Isotopes of beryllium
Although beryllium (Be) has 12 known isotopes, only one of these isotopes (9
Be
) is stable and a primordial nuclide. As such, it is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes are short-lived that none are primordial and their abundance is very low. Most 9
Be
in the universe is thought to be formed by cosmic ray nucleosynthesis from cosmic ray spallation in the period between the Big Bang and the formation of the solar system.
Of the 11 known radioisotopes, the most stable are 10Be with a half-life of 1.39 million years and 7Be with a half-life of 53.22 days. All other radioisotopes have half-lives under 1 minute.
Beryllium is unique as being the only monoisotopic element with an even number of protons. It is also the only one with an odd number of neutrons. There are 25 other monoisotopic elements, but all have odd atomic numbers, and even numbers of neutrons.
The natural light-element ratio of equal protons and neutron numbers is prevented in beryllium by the extreme instability of 8
Be
toward double-alpha decay, which is favored due to the extremely tight binding of 4
He
nuclei. The half-life for the decay of 8
Be
is only 6.7(17)×10−17 seconds.
Beryllium is prevented from having a stable isotope with 4 protons and 6 neutrons by the very large mismatch in proton/neutron ratio for such a light element. Nevertheless, this isotope, 10
Be
, has a half-life of 1.39 million years, which indicates unusual stability for a light isotope with such a large neutron/proton imbalance.
Still other possible beryllium isotopes have even more severe mismatches in neutron and proton number, and thus are even less stable.
The isotopes 7
Be
, with a half-life of 53 days, and 10
Be
are both cosmogenic nuclide because they are made on a recent timescale in the solar system by cosmic ray spallations, like 14
C
. These two radioisotopes of beryllium in the atmosphere track the sun spot cycle and solar activity, since this affects the magnetic field that shields the Earth from cosmic rays. The rate at which the short-lived 7
Be
is transferred from the air to the ground is controlled in part by the weather.
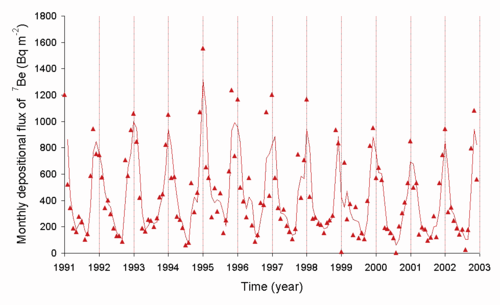
Be
from the air to the ground in Japan (source M. Yamamoto et al., Journal of Environmental Radioactivity, 2006, 86, 110-131)
7
Be
decay in the sun is one of the sources of solar neutrinos, and the first type ever detected using the Homestake experiment.
Beryllium has a standard atomic mass of: 9.012182(3) u
Table
| nuclide symbol |
Z(p) | N(n) | isotopic mass (u) |
half-life | decay mode(s)[1][n 1] |
daughter isotope(s)[n 2] |
nuclear spin |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|
| 5 Be |
4 | 1 | 5.04079(429)# | p | 4 Li |
(1/2+)# | |||
| 6 Be |
4 | 2 | 6.019726(6) | 5.0(3)×10−21 s [0.092(6) MeV] |
2p | 4 He |
0+ | ||
| 7 Be [n 3] |
4 | 3 | 7.01692983(11) | 53.22(6) d | EC | 7 Li |
3/2- | Trace[n 4] | |
| 8 Be [n 5] |
4 | 4 | 8.00530510(4) | 6.7(17)×10−17 s [6.8(17) eV] |
fission | 2 4 He |
0+ | ||
| 9 Be |
4 | 5 | 9.0121822(4) | Stable | 3/2- | 1.0000 | |||
| 10 Be |
4 | 6 | 10.0135338(4) | 1.39×106 a | β− | 10 B |
0+ | Trace[n 4] | |
| 11 Be [n 6] |
4 | 7 | 11.021658(7) | 13.81(8) s | β− (97.1%) | 11 B |
1/2+ | ||
| β−, α (2.9%) | 7 Li | ||||||||
| 12 Be |
4 | 8 | 12.026921(16) | 21.49(3) ms | β− (99.48%) | 12 B |
0+ | ||
| β−, n (0.52%) | 11 B | ||||||||
| 13 Be |
4 | 9 | 13.03569(8) | .5(1) ns | n | 12 Be |
1/2+ | ||
| 14 Be [n 7] |
4 | 10 | 14.04289(14) | 4.84(10) ms | β−, n (81.0%) | 13 B |
0+ | ||
| β− (14.0%) | 14 B | ||||||||
| β−, 2n (5.0%) | 12 B | ||||||||
| 15 Be |
4 | 11 | 15.05346(54)# | <200 ns | |||||
| 16 Be |
4 | 12 | 16.06192(54)# | <200 ns | 0+ | ||||
- ^ Abbreviations:
EC: Electron capture - ^ Bold for stable isotopes
- ^ Produced in Big Bang nucleosynthesis, but not primordial, as it all quickly decayed to 7Li
- ^ a b cosmogenic nuclide
- ^ Intermediate product of triple alpha process in stellar nucleosynthesis as part of the path producing 12C
- ^ Has 1 halo neutron
- ^ Has 4 halo neutrons
Notes
- Values marked # are not purely derived from experimental data, but at least partly from systematic trends. Spins with weak assignment arguments are enclosed in parentheses.
See also
References
- Isotope masses from:
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- Isotopic compositions and standard atomic masses from:
- J. R. de Laeter, J. K. Böhlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - M. E. Wieser (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure and Applied Chemistry. 78 (11): 2051–2066. doi:10.1351/pac200678112051.
{{cite journal}}: Unknown parameter|laysummary=ignored (help)
- J. R. de Laeter, J. K. Böhlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
- Half-life, spin, and isomer data selected from the following sources. See editing notes on this article's talk page.
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - National Nuclear Data Center, Brookhaven National Laboratory. Information extracted from the NuDat 2.1 database (retrieved Sept. 2005).
- N. E. Holden (2004). "Table of the Isotopes". In D. R. Lide (ed.). CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. Section 11. ISBN 978-0849304859.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help)
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
