 90% if the mother is also positive for the hepatitis B e antigen. HBV can be transmitted between family members within households, possibly by contact of nonintact skin or mucous membrane with secretions or saliva containing HBV.[14] However, at least 30% of reported hepatitis B among adults cannot be associated with an identifiable risk factor.[15]
The primary method of transmission reflects the prevalence of chronic HBV infection in a given area. In low prevalence areas such as the continental United States and Western Europe, where less than 2% of the population is chronically infected, injection drug abuse and unprotected sex are the primary methods, although other factors may be important.[16] In moderate prevalence areas, which include Eastern Europe, Russia, and Japan, where 2-7% of the population is chronically infected, the disease is predominantly spread among children. In high prevalence areas such as China and South East Asia, transmission during childbirth is most common, although in other areas of high endemicity such as Africa, transmission during childhood is a significant factor.[17] The prevalence of chronic HBV infection in areas of high endemicity is at least 8%.
Pathogenesis
The hepatitis B virus primarily interferes with the functions of the liver by replicating in liver cells, known as hepatocytes. During HBV infection, the host immune response causes both hepatocellular damage and viral clearance. Although the innate immune response does not play a significant role in these processes, the adaptive immune response, particularly virus-specific cytotoxic T lymphocytes (CTLs), contributes to most of the liver injury associated with HBV infection. By killing infected cells and by producing antiviral cytokines capable of purging HBV from viable hepatocytes, CTLs eliminate the virus.[18] Although liver damage is initiated and mediated by the CTLs, antigen-nonspecific inflammatory cells can worsen CTL-induced immunopathology, and platelets activated at the site of infection may facilitate the accumulation of CTLs into the liver.[19]
Reactivation
Hepatitis B virus DNA persists in the body after infection and in some people the disease re-occurs.[20] Although rare, reactivation is seen most often in people with impaired immunity.[21]
Treatment
Acute hepatitis B infection does not usually require treatment because most adults clear the infection spontaneously.[22] Early antiviral treatment may only be required in fewer than 1% of patients, whose infection takes a very aggressive course ("fulminant hepatitis") or who are immunocompromised. On the other hand, treatment of chronic infection may be necessary to reduce the risk of cirrhosis and liver cancer. Chronically infected individuals with persistently elevated serum alanine aminotransferase, a marker of liver damage, and HBV DNA levels are candidates for therapy.[23]
Although none of the available drugs can clear the infection, they can stop the virus from replicating, and prevent liver damage such as cirrhosis and liver cancer. Treatments include antiviral drugs such as lamivudine, adefovir and entecavir, and immune system modulators such as interferon alpha. However, some individuals are much more likely to respond than others and this might be because of the genotype of the infecting virus or the patient's heredity. The treatment works by reducing the viral load, (the amount of virus particles as measured in the blood), which in turn reduces viral replication in the liver.
On March 29, 2005, the US Food and Drug Administration (FDA) approved Entecavir for the treatment of Hepatitis B.[24] On February 25, 2005, the EU Commission approved Peginterferon alfa-2a (Pegasys).[25][26] On October 27, 2006, telbivudine gained FDA approval. It is marketed under the brand name Tyzeka in the US and Sebivo outside the US. It is approved in Switzerland.[27]
Infants born to mothers known to carry hepatitis B can be treated with antibodies to the hepatitis B virus (hepatitis B immune globulin or HBIg). When given with the vaccine within twelve hours of birth, the risk of acquiring hepatitis B is reduced 95%. This treatment allows a mother to safely breastfeed her child.
Vaccination

Several vaccines have been developed for the prevention of hepatitis B virus infection. These rely on the use of one of the viral envelope proteins (hepatitis B surface antigen or HBsAg). The vaccine was originally prepared from plasma obtained from patients who had long-standing hepatitis B virus infection. However, currently, these are more often made using recombinant DNA technology, though plasma-derived vaccines continue to be used; the two types of vaccines are equally effective and safe.[28]
Following vaccination Hepatitis B Surface antigen may be detected in serum for several days; this is known as vaccine antigenaemia.[29]
Microbiology
Structure

Hepatitis B virus (HBV) is a member of the Hepadnavirus family.[10] The virus particle, (virion) consists of an outer lipid envelope and an icosahedral nucleocapsid core composed of protein. The nucleocapsid encloses the viral DNA and a DNA polymerase that has reverse transcriptase activity.[30] The outer envelope contains embedded proteins which are involved in viral binding of, and entry into, susceptible cells. The virus is one of the smallest enveloped animal viruses with a virion diameter of 42nm, but pleomorphic forms exist, including filamentous and spherical bodies lacking a core. These particles are not infectious and are composed of the lipid and protein that forms part of the surface of the virion, which is called the surface antigen (HBsAg), and is produced in excess during the life cycle of the virus.[31]
Genome

The genome of HBV is made of circular DNA, but it is unusual because the DNA is not fully double-stranded. One end of the full length strand is linked to the viral DNA polymerase. The genome is 3020-3320 nucleotides long (for the full length strand) and 1700-2800 nucleotides long (for the short length strand).[32] The negative-sense, (non-coding), is complementary to the viral mRNA. The viral DNA is found in the nucleus soon after infection of the cell. The partially double-stranded DNA is rendered fully double-stranded by completion of the (+) sense strand and removal of a protein molecule from the (-) sense strand and a short sequence of RNA from the (+) sense strand. Non-coding bases are removed from the ends of the (-)sense strand and the ends are rejoined. There are four known genes encoded by the genome called C, X, P, and S. The core protein is coded for by gene C (HBcAg), and its start codon is preceded by an upstream in-frame AUG start codon from which the pre-core protein is produced. HBeAg is produced by proteolytic processing of the pre-core protein. The DNA polymerase is encoded by gene P. Gene S is the gene that codes for the surface antigen (HBsAg). The HBsAg gene is one long open reading frame but contains three in frame "start" (ATG) codons that divide the gene into three sections, pre-S1, pre-S2, and S. Because of the multiple start codons, polypeptides of three different sizes called large, middle, and small (pre-S1 + pre-S2 + S, pre-S2 + S, or S) are produced.[33] The function of the protein coded for by gene X is not fully understood.[34]
Replication

The life cycle of Hepatitis B virus is complex. Hepatitis B is one of a few known non-retroviral viruses which use reverse transcription as a part of its replication process. The virus gains entry into the cell by binding to a receptor on the surface of the cell and enters it by endocytosis. Because the virus multiplies via RNA made by a host enzyme, the viral genomic DNA has to be transferred to the cell nucleus by host proteins called chaperones. The partially double stranded viral DNA is then made fully double stranded and transformed into closed circular supercoiled DNA (cccDNA) that serves as a template for transcription of four viral mRNAs. The largest mRNA, (which is longer than the viral genome), is used to make the new copies of the genome and to make the capsid core protein and the viral DNA polymerase. These four viral transcripts undergo additional processing and go on to form progeny virions which are released from the cell or returned to the nucleus and re-cycled to produce even more copies.[33][35] The long mRNA is then transported back to the cytoplasm where the virion P protein synthesizes DNA via its reverse transcriptase activity.
Serotypes
The virus is divided into four major serotypes (adr, adw, ayr, ayw) based on antigenic epitopes present on its envelope proteins, and into eight genotypes (A-H) according to overall nucleotide sequence variation of the genome. The genotypes have a distinct geographical distribution and are used in tracing the evolution and transmission of the virus. Differences between genotypes affect the disease severity, course and likelihood of complications, and response to treatment and possibly vaccination.[36][37]
History
The earliest record of an epidemic caused by Hepatitis B virus was made by Lurman in 1885.[38] An outbreak of smallpox occurred in Bremen in 1883 and 1,289 shipyard employees were vaccinated with lymph from other people. After several weeks, and up to eight months later, 191 of the vaccinated workers became ill with jaundice and were diagnosed as suffering from serum hepatitis. Other employees who had been inoculated with different batches of lymph remained healthy. Lurman's paper, now regarded as a classical example of an epidemiological study, proved that contaminated lymph was the source of the outbreak. Later, numerous similar outbreaks were reported following the introduction, in 1909, of hypodermic needles that were used, and more importantly reused, for administering Salvarsan for the treatment of syphilis. The virus was not discovered until 1965 when Baruch Blumberg, then working at the National Institutes of Health (NIH), discovered the Australia antigen (later known to be Hepatitis B surface antigen, or HBsAg) in the blood of Australian aboriginal people.[39] Although a virus had been suspected since the research published by MacCallum in 1947.[40] In 1970, D.S. Dane and others discovered the virus particle by electron microscopy.[41] By the early 1980s the genome of the virus had been sequenced,[42] and the first vaccines were being tested.[43]
See also
- Hepatitis A
- Hepatitis B in China
- Hepatitis C
- Hepatitis D
- Hepatitis E
- Hepatitis F
- Hepatitis G
- Jade Ribbon Campaign
- Maurice Hilleman
- Baruch S. Blumberg
References
- ^ Barker LF, Shulman NR, Murray R; et al. (1996). "Transmission of serum hepatitis. 1970". JAMA. 276 (10): 841–4. PMID 8769597.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Williams R (2006). "Global challenges in liver disease". Hepatology. 44 (3): 521–6. doi:10.1002/hep.21347. PMID 16941687.
- ^ Chang MH (2007). "Hepatitis B virus infection". Semin Fetal Neonatal Med. 12 (3): 160–7. doi:10.1016/j.siny.2007.01.013. PMID 17336170.
- ^ Pungpapong S, Kim WR, Poterucha JJ (2007). "Natural history of hepatitis B virus infection: an update for clinicians". Mayo Clin. Proc. 82 (8): 967–75. PMID 17673066.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kerkar N (2005). "Hepatitis B in children: complexities in management". Pediatric transplantation. 9 (5): 685–91. doi:10.1111/j.1399-3046.2005.00393.x. PMID 16176431.
- ^ Lai KN, Li PK, Lui SF; et al. (1991). "Membranous nephropathy related to hepatitis B virus in adults". N. Engl. J. Med. 324 (21): 1457–63. PMID 2023605.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Taylor JM (2006). "Hepatitis delta virus". Virology. 344 (1): 71–6. doi:10.1016/j.virol.2005.09.033. PMID 16364738.
- ^ Oliveri F, Brunetto MR, Actis GC, Bonino F (1991). "Pathobiology of chronic hepatitis virus infection and hepatocellular carcinoma (HCC)". The Italian journal of gastroenterology. 23 (8): 498–502. PMID 1661197.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bonino F, Chiaberge E, Maran E, Piantino P (1987). "Serological markers of HBV infectivity". Ann. Ist. Super. Sanita. 24 (2): 217–23. PMID 3331068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Zuckerman AJ (1996). Hepatitis Viruses. In: Baron's Medical Microbiology (Baron S et al, eds.) (4th ed. ed.). Univ of Texas Medical Branch. ISBN 0-9631172-1-1.
{{cite book}}:|edition=has extra text (help) - ^ Lok AS, McMahon BJ (2007). "Chronic hepatitis B". Hepatology. 45 (2): 507–39. doi:10.1002/hep.21513. PMID 17256718.
- ^ Chu CM, Liaw YF (2007). "Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B". Gastroenterology. 133 (5): 1458–65. doi:10.1053/j.gastro.2007.08.039. PMID 17935720.
- ^ Zoulim F (2006). "New nucleic acid diagnostic tests in viral hepatitis". Semin. Liver Dis. 26 (4): 309–17. doi:10.1055/s-2006-951602. PMID 17051445.
- ^ Petersen NJ, Barrett DH, Bond WW, Berquist KR, Favero MS, Bender TR, Maynard JE (1976). "Hepatitis B surface antigen in saliva, impetiginous lesions, and the environment in two remote Alaskan villages". Appl. Environ. Microbiol. 32 (4): 572–574. PMID 791124.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shapiro CN (1993). "Epidemiology of hepatitis B". Pediatr. Infect. Dis. J. 12 (5): 433–7. PMID 8392167.
- ^ Redd JT, Baumbach J, Kohn W; et al. (2007). "Patient-to-patient transmission of hepatitis B virus associated with oral surgery" (PDF). J Infect Dis. 195 (9): 1311–4. Retrieved 2007-12-12.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Alter MJ (2003). "Epidemiology and prevention of hepatitis B". Semin. Liver Dis. 23 (1): 39–46. doi:10.1055/s-2003-37583. PMID 12616449.
- ^ Iannacone M, Sitia G, Ruggeri ZM, Guidotti LG (2007). "HBV pathogenesis in animal models: recent advances on the role of platelets". J. Hepatol. 46 (4): 719–26. doi:10.1016/j.jhep.2007.01.007. PMID 17316876.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Iannacone M, Sitia G, Isogawa M, Marchese P, Castro M, Lowenstein P, Chisari F, Ruggeri Z, Guidotti L (2005). "Platelets mediate cytotoxic T lymphocyte-induced liver damage". Nature Medicine. 11 (11): 1167–1169. PMID 16258538.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vierling JM (2007). "The immunology of hepatitis B". Clin Liver Dis. 11 (4): 727–59, vii–viii. doi:10.1016/j.cld.2007.08.001. PMID 17981227.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Katz LH, Fraser A, Gafter-Gvili A, Leibovici L, Tur-Kaspa R (2008). "Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis". J. Viral Hepat. 15 (2): 89–102. doi:10.1111/j.1365-2893.2007.00902.x. PMID 18184191.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hollinger FB, Lau DT (2006). "Hepatitis B: the pathway to recovery through treatment". Gastroenterol. Clin. North Am. 35 (4): 895–931. doi:10.1016/j.gtc.2006.10.002. PMID 17129820.
- ^ Lai CL, Yuen MF (2007). "The natural history and treatment of chronic hepatitis B: a critical evaluation of standard treatment criteria and end points". Ann. Intern. Med. 147 (1): 58–61. PMID 17606962.
- ^ U.S. Food and Drug Administration. March 30, 2005. FDA Talk Paper: FDA Approves New Treatment for Chronic Hepatitis B. fda.gov. Retrieved on September 11, 2007.
- ^ Hui CK, Lau GK (2005). "Peginterferon-alpha2a (40 kDa) (Pegasys) for hepatitis B". Expert review of anti-infective therapy. 3 (4): 495–504. doi:10.1586/14787210.3.4.495. PMID 16107195.
- ^ February 25, 2005. [http://www.roche.com/med-cor-2005-02-25 Pegasys approved in the European Union for the treatment of chronic hepatitis B: Only pegylated interferon approved for the treatment of chronic hepatitis B Retrieved on September 11, 2007.
- ^ October 27, 2006. FDA Approves Telbivudine for Treatment of Chronic Hepatitis B. hivandhepatitis.com. Retrieved on September 11, 2007.
- ^ Zuckerman JN (2006). "Vaccination against hepatitis A and B: developments, deployment and delusions". Curr. Opin. Infect. Dis. 19 (5): 456–9. doi:10.1097/01.qco.0000244051.23511.09. PMID 16940869.
- ^ Martín-Ancel A, Casas ML, Bonet B (2004). "Implications of postvaccination hepatitis B surface antigenemia in the management of exposures to body fluids". Infect Control Hosp Epidemiol. 25 (7): 611–3. PMID 15301037.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Locarnini S (2004). "Molecular virology of hepatitis B virus". Semin. Liver Dis. 24 Suppl 1: 3–10. doi:10.1055/s-2004-828672. PMID 15192795.
- ^ Howard CR (1986). "The biology of hepadnaviruses". J. Gen. Virol. 67 ( Pt 7): 1215–35. PMID 3014045.
- ^ Kay A, Zoulim F (2007). "Hepatitis B virus genetic variability and evolution". Virus Res. 127 (2): 164–76. PMID 17383765.
- ^ a b Beck J, Nassal M (2007). "Hepatitis B virus replication". World J. Gastroenterol. 13 (1): 48–64. PMID 17206754.
- ^ Bouchard MJ, Schneider RJ (2004). "The enigmatic X gene of hepatitis B virus". J. Virol. 78 (23): 12725–34. doi:10.1128/JVI.78.23.12725-12734.2004. PMID 15542625.
- ^ Bruss V (2007). "Hepatitis B virus morphogenesis". World J. Gastroenterol. 13 (1): 65–73. PMID 17206755.
- ^ Kramvis A, Kew M, François G (2005). "Hepatitis B virus genotypes". Vaccine. 23 (19): 2409–23. doi:10.1016/j.vaccine.2004.10.045. PMID 15752827.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Magnius LO, Norder H (1995). "Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene". Intervirology. 38 (1–2): 24–34. PMID 8666521.
- ^ Lurman A. (1885) Eine icterus epidemic. (In German). Berl Klin Woschenschr 22:20–3.
- ^ Alter HJ, Blumberg BS (1966). "Further studies on a "new" human isoprecipitin system (Australia antigen)". Blood. 27 (3): 297–309. PMID 5930797.
- ^ MacCallum, F.O., Homologous serum hepatitis. Lancet 2, 691, (1947)
- ^ Dane DS, Cameron CH, Briggs M (1970). "Virus-like particles in serum of patients with Australia-antigen-associated hepatitis". Lancet. 1 (7649): 695–8. PMID 4190997.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P (1979). "Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli". Nature. 281 (5733): 646–50. PMID 399327.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Hepatitis B vaccine". Lancet. 2 (8206): 1229–30. 1980. PMID 6108398.
External links
| Hepatitis B | |
|---|---|
| Specialty | Infectious diseases |
