Mefloquine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lariam, Mephaquin, Mefliam |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Extensive liver; main metabolite is inactive |
| Elimination half-life | 2 to 4 weeks |
| Excretion | Primarily bile and feces; urine (9% as unchanged drug, 4% as primary metabolite) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H16F6N2O |
| Molar mass | 378.312 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Mefloquine, sold under the brand names Lariam among others, is a medication used to prevent or treat malaria.[1] When used for prevention it is taken once a week and should be begun one or two weeks before potential exposure and continued for four weeks after potential exposure. It can be used to treat mild or moderate malaria but should not be used to treat severe malaria. It is taken by mouth.[1]
Serious side effects include potentially long-term mental health problems such as depression, hallucinations, and anxiety and neurological side effects such as poor balance, seizures, and ringing in the ears. It is therefore not recommended in people with a history of mental health problems or epilepsy. Common side effects include vomiting, diarrhea, headaches, and a rash. It is not recommended in pregnancy unless other options are not available. It should not be used during breast feeding.[1]
Mefloquine was developed by the United States Army in the 1970s and came into use in the mid 1980s.[2][3][4] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[5] It is available as a generic medication.[1] The wholesale price in the developing world is about 0.6 to 1.3 USD per dose.[6] In the United States it costs about 10 USD a dose.[1]
Medical uses

Mefloquine is used to both prevent and treat certain forms of malaria.[7]
Malaria prevention
Mefloquine is useful for the prevention of malaria in all areas except for those where parasites may have resistance to multiple drugs,[8] and is one of several anti-malarial drugs recommended by the United States Centers for Disease Control for this purpose. It is also recommended by the Infectious Disease Society of America for malaria prophylaxis as a first or second line agent, depending on resistance patterns in the malaria found in the geographic region visited.[9][10] It is typically taken for one to two weeks before entering an area with malaria.[7] Doxycycline and atovaquone/proguanil provide protection within one to two days and may be better tolerated.[11][12] If a person becomes ill with malaria despite prophylaxis with mefloquine, the use of halofantrine and quinine for treatment may be ineffective.[13]: 4
Malaria treatment
Mefloquine is used as a treatment for chloroquine-sensitive or resistant Plasmodium falciparum malaria, and is deemed a reasonable alternative for uncomplicated chloroquine-resistant Plasmodium vivax malaria.[7][13] It is one of several drugs recommended by the United States Centers for Disease Control [14] It is not recommended for severe malaria infections, particularly infections from P. falciparum, which should be treated with intravenous antimalarials.[7][13] Mefloquine does not eliminate parasites in the liver phase of the disease, and people with P. vivax malaria should be treated with a second drug that is effective for the liver phase, such as primaquine.[13]: 4
Mefloquine is widely used for the treatment of malaria in pregnancy. Limited data suggests that it is safe for this purpose.[15]
Resistance to mefloquine
Resistance to mefloquine is now common around the west border in Cambodia and other parts of Southeast Asia.[16] The mechanism of resistance is by increase in Pfmdr1 copy number.[17]
Adverse effects
Mefloquine is contraindicated in those with a previous history of seizures or a recent history of psychiatric disorders.[7] Severe side effects requiring hospitalization are rare.[8] Compared to other malaria prophylaxis regimens, mefloqinone may produce more side effects in non-pregnant travelers. In pregnant travelers, it appears to pose minimal risk to the fetus. [15][18]
Neurologic and psychiatric
In 2013 the United States Food and Drug Administration added a boxed warning to the U.S. label of mefloquine regarding the potential for neuropsychiatric side effects that may persist even after discontinuing administration of the drug.[19][20] Psychiatric effects include nightmares, visual and auditory hallucinations, anxiety, depression, unusual behavior, and suicidal ideations, among others. Neurologic effects include dizziness, loss of balance, and tinnitus. The label warns that mild symptoms may presage more serious ones, and that the drug should be discontinued at the first sign of symptoms. Mefloquine should not be used in people with a history of psychiatric problems.
Central nervous system events requiring hospitalization occur in about one in 10,000 people taking mefloquine for malaria prevention, with milder events (e.g., dizziness, headache, insomnia, and vivid dreams) in up to 25%.[21] When some measure of subjective severity is applied to the rating of adverse events, about 11-17% of travelers are incapacitated to some degree.[11]
Cardiac
Mefloquine may cause abnormalities with heart rhythms that are visible on electrocardiograms. Combining mefloquine with other drugs that cause similar effects, such as quinine or quinidine, can increase these effects. Combining mefloquine with halofantrine can cause significant increases in QTc intervals.[13]: 10
Contraindications
Mefloquine is contraindicated in those with a previous history of seizures or a recent history of psychiatric disorders.[7] Women should not become pregnant and should use effective birth control while taking mefloquine.
Pregnancy and breastfeeding
A retrospective analysis of outcomes in more than 2,500 women found no evidence that mefloquine was associated with an increased risk of birth defects or miscarriages.[22] The drug may be used during breastfeeding, though it appears in breast milk in low concentrations.[8][13]: 9 The World Health Organization gives approval for the use of mefloquine in the second and third trimesters of pregnancy and use in the first trimester does not mandate termination of pregnancy.[8]
Elimination
Mefloquine is metabolized primarily through the liver. Its elimination in persons with impaired liver function may be prolonged, resulting in higher plasma levels and an increased risk of adverse reactions. The mean elimination plasma half-life of mefloquine is between two and four weeks. Total clearance is through the liver, and the primary means of excretion is through the bile and feces, as opposed to only 4% to 9% excreted through the urine. During long-term use, the plasma half-life remains unchanged.[23][24]
Liver function tests should be performed during long-term administration of mefloquine.[25] Alcohol use should be avoided during treatment with mefloquine.[26]
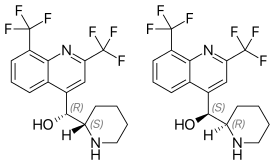
Chirality and structure activity relationships
Mefloquine is a chiral molecule with two asymmetric carbon centres, which means it has four different stereoisomers. The drug is currently manufactured and sold as a racemate of the (R,S)- and (S,R)-enantiomers by Hoffman-LaRoche, a Swiss pharmaceutical company. Essentially, it is two drugs in one. Plasma concentrations of the (–)-enantiomer are significantly higher than those for the (+)-enantiomer, and the pharmacokinetics between the two enantiomers are significantly different. The (+)-enantiomer has a shorter half-life than the (–)-enantiomer.[11]
According to some research,[27] the (+)-enantiomer is more effective in treating malaria, and the (–)-enantiomer specifically binds to adenosine receptors in the central nervous system, which may explain some of its psychotropic effects.
History
Mefloquine was invented at Walter Reed Army Institute of Research (WRAIR) in the 1970s shortly after the end of the Vietnam war. Mefloquine was number 142,490 of a total of 250,000 antimalarial compounds screened during the study.[2]
Mefloquine was the first Public-Private Venture (PPV) between the US Department of Defense and a pharmaceutical company. WRAIR transferred all its phase I and phase II clinical trial data to Hoffman LaRoche and Smith Kline. FDA approval as a treatment for malaria was swift. Most notably, phase III safety and tolerability trials were skipped.[2]
However, mefloquine was not approved by the FDA for prophylactic use until 1989. This approval was based primarily on compliance, while safety and tolerability were overlooked.[2] Because of the drug's very long half-life, the Centers for Disease Control originally recommended a mefloquine dosage of 250 mg every two weeks; however, this caused an unacceptably high malaria rate in the Peace Corps volunteers who participated in the approval study, so the drug regimen was switched to once a week.[11]
The first randomized, controlled trial on a mixed population was performed in 2001. Prophylaxis with mefloquine was compared to prophylaxis with atovaquone-proguanil. Roughly 67% of participants in the mefloquine arm reported greater than or equal to one adverse event, vs 71% in the atovaquone-proguanil arm. In the mefloquine arm, 5% of the users reported severe events requiring medical attention, vs 1.2% in the atovaquone-proguanil arm.[2][28]
The brand name drug, Lariam, is manufactured by the Swiss company Hoffmann–La Roche. In August 2009, Roche stopped marketing Lariam in the United States.
Research
In June 2010, the first case report appeared of a progressive multifocal leukoencephalopathy being successfully treated with mefloquine. Mefloquine can also act against the JC virus. Administration of mefloquine seemed to eliminate the virus from the patient's body and prevented further neurological deterioration.[29]
WRAIR has published several papers outlining ongoing efforts at that institution to make mefloquine safer by producing a drug composed of only the (+)-enantiomer.
Mefloquine alters cholinergic synaptic transmission through both postsynaptic [30] and presynaptic actions.[31] The postsynaptic action to inhibit acetylcholinesterase changes transmission across synapses in the brain.[32]
References
- ^ a b c d e "Lariam". The American Society of Health-System Pharmacists. Retrieved 27 Nov 2015.
- ^ a b c d e Croft, AM (2007). "A lesson learnt: the rise and fall of Lariam and Halfan". J R Soc Med. 4 (4): 170–4. doi:10.1258/jrsm.100.4.170. PMC 1847738. PMID 17404338.
- ^ Ravina, Enrique (2011). The evolution of drug discovery : from traditional medicines to modern drugs (1. Aufl. ed.). Weinheim: Wiley-VCH. p. 136. ISBN 9783527326693.
- ^ Junghanss, Jeremy Farrar, Peter J. Hotez, Thomas (2013). Manson's tropical diseases (23rd ed.). Oxford: Elsevier/Saunders. p. 569. ISBN 9780702053061.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ "Mefloquine". International Drug Price Indicator Guide. Retrieved 27 November 2015.
- ^ a b c d e f "Lariam". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ a b c d Schlagenhauf, P; Adamcova, M; Regep, L; Schaerer, MT; Rhein, HG (2010-12-09). "The position of mefloquine as a 21st century malaria chemoprophylaxis". Malaria journal. 9: 357. doi:10.1186/1475-2875-9-357. PMC 3224336. PMID 21143906.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "www.idsociety.org" (PDF).
- ^ "Malaria - Chapter 3 - 2014 Yellow Book | Travelers' Health | CDC".
- ^ a b c d Schlagenhauf, P. (1999). "Mefloquine for malaria chemoprophylaxis 1992-1998". Travel Med. 6 (2): 122–123. doi:10.1111/j.1708-8305.1999.tb00843.x. PMID 10381965.
- ^ Jacquerioz, FA; Croft, AM (2009-10-07). Jacquerioz, Frederique A (ed.). "Drugs for preventing malaria in travellers". Cochrane database of systematic reviews (Online) (4): CD006491. doi:10.1002/14651858.CD006491.pub2. PMID 19821371.
- ^ a b c d e f "Lariam medication guide" (PDF). Hoffman La Roche. Retrieved 27 September 2013.
- ^ "www.cdc.gov" (PDF).
- ^ a b González R, Hellgren U, Greenwood B, Menéndez C (2014). "Mefloquine safety and tolerability in pregnancy: a systematic literature review". Malar. J. 13: 75. doi:10.1186/1475-2875-13-75. PMC 3942617. PMID 24581338.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Traveldoctor http://www.traveldoctor.co.uk/malaria.htm. Retrieved 9 July 2016.
{{cite web}}: Missing or empty|title=(help) - ^ Price RN, Uhlemann AC, Brockman A; et al. (2004). "Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number". Lancet. 364 (9432): 438–47. doi:10.1016/S0140-6736(04)16767-6. PMC 4337987. PMID 15288742.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Croft AM (2010). "Malaria: prevention in travellers". Clin Evid (Online). 2010. PMC 3217660. PMID 21418669.
- ^ "www.fda.gov" (PDF).
- ^ "www.accessdata.fda.gov" (PDF).
- ^ AlKadi, HO (2007). "Antimalarial drug toxicity: a review". Chemotherapy. 53 (6): 385–91. doi:10.1159/000109767. PMID 17934257.
- ^ Schlagenhauf P, Blumentals WA, Suter P, et al. (2012). "Pregnancy and fetal outcomes after exposure to mefloquine in the pre- and periconception period and during pregnancy". Clin Infect Dis. 54 (11): e124–31. doi:10.1093/cid/cis215. PMC 3348951. PMID 22495078.
- ^ "Lariam product monogram" (PDF). Hoffman La Roche Limited. p. 3. Retrieved 24 April 2011.
- ^ "Lariam product monogram" (PDF). Hoffman La Roche Limited. p. 4. Retrieved 24 April 2011.
- ^ "Lariam product monogram" (PDF). Hoffman La Roche Limited. p. 6. Retrieved 24 April 2011.
- ^ "Lariam product monogram" (PDF). Hoffman La Roche Limited. p. 18. Retrieved 24 April 2011.
- ^ Fletcher, A., and Shepherd, R. Use of (+)-mefloquine for the treatment of malaria. US 6664397.
- ^ Overbosch D, Schilthuis H, Bienzle U, et al. (October 2001). "Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study". Clin. Infect. Dis. 33 (7): 1015–21. doi:10.1086/322694. PMID 11528574.
- ^ Gofton TE, Al-Khotani1 A, O'Farrell B, Ang LC, McLachlan RS (June 2010). "Mefloquine in the treatment of progressive multifocal leukoencephalopathy". J Neurol Neurosurg Psychiatry. 82 (4): 452–455. doi:10.1136/jnnp.2009.190652. PMID 20562463.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ McArdle JJ, Sellin LC, Coakley KM, Potian JG, Quinones-Lopez MC, Rosenfeld CA, Sutatos LG, Hognason K (June 2005). "Mefloquine inhibits cholinesterases at the mouse neuromuscular junction". Neuropharmacology. 49 (8): 1132–1139. doi:10.1016/j.neuropharm.2005.06.011. PMID 16081111.
- ^ McArdle JJ, Sellin LC, Coakley KM, Potian JG, Hognason K (September 2006). "Mefloquine selectively increases asynchronous acetylcholine release from motor nerve terminals". Neuropharmacology. 50 (3): 345–353. doi:10.1016/j.neuropharm.2005.09.011. PMID 16288931.
- ^ Zhou C, Xiao C, McArdle JJ, Ye JH (February 2006). "Mefloquine enhances nigral gamma-aminobutyric acid release via inhibition of cholinesterase". JPET. 317 (3): 1155–1160. doi:10.1124/JPET.106.101923. PMID 16501066.
External links
- Manufacturer's information
- Roche Medication Guide for Lariam
- Mefloquine (Lariam) Action, Clearinghouse for information on mefloquine news, research, toxicity
- Controversies and Misconceptions in Malaria Chemoprophylaxis for Travelers
- The position of mefloquine as a 21st century malaria chemoprophylaxis (Paper based on data collated for an F. Hoffmann-La Roche regulatory update)
- Crazy Pills - NYTimes Op-Ed
- The Lariam Files - Keith Epstein, Washington Post
