Menopause
| Menopause | |
|---|---|
| Specialty | Gynaecology |
Menopause, also known as the climacteric, is the time in most women's lives when menstrual periods stop permanently, and they are no longer able to bear children.[2][3] Menopause typically occurs between 49 and 52 years of age.[4] Medical professionals often define menopause as having occurred when a woman has not had any vaginal bleeding for a year.[5] It may also be defined by a decrease in hormone production by the ovaries.[6] In those who have had surgery to remove their uterus but they still have ovaries, menopause may be viewed to have occurred at the time of the surgery or when their hormone levels fell.[6] Following the removal of the uterus, symptoms typically occur earlier, at an average of 45 years of age.[7]
Before menopause, a woman's periods typically become irregular, which means that periods may be longer or shorter in duration or be lighter or heavier in the amount of flow. During this time, women often experience hot flashes; these typically last from 30 seconds to ten minutes and may be associated with shivering, sweating, and reddening of the skin.[8] Hot flashes often stop occurring after a year or two.[3] Other symptoms may include vaginal dryness, trouble sleeping, and mood changes.[8] The severity of symptoms varies between women.[3] While menopause is often thought to be linked to an increase in heart disease, this primarily occurs due to increasing age and does not have a direct relationship with menopause. In some women, problems that were present like endometriosis or painful periods will improve after menopause.[3]
Menopause is usually a natural change.[9] It can occur earlier in those who smoke tobacco.[5][10] Other causes include surgery that removes both ovaries or some types of chemotherapy.[5] At the physiological level, menopause happens because of a decrease in the ovaries' production of the hormones estrogen and progesterone.[2] While typically not needed, a diagnosis of menopause can be confirmed by measuring hormone levels in the blood or urine.[11] Menopause is the opposite of menarche, the time when a girl's periods start.[12]
Specific treatment is not usually needed. Some symptoms, however, may be improved with treatment. With respect to hot flashes, avoiding smoking, caffeine, and alcohol is often recommended. Sleeping in a cool room and using a fan may help.[13] The following medications may help: menopausal hormone therapy (MHT), clonidine, gabapentin, or selective serotonin reuptake inhibitors.[13][14] Exercise may help with sleeping problems. While MHT was once routinely prescribed, it is now only recommended in those with significant symptoms, as there are concerns about side effects.[13] High-quality evidence for the effectiveness of alternative medicine has not been found.[3] There is tentative evidence for soy isoflavones.[15]
Signs and symptoms

During early menopause transition, the menstrual cycles remain regular but the interval between cycles begins to lengthen. Hormone levels begin to fluctuate. Ovulation may not occur with each cycle.[16]
The date of the final menstrual period is usually taken as the point when menopause has occurred.[16] During the menopausal transition and after menopause, women can experience a wide range of symptoms.
Vagina and uterus
During the transition to menopause, menstrual patterns can show shorter cycling (by 2–7 days);[16] longer cycles remain possible.[16] There may be irregular bleeding (lighter, heavier, spotting).[16] Dysfunctional uterine bleeding is often experienced by women approaching menopause due to the hormonal changes that accompany the menopause transition. Spotting or bleeding may simply be related to vaginal atrophy, a benign sore (polyp or lesion), or may be a functional endometrial response. The European Menopause and Andropause Society has released guidelines for assessment of the endometrium, which is usually the main source of spotting or bleeding.[17]
In post-menopausal women, however, any genital bleeding is an alarming symptom that requires an appropriate study to rule out the possibility of malignant diseases.
Symptoms that may appear during menopause and continue through postmenopause include:
- painful intercourse[16]
- vaginal dryness[16]
- atrophic vaginitis — thinning of the membranes of the vulva, the vagina, the cervix, and the outer urinary tract, along with considerable shrinking and loss in elasticity of all of the outer and inner genital areas.
Other physical
Other physical symptoms of menopause include lack of energy, joint soreness, stiffness,[16] back pain,[16] breast enlargement,[16] breast pain,[16] heart palpitations,[16] headache,[16] dizziness,[16] dry, itchy skin,[16] thinning, tingling skin, weight gain,[16] urinary incontinence,[16][18] urinary urgency,[16] interrupted sleeping patterns,[16][19][20][21] heavy night sweats,[16] hot flashes.[16]
Psychological
Psychological symptoms include anxiety,[22] poor memory,[16] inability to concentrate,[16] depressive mood,[16][22] irritability,[16] mood swings,[16] less interest in sexual activity.[16]
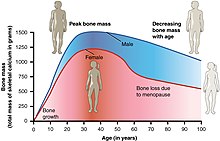
Long term effects
Menopause confers:
- A possible but contentious increased risk of atherosclerosis.[23] The risk of acute myocardial infarction and other cardiovascular diseases rises sharply after menopause, but the risk can be reduced by managing risk factors, such as tobacco smoking, hypertension, increased blood lipids and body weight.[24][25]
- Increased risk of osteopenia and osteoporosis[citation needed]
Women who experience menopause before 45 years of age have an increased risk of heart disease and death.[26]
Causes
Age
In the Western world, the typical age of menopause (last period from natural causes) is between 40 and 61[27] and the average age for last period is 51 years.[28] The average age of natural menopause in Australia is 51.7 years.[29] In India and the Philippines, the median age of natural menopause is considerably earlier, at 44 years.[30]
In rare cases, a woman's ovaries stop working at a very early age, ranging anywhere from the age of puberty to age 40. This is known as premature ovarian failure and affects 1 to 2% of women by age 40.[31]
Undiagnosed and untreated coeliac disease is a risk factor for early menopause. Coeliac disease can present with several non-gastrointestinal symptoms, in the absence of gastrointestinal symptoms, and most cases escape timely recognition and go undiagnosed, leading to a risk of long-term complications. A strict gluten-free diet reduces the risk. Women with early diagnosis and treatment of coeliac disease present a normal duration of fertile life span.[32][33]
Women who have undergone hysterectomy with ovary conservation go through menopause on average 3.7 years earlier than the expected age. Other factors that can promote an earlier onset of menopause (usually 1 to 3 years early) are smoking cigarettes or being extremely thin.[34]
Premature ovarian failure
Premature ovarian failure (POF) is diagnosed or confirmed by high blood levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH) on at least three occasions at least four weeks apart.[35]
Known causes of premature ovarian failure include autoimmune disorders, thyroid disease, diabetes mellitus, chemotherapy, being a carrier of the fragile X syndrome gene, and radiotherapy. However, in the majority of spontaneous cases of premature ovarian failure, the cause is unknown, i.e., it is generally idiopathic.[35]
Women who have a functional disorder affecting the reproductive system (e.g., endometriosis, polycystic ovary syndrome, cancer of the reproductive organs) can go into menopause at a younger age than the normal timeframe. The functional disorders often significantly speed up the menopausal process.
An early menopause can be related to cigarette smoking, higher body mass index, racial and ethnic factors, illnesses, and the surgical removal of the ovaries, with or without the removal of the uterus.[36]
Rates of premature menopause have been found to be significantly higher in fraternal and identical twins; approximately 5% of twins reach menopause before the age of 40. The reasons for this are not completely understood. Transplants of ovarian tissue between identical twins have been successful in restoring fertility.
Surgical menopause
Menopause can be surgically induced by bilateral oophorectomy (removal of ovaries), which is often, but not always, done in conjunction with removal of the Fallopian tubes (salpingo-oophorectomy) and uterus (hysterectomy).[37] Cessation of menses as a result of removal of the ovaries is called "surgical menopause". The sudden and complete drop in hormone levels usually produces extreme withdrawal symptoms such as hot flashes, etc.
Removal of the uterus without removal of the ovaries does not directly cause menopause, although pelvic surgery of this type can often precipitate a somewhat earlier menopause, perhaps because of a compromised blood supply to the ovaries.[citation needed]
Mechanism
The menopausal transition, and postmenopause itself, is a natural change, not usually a disease state or a disorder. The main cause of this transition is the natural depletion and aging of the finite amount of oocytes (ovarian reserve). This process is sometimes accelerated by other conditions and is known to occur earlier after a wide range of gynecologic procedures such as hysterectomy (with and without ovariectomy), endometrial ablation and uterine artery embolisation. The depletion of the ovarian reserve causes an increase in circulating follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels because there are a decreased number of oocytes and follicles responding to these hormones and producing estrogen.
The transition has a variable degree of effects.[38]
The stages of the menopause transition have been classified according to a woman's reported bleeding pattern, supported by changes in the pituitary follicle-stimulating hormone (FSH) levels.[39]
In younger women, during a normal menstrual cycle the ovaries produce estradiol, testosterone and progesterone in a cyclical pattern under the control of FSH and luteinising hormone (LH) which are both produced by the pituitary gland. During perimenopause (approaching menopause), estradiol levels and patterns of production remain relatively unchanged or may increase compared to young women, but the cycles become frequently shorter or irregular.[40] The often observed increase in estrogen is presumed to be in response to elevated FSH levels that, in turn, is hypothesized to be caused by decreased feedback by inhibin.[41] Similarly, decreased inhibin feedback after hysterectomy is hypothesized to contribute to increased ovarian stimulation and earlier menopause.[42][43]
The menopausal transition is characterized by marked, and often dramatic, variations in FSH and estradiol levels. Because of this, measurements of these hormones are not considered to be reliable guides to a woman's exact menopausal status.[44]
Menopause occurs because of the sharp decrease of estradiol and progesterone production by the ovaries. After menopause, estrogen continues to be produced mostly by aromatase in fat tissues and is produced in small amounts in many other tissues such as ovaries, bone, blood vessels, and the brain where it acts locally.[45] The substantial fall in circulating estradiol levels at menopause impacts many tissues, from brain to skin.
In contrast to the sudden fall in estradiol during menopause, the levels of total and free testosterone, as well as dehydroepiandrosterone sulfate (DHEAS) and androstenedione appear to decline more or less steadily with age. An effect of natural menopause on circulating androgen levels has not been observed.[46] Thus specific tissue effects of natural menopause cannot be attributed to loss of androgenic hormone production.[47]
Hot flushes and other vasomotor symptoms accompany the menopausal transition. While many sources continue to claim that hot flashes during the menopausal transition are caused by low estrogen levels, this assertion was shown incorrect in 1935 and, in most cases, hot flushes are observed despite elevated estrogen levels. The exact cause of these symptoms is not yet understood, possible factors considered are higher and erratic variation of estradiol level during the cycle, elevated FSH levels which may indicate hypothalamic dysregulation perhaps caused by missing feedback by inhibin. It has been also observed that the vasomotor symptoms differ during early perimenopause and late menopausal transition and it is possible that they are caused by a different mechanism.[40]
Long-term effects of menopause may include osteoporosis, vaginal atrophy as well as changed metabolic profile resulting in cardiac risks.
Ovarian aging
Decreased inhibin feedback after hysterectomy is hypothesized to contribute to increased ovarian stimulation and earlier menopause. Hastened ovarian aging has been observed after endometrial ablation. While it is difficult to prove that these surgeries are causative, it has been hypothesized that the endometrium may be producing endocrine factors contributing to the endocrine feedback and regulation of the ovarian stimulation. Elimination of this factors contributes to faster depletion of the ovarian reserve. Reduced blood supply to the ovaries that may occur as a consequence of hysterectomy and uterine artery embolisation has been hypothesized to contribute to this effect.[42][43]
Impaired DNA repair mechanisms may contribute to earlier depletion of the ovarian reserve during aging.[48] As women age, double-strand breaks accumulate in the DNA of their primordial follicles. Primordial follicles are immature primary oocytes surrounded by a single layer of granulosa cells. An enzyme system is present in oocytes that ordinarily accurately repairs DNA double-strand breaks. This repair system is called "homologous recombinational repair", and it is especially effective during meiosis. Meiosis is the general process by which germ cells are formed in all sexual eukaryotes; it appears to be an adaptation for efficiently removing damages in germ line DNA.[49] (See Meiosis.)
Human primary oocytes are present at an intermediate stage of meiosis, termed prophase I (see Oogenesis). Expression of four key DNA repair genes that are necessary for homologous recombinational repair during meiosis (BRCA1, MRE11, Rad51, and ATM) decline with age in oocytes.[48] This age-related decline in ability to repair DNA double-strand damages can account for the accumulation of these damages, that then likely contributes to the depletion of the ovarian reserve.
Diagnosis
One way of assessing the impact on women of some of these menopause effects are the Greene Climacteric Scale questionnaire,[50] the Cervantes Scale[51] and the Menopause Rating Scale.[19]
Premenopause
Premenopause is a term used to mean the years leading up to the last period, when the levels of reproductive hormones are becoming more variable and lower, and the effects of hormone withdrawal are present.[37] Premenopause starts some time before the monthly cycles become noticeably irregular in timing.[52]
Perimenopause
The term "perimenopause", which literally means "around the menopause", refers to the menopause transition years, a time before and after the date of the final episode of flow. According to the North American Menopause Society, this transition can last for four to eight years.[53] The Centre for Menstrual Cycle and Ovulation Research describes it as a six- to ten-year phase ending 12 months after the last menstrual period.[54]
During perimenopause, estrogen levels average about 20–30% higher than during premenopause, often with wide fluctuations.[54] These fluctuations cause many of the physical changes during perimenopause as well as menopause.[55] Some of these changes are hot flashes, night sweats, difficulty sleeping, vaginal dryness or atrophy, incontinence, osteoporosis, and heart disease.[54] During this period, fertility diminishes but is not considered to reach zero until the official date of menopause. The official date is determined retroactively, once 12 months have passed after the last appearance of menstrual blood.
The menopause transition typically begins between 40 and 50 years of age (average 47.5).[56][57] The duration of perimenopause may be for up to eight years.[57] Women will often, but not always, start these transitions (perimenopause and menopause) about the same time as their mother did.[58]
In some women, menopause may bring about a sense of loss related to the end of fertility. In addition, this change often occurs when other stressors may be present in a woman's life:
- Caring for, and/or the death of, elderly parents
- Empty nest syndrome when children leave home
- The birth of grandchildren, which places people of "middle age" into a new category of "older people" (especially in cultures where being older is a state that is looked down on)
Some research appears to show that melatonin supplementation in perimenopausal women can improve thyroid function and gonadotropin levels, as well as restoring fertility and menstruation and preventing depression associated with menopause.[59]
Postmenopause
The term "postmenopausal" describes women who have not experienced any menstrual flow for a minimum of 12 months, assuming that they have a uterus and are not pregnant or lactating.[37] In women without a uterus, menopause or postmenopause can be identified by a blood test showing a very high FSH level. Thus postmenopause the time in a woman's life that take place after her last period or, more accurately, after the point when her ovaries become inactive.
The reason for this delay in declaring postmenopause is because periods are usually erratic at this time of life. Therefore, a reasonably long stretch of time is necessary to be sure that the cycling has ceased. At this point a woman is considered infertile; however, the possibility of becoming pregnant has usually been very low (but not quite zero) for a number of years before this point is reached.
A woman's reproductive hormone levels continue to drop and fluctuate for some time into post-menopause, so hormone withdrawal effects such as hot flashes may take several years to disappear.
Any period-like flow during postmenopause, even spotting, must be reported to a doctor. The cause may be minor, but the possibility of endometrial cancer must be checked for.
Management
Perimenopause is a natural stage of life. It is not a disease or a disorder. Therefore, it does not automatically require any kind of medical treatment. However, in those cases where the physical, mental, and emotional effects of perimenopause are strong enough that they significantly disrupt the life of the woman experiencing them, palliative medical therapy may sometimes be appropriate.
Hormone replacement therapy
In the context of the menopause, hormone replacement therapy (HRT) is the use of estrogen in women without a uterus and estrogen plus progestin in women who have an intact uterus.[60]
HRT may be reasonable for the treatment of menopausal symptoms such as hot flashes.[61] Its use appears to increase the risk of strokes and blood clots.[62][needs update] When used for menopausal symptoms it should be used for the shortest time possible and at the lowest dose possible.[62] The response to HRT in each postmenopausal woman may not be the same. Genetic polymorphism in estrogen receptors appears to be associated with inter-individual variability in metabolic response to HRT in postmenopausal women.[63]
It also appears effective for preventing bone loss and osteoporotic fracture.[64] It is often seen as a second line agent for this purpose.[65] There is some concern that this treatment increases the risk of breast cancer.[66]
Selective estrogen receptor modulators
SERMs are a category of drugs, either synthetically produced or derived from a botanical source, that act selectively as agonists or antagonists on the estrogen receptors throughout the body. The most commonly prescribed SERMs are raloxifene and tamoxifen. Raloxifene exhibits oestrogen agonist activity on bone and lipids, and antagonist activity on breast and the endometrium.[67] Tamoxifen is in widespread use for treatment of hormone sensitive breast cancer. Raloxifene prevents vertebral fractures in postmenopausal, osteoporotic women and reduces the risk of invasive breast cancer.[68]
Other medication
Some of the SSRIs and SNRIs appear to provide some relief.[14] Low dose paroxetine has been FDA-approved for hot moderate-to-severe vasomotor symptoms associated with menopause.[69] They may, however, be associated with sleeping problems.[14]
Gabapentin or clonidine may help but does not work as well as hormone therapy.[14] Clonidine may be associated with constipation and sleeping problems.[14]
Alternative medicine
There is no evidence of consistent benefit of alternative therapies for menopausal symptoms despite their popularity.[70] The effect of soy isoflavones on menopausal symptoms is promising for reduction of hot flashes and vaginal dryness.[15][71] Evidence does not support a benefit from phytoestrogens such as coumestrol,[72] femarelle,[73] or black cohosh.[15][74] There is no evidence to support the efficacy of acupuncture as a management for menopausal symptoms.[75] As of 2011 there is no support for herbal or dietary supplements in the prevention or treatment of the mental changes that occur around menopause.[76] A 2016 Cochrane review found not enough evidence to show a difference between Chinese herbal medicine and placebo for the vasomotor symptoms.[77]
Other therapies
- Lack of lubrication is a common problem during and after perimenopause. Vaginal moisturizers can help women with overall dryness, and lubricants can help with lubrication difficulties that may be present during intercourse. It is worth pointing out that moisturizers and lubricants are different products for different issues: some women complain that their genitalia are uncomfortably dry all the time, and they may do better with moisturizers. Those who need only lubricants do well using them only during intercourse.
- Low-dose prescription vaginal estrogen products such as estrogen creams are generally a safe way to use estrogen topically, to help vaginal thinning and dryness problems (see vaginal atrophy) while only minimally increasing the levels of estrogen in the bloodstream.
- In terms of managing hot flashes, lifestyle measures such as drinking cold liquids, staying in cool rooms, using fans, removing excess clothing, and avoiding hot flash triggers such as hot drinks, spicy foods, etc., may partially supplement (or even obviate) the use of medications for some women.
- Individual counseling or support groups can sometimes be helpful to handle sad, depressed, anxious or confused feelings women may be having as they pass through what can be for some a very challenging transition time.
- Osteoporosis can be minimized by smoking cessation, adequate vitamin D intake and regular weight-bearing exercise. The bisphosphate drug alendronate may decrease the risk of a fracture, in women that have both bone loss and a previous fracture and less so for those with just osteoporosis.[78]
Society and culture
The cultural context within which a woman lives can have a significant impact on the way she experiences the menopausal transition. Menopause has been described as a subjective experience, with social and cultural factors playing a prominent role in the way menopause is experienced and perceived.
Within the United States, social location affects the way women perceive menopause and its related biological effects. Research indicates that whether a woman views menopause as a medical issue or an expected life change is correlated with her socio-economic status.[79] The paradigm within which a woman considers menopause influences the way she views it: Women who understand menopause as a medical condition rate it significantly more negatively than those who view it as a life transition or a symbol of aging.[80]
Ethnicity and geography play roles in the experience of menopause. American women of different ethnicities report significantly different types of menopausal effects. One major study found Caucasian women most likely to report what are sometimes described as psychosomatic symptoms, while African-American women were more likely to report vasomotor symptoms.[81]
It seems that Japanese women experience menopause effects, or konenki, in a different way from American women.[82] Japanese women report lower rates of hot flashes and night sweats; this can be attributed to a variety of factors, both biological and social. Historically, konenki was associated with wealthy middle-class housewives in Japan, i.e., it was a "luxury disease" that women from traditional, inter-generational rural households did not report. Menopause in Japan was viewed as a symptom of the inevitable process of aging, rather than a "revolutionary transition", or a "deficiency disease" in need of management.[82]
In Japanese culture, reporting of vasomotor symptoms has been on the increase, with research conducted by Melissa Melby in 2005 finding that of 140 Japanese participants, hot flashes were prevalent in 22.1%.[83] This was almost double that of 20 years prior.[84] Whilst the exact cause for this is unknown, possible contributing factors include significant dietary changes, increased medicalisation of middle-aged women and increased media attention on the subject.[84] However, reporting of vasomotor symptoms is still significantly lower than North America.[85]
Additionally, while most women in the United States apparently have a negative view of menopause as a time of deterioration or decline, some studies seem to indicate that women from some Asian cultures have an understanding of menopause that focuses on a sense of liberation and celebrates the freedom from the risk of pregnancy.[86] Postmenopausal Indian women can enter Hindu temples and participate in rituals, marking it as a celebration for reaching an age of wisdom and experience.
Diverging from these conclusions, one study appeared to show that many American women "experience this time as one of liberation and self-actualization".[87]
Generally speaking, women raised in the Western world or developed countries in Asia live long enough so that a third of their life is spent in post-menopause. For some women, the menopausal transition represents a major life change, similar to menarche in the magnitude of its social and psychological significance. Although the significance of the changes that surround menarche is fairly well recognized, in countries such as the United States, the social and psychological ramifications of the menopause transition are frequently ignored or underestimated.[citation needed]
Medicalization
The medicalization of menopause within biomedical practice began in the early 19th century and has affected the way menopause is viewed within society. By the 1930s in North America and Europe, biomedicine practitioners began to think of menopause as a disease-like state. This idea coincided with the concept of the "standardization of the body". The bodies of young premenopausal women began to be considered the "normal", against which all female bodies were compared.[88]
Etymology
Menopause literally means the "end of monthly cycles" (the end of monthly periods or menstruation), from the Greek word pausis ("pause") and mēn ("month"). This is a medical calque; the Greek word for menses is actually different. In Ancient Greek, the menses were described in the plural, ta emmēnia, ("the monthlies"), and its modern descendant has been clipped to ta emmēna. The Modern Greek medical term is emmenopausis in Katharevousa or emmenopausi in Demotic Greek.
The word "menopause" was coined specifically for human females, where the end of fertility is traditionally indicated by the permanent stopping of monthly menstruations. However, menopause exists in some other animals, many of which do not have monthly menstruation;[89] in this case, the term means a natural end to fertility that occurs before the end of the natural lifespan.
Evolutionary rationale
Various theories have been suggested that attempt to suggest evolutionary benefits to the human species stemming from the cessation of women's reproductive capability before the end of their natural lifespan. Explanations can be categorized as adaptive and non-adaptive:
Non-adaptive hypotheses
The high cost of female investment in offspring may lead to physiological deteriorations that amplify susceptibility to becoming infertile. This hypothesis suggests the reproductive lifespan in humans has been optimized, but it has proven more difficult in females and thus their reproductive span is shorter. If this hypothesis were true, however, age at menopause should be negatively correlated with reproductive effort[90] and the available data does not support this.[91]
A recent increase in female longevity due to improvements in the standard of living and social care has also been suggested.[92] It is difficult for selection, however, to favour aid to offspring from parents and grandparents.[93] Irrespective of living standards, adaptive responses are limited by physiological mechanisms. In other words, senescence is programmed and regulated by specific genes.[94]
Adaptive hypotheses
"Survival of the fittest" hypothesis
This hypothesis suggests that younger mothers and offspring under their care will fare better in a difficult and predatory environment because a younger mother will be stronger and more agile in providing protection and sustenance for herself and a nursing baby. The various biological factors associated with menopause had the effect of male members of the species investing their effort with the most viable of potential female mates.[95][page needed] One problem with this hypothesis is that we would expect to see menopause exhibited in the animal kingdom.[89]
Mother hypothesis
The mother hypothesis suggests that menopause was selected for humans because of the extended development period of human offspring and high costs of reproduction so that mothers gain an advantage in reproductive fitness by redirecting their effort from new offspring with a low survival chance to existing children with a higher survival chance.[96]
Grandmother hypothesis
The Grandmother hypothesis suggests that menopause was selected for humans because it promotes the survival of grandchildren. According to this hypothesis, post-reproductive women feed and care for children, adult nursing daughters, and grandchildren whose mothers have weaned them. Human babies require large and steady supplies of glucose to feed the growing brain. In infants in the first year of life, the brain consumes 60% of all calories, so both babies and their mothers require a dependable food supply. Some evidence suggests that hunters contribute less than half the total food budget of most hunter-gatherer societies, and often much less than half, so that foraging grandmothers can contribute substantially to the survival of grandchildren at times when mothers and fathers are unable to gather enough food for all of their children. In general, selection operates most powerfully during times of famine or other privation. So although grandmothers might not be necessary during good times, many grandchildren cannot survive without them during times of famine. Arguably, however, there is no firm consensus on the supposed evolutionary advantages (or simply neutrality) of menopause to the survival of the species in the evolutionary past.
Indeed, analysis of historical data found that the length of a female's post-reproductive lifespan was reflected in the reproductive success of her offspring and the survival of her grandchildren.[97] Interestingly, another study found comparative effects but only in the maternal grandmother—paternal grandmothers had a detrimental effect on infant mortality (probably due to paternity uncertainty).[98] Differing assistance strategies for maternal and paternal grandmothers have also been demonstrated. Maternal grandmothers concentrate on offspring survival, whereas paternal grandmothers increase birth rates.[99]
Some believe a problem concerning the grandmother hypothesis is that it requires a history of female philopatry while in the present day the majority of hunter-gatherer societies are patriarchal.[100] However, there is disagreement split along ideological lines about whether patrilineality would have existed before modern times.[101] Some believe variations on the mother, or grandmother effect fail to explain longevity with continued spermatogenesis in males (oldest verified paternity is 94 years, 35 years beyond the oldest documented birth attributed to females).[102] Notably, the survival time past menopause is roughly the same as the maturation time for a human child. That a mother's presence could aid in the survival of a developing child, while an unidentified father's absence might not have affected survival, could explain the paternal fertility near the end of the father's lifespan.[103] A man with no certainty of which children are his may merely attempt to father additional children, with support of existing children present but small. Note the existence of partible paternity supporting this.[104] Some argue that the mother and grandmother hypotheses fail to explain the detrimental effects of losing ovarian follicular activity, such as osteoporosis, osteoarthritis, Alzheimer's disease and coronary artery disease.[105]
The theories discussed above assume that evolution directly selected for menopause. Another theory states that menopause is the byproduct of the evolutionary selection for follicular atresia, a factor that causes menopause. Menopause results from having too few ovarian follicles to produce enough estrogen to maintain the ovarian-pituitary-hypothalamic loop, which results in the cessation of menses and the beginning of menopause. Human females are born with approximately a million oocytes, and approximately 400 oocytes are lost to ovulation throughout life.[106][107]
Other animals
Menopause in the animal kingdom appears to be uncommon, but the presence of this phenomenon in different species has not been thoroughly researched. Life histories show a varying degree of senescence; rapid senescing organisms (e.g., Pacific salmon and annual plants) do not have a post-reproductive life-stage. Gradual senescence is exhibited by all placental mammalian life histories.
Menopause has been observed in several species of nonhuman primates,[89] including rhesus monkeys,[108] and chimpanzees.[109] Menopause also has been reported in a variety of other vertebrate species including elephants,[110] short-finned pilot whales,[111] killer whales,[112] and other cetaceans,[113][114] the guppy,[115] the platyfish, the budgerigar, the laboratory rat and mouse, and the opossum. However, with the exception of the short-finned pilot whale, such examples tend to be from captive individuals, and thus they are not necessarily representative of what happens in natural populations in the wild.
Dogs do not experience menopause; the canine estrus cycle simply becomes irregular and infrequent. Although older female dogs are not considered good candidates for breeding, offspring have been produced by older animals.[116] Similar observations have been made in cats.[117]
See also
References
- ^ Chuku, Gloria (2005). Igbo women and economic transformation in southeastern Nigeria, 1900–1960. Paragraph 3: Routledge. p. 73. ISBN 0415972108.
{{cite book}}: CS1 maint: location (link) - ^ a b "Menopause: Overview". Eunice Kennedy Shriver National Institute of Child Health and Human Development. 28 June 2013. Retrieved 8 March 2015.
- ^ a b c d e "Menopause: Overview". PubMedHealth. 29 August 2013. Retrieved 8 March 2015.
- ^ Takahashi, TA; Johnson, KM (May 2015). "Menopause". The Medical clinics of North America. 99 (3): 521–34. doi:10.1016/j.mcna.2015.01.006. PMID 25841598.
- ^ a b c "What is menopause?". Eunice Kennedy Shriver National Institute of Child Health and Human Development. 28 June 2013. Retrieved 8 March 2015.
- ^ a b Sievert, Lynnette Leidy (2006). Menopause : a biocultural perspective ([Online-Ausg.] ed.). New Brunswick, N.J.: Rutgers University Press. p. 81. ISBN 9780813538563.
- ^ International position paper on women's health and menopause : a comprehensive approach. DIANE Publishing. 2002. p. 36. ISBN 9781428905214.
- ^ a b "What are the symptoms of menopause?". Eunice Kennedy Shriver National Institute of Child Health and Human Development. 6 May 2013. Retrieved 8 March 2015.
- ^ "What causes menopause?". Eunice Kennedy Shriver National Institute of Child Health and Human Development. 6 May 2013. Retrieved 8 March 2015.
- ^ Warren, volume editors, Claudio N. Soares, Michelle (2009). The menopausal transition : interface between gynecology and psychiatry ([Online-Ausg.] ed.). Basel: Karger. p. 73. ISBN 978-3805591010.
{{cite book}}:|first1=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ "How do health care providers diagnose menopause?". Eunice Kennedy Shriver National Institute of Child Health and Human Development. 6 May 2013. Retrieved 8 March 2015.
- ^ Wood, James. "9". Dynamics of Human Reproduction: Biology, Biometry, Demography. Transaction Publishers. p. 401. ISBN 9780202365701.
- ^ a b c "What are the treatments for other symptoms of menopause?". Eunice Kennedy Shriver National Institute of Child Health and Human Development. 28 June 2013. Retrieved 8 March 2015.
- ^ a b c d e Krause, MS; Nakajima, ST (March 2015). "Hormonal and Nonhormonal Treatment of Vasomotor Symptoms". Obstetrics and Gynecology Clinics of North America. 42 (1): 163–179. doi:10.1016/j.ogc.2014.09.008. PMID 25681847.
- ^ a b c Franco, Oscar H.; Chowdhury, Rajiv; Troup, Jenna; Voortman, Trudy; Kunutsor, Setor; Kavousi, Maryam; Oliver-Williams, Clare; Muka, Taulant (21 June 2016). "Use of Plant-Based Therapies and Menopausal Symptoms". JAMA. 315 (23): 2554–63. doi:10.1001/jama.2016.8012. PMID 27327802.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa Hoffman, Barbara (2012). Williams gynecology. New York: McGraw-Hill Medical. pp. 555–556. ISBN 9780071716727.
- ^ Dreisler, E; Poulsen, LG; Antonsen, SL; Ceausu, I; Depypere, H; Erel, CT; Lambrinoudaki, I; Pérez-López, FR; Simoncini, T; Tremollieres, F; Rees, M; Ulrich, LG (2013). "EMAS clinical guide: Assessment of the endometrium in peri and postmenopausal women". Maturitas. 75 (2): 181–90. doi:10.1016/j.maturitas.2013.03.011. PMID 23619009.
- ^ Pérez-López, FR; Cuadros, JL; Fernández-Alonso, AM; Chedraui, P; Sánchez-Borrego, R; Monterrosa-Castro, A (2012). "Quality of life in a large cohort of mid-aged Colombian women assessed using the Cervantes Scale". Maturitas. 73 (4): 369–72. doi:10.1016/j.maturitas.2012.09.004. PMID 23041251.
- ^ a b Chedraui, P; Pérez-López, FR; Mendoza, M; Leimberg, ML; Martínez, MA; Vallarino, V; Hidalgo, L (2010). "Factors related to increased daytime sleepiness during the menopausal transition as evaluated by the Epworth sleepiness scale". Maturitas. 65 (1): 75–80. doi:10.1016/j.maturitas.2009.11.003. PMID 19945237.
- ^ Arakane, M; Castillo, C; Rosero, MF; Peñafiel, R; Pérez-López, FR; Chedraui, P (2011). "Factors relating to insomnia during the menopausal transition as evaluated by the Insomnia Severity Index". Maturitas. 69 (2): 157–161. doi:10.1016/j.maturitas.2011.02.015. PMID 21444163.
- ^ Monterrosa-Castro, A; Marrugo-Flórez, M; Romero-Pérez, I; Chedraui, P; Fernández-Alonso, AM; Pérez-López, FR (2013). "Prevalence of insomnia and related factors in a large mid-aged female Colombian sample". Maturitas. 74 (4): 346–51. doi:10.1016/j.maturitas.2013.01.009. PMID 23391501.
- ^ a b Llaneza, P; García-Portilla, MP; Llaneza-Suárez, D; Armott, B; Pérez-López, FR (2012). "Depressive disorders and the menopause transition". Maturitas. 71 (2): 120–30. doi:10.1016/j.maturitas.2011.11.017. PMID 22196311.
- ^ Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology: With Student Consult Online Access. Philadelphia: Saunders. p. 344. ISBN 1-4160-2973-7. 8th edition
- ^ Souza, Hugo (2013). "Autonomic Cardiovascular Damage during Post-menopause: the Role of Physical Training". Aging and Disease. 4 (6): 320–328. doi:10.14336/AD.2013.0400320. ISSN 2152-5250.
- ^ "Perimenopausal risk factors and future health". Human Reproduction Update. 17 (5): 706–717. 2011. doi:10.1093/humupd/dmr020. PMID 21565809.
- ^ Muka, Taulant; Oliver-Williams, Clare; Kunutsor, Setor; Laven, Joop S. E.; Fauser, Bart C. J. M.; Chowdhury, Rajiv; Kavousi, Maryam; Franco, Oscar H. (14 September 2016). "Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality". JAMA Cardiology. doi:10.1001/jamacardio.2016.2415.
- ^ Minkin, Mary Jane; et al. (1997). What Every Woman Needs to Know about Menopause. Yale University Press. ISBN 0-300-07261-9.
- ^ Kato, I; Toniolo, P; Akhmedkhanov, A; Koenig, KL; Shore, R; Zeleniuch-Jacquotte, A (1998). "Prospective study of factors influencing the onset of natural menopause". J Clin Epidemiol. 51 (12): 1271–1276. doi:10.1016/S0895-4356(98)00119-X. PMID 10086819.
- ^ Do, KA; Treloar, SA; Pandeya, N; Purdie, D; Green, AC; Heath, AC; Martin, NG (1998). "Predictive factors of age at menopause in a large Australian twin study". Hum Biol. 70 (6): 1073–91. PMID 9825597.
- ^ Ringa, V. (2000). "Menopause and treatments". Quality of Life Research. 9 (6): 695–707. doi:10.1023/A:1008913605129. JSTOR 4036942.
- ^ Podfigurna-Stopa, A; et al. (September 2016). "Premature ovarian insufficiency: the context of long-term effects". Journal of endocrinological investigation. 39 (9): 983–90. PMC 4987394. PMID 27091671.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ Tersigni C, Castellani R, de Waure C, Fattorossi A, De Spirito M, Gasbarrini A, Scambia G, Di Simone N (2014). "Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms". Hum Reprod Update. 20 (4): 582–93. doi:10.1093/humupd/dmu007. PMID 24619876.
- ^ Lasa, JS; Zubiaurre, I; Soifer, LO (2014). "Risk of infertility in patients with celiac disease: a meta-analysis of observational studies". Arq Gastroenterol. 51 (2): 144–50. doi:10.1590/S0004-28032014000200014. PMID 25003268.
- ^ Healthline. "What causes early menopause". Healthline.
- ^ a b Kalantaridou SN, Davis SR, Nelson LM. Endocrinology Metabolism Clinics of North America, December 1998; 27(4) 989–1006.
- ^ Bucher, et al. 1930
- ^ a b c Harlow, SD; Gass, M; Hall, JE; Lobo, R; Maki, P; Rebar, RW; Sherman, S; Sluss, PM; de Villiers, TJ (2012). "Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging". Fertility and Sterility. 97 (4): 398–406. doi:10.1016/j.fertnstert.2012.01.128. PMID 22341880.
- ^ Lee S. Cohen; Claudio N. Soares; Allison F. Vitonis; Michael W. Otto; Bernard L. Harlow; et al. (April 2006). "Risk for New Onset of Depression During the Menopausal Transition". Archives of General Psychiatry. The Harvard Study of Moods and Cycles. 63 (4): 385–390. doi:10.1001/archpsyc.63.4.385. Retrieved 28 September 2013.
- ^ Soules, MR; Sherman, S; Parrott, E; Rebar, R; Santoro, N; Utian, W; Woods, N (2001). "Executive summary: Stages of Reproductive Aging Workshop (STRAW)". Climacteric. 4 (4): 267–72. doi:10.1080/cmt.4.4.267.272. PMID 11770182.
- ^ a b Prior, JC. "Perimenopause: The Complex Endocrinology of the Menopausal Transition". Endocrine Reviews. 19: 397–428. doi:10.1210/edrv.19.4.0341.
- ^ Burger, HG (1994). "Diagnostic role of follicle stimulating hormone (FSH) measurements during menopausal transition – an analysis of FSH, oestradiol and inhibin". European Journal of Endocrinology. 130 (1): 38–42. doi:10.1530/eje.0.1300038. PMID 8124478.
- ^ a b Nahás E, Pontes A, Traiman P, NahásNeto J, Dalben I, De Luca L (2003). "Inhibin B and ovarian function after total abdominal hysterectomy in women of reproductive age". Gynecol. Endocrinol. 17: 125–31. doi:10.1080/713603218. PMID 12737673.
- ^ a b Petri Nahás EA, Pontes A, Nahas-Neto J, Borges VT, Dias R, Traiman P (February 2005). "Effect of total abdominal hysterectomy on ovarian blood supply in women of reproductive age". J Ultrasound Med. 24: 169–74. PMID 15661947.
- ^ Burger, HG (1994). "Diagnostic role of follicle stimulating hormone (FSH) measurements during menopausal transition – an analysis of FSH, oestraiol and inhibin". European Journal of Endocrinology. 130 (1): 38–42. doi:10.1530/eje.0.1300038. PMID 8124478.
- ^ Simpson, ER; Davis, SR (2001). "Minireview: aromatase and the regulation of estrogen biosynthesis – some new perspectives". Endocrinology. 142 (11): 4589–94. doi:10.1210/en.142.11.4589. PMID 11606422.
- ^ Davison, SL; Bell, R; Donath, S; Montalto, JG; Davis, SR (2005). "Androgen levels in adult females: changes with age, menopause, and oophorectomy". J Clin Endocrinol Metab. 90 (7): 3847–53. doi:10.1210/jc.2005-0212. PMID 15827095.
- ^ Robin H. Fogle; Frank Z. Stanczyk; Xiaohua Zhang; Richard J. Paulson. "Ovarian Androgen Production in Postmenopausal Women". The Journal of Clinical Endocrinology & Metabolism. 92 (8): 3040–3043. doi:10.1210/jc.2007-0581. Retrieved 27 September 2013.
- ^ a b Titus, S; Li, F; Stobezki, R; Akula, K; Unsal, E; Jeong, K; Dickler, M; Robson, M; Moy, F; Goswami, S; Oktay, K (2013). "Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans". Science Translational Medicine. 5 (172): 172ra21. doi:10.1126/scitranslmed.3004925. PMID 23408054.
- ^ Harris Bernstein, Carol Bernstein and Richard E. Michod (2011). Meiosis as an Evolutionary Adaptation for DNA Repair. Chapter 19 in DNA Repair. Inna Kruman editor. InTech Open Publisher. DOI: 10.5772/25117 http://www.intechopen.com/books/dna-repair/meiosis-as-an-evolutionary-adaptation-for-dna-repair
- ^ Greene, JG (1998). "Constructing a standard climacteric scale". Maturitas. 29 (1): 25–31. doi:10.1016/s0378-5122(98)00025-5. PMID 9643514.
- ^ Monterrosa-Castro, A; Romero-Pérez, I; Marrugo-Flórez, M; Fernández-Alonso, AM; Chedraui, P; Pérez-López, FR (2012). "Quality of life in a large cohort of mid-aged Colombian women assessed using the Cervantes Scale". Menopause. 19 (8): 924–30. doi:10.1097/gme.0b013e318247908d. PMID 22549166.
- ^ Schneider, Hermann P.G.; Naftolin, Frederick (2005). Climacteric medicine where do we go?. London: Taylor & Francis. p. 28. ISBN 9780203024966.
- ^ "Menopause 101". A primer for the perimenopausal. The North American Menopause Society. Retrieved 11 April 2013.
- ^ a b c Prior, Jerilynn. "Perimenopause". Centre for Menstrual Cycle and Ovulation Research (CeMCOR). Retrieved 10 May 2013.
- ^ Chichester, Melanie; Ciranni, Patricia (August–September 2011). "Approaching Menopause (But Not There Yet!)". Nursing for Women's Health. 15 (4): 320. doi:10.1111/j.175-486X.2011.01652.x. Retrieved 11 April 2013.
- ^ Hurst, Bradley S. (2011). Disorders of menstruation. Chichester, West Sussex: Wiley-Blackwell. ISBN 9781444391817.
- ^ a b McNamara, M; Batur, P; DeSapri, KT (3 February 2015). "In the clinic. Perimenopause". Annals of Internal Medicine. 162 (3): ITC1-15. doi:10.7326/AITC201502030. PMID 25643316.
- ^ Kessenich, Cathy. "Inevitable Menopause". Retrieved 11 April 2013.
- ^ Bellipanni, G; Di Marzo, F; Blasi, F; Di Marzo, A (December 2005). "Effects of melatonin in perimenopausal and menopausal women: our personal experience. 2005". Annals of the New York Academy of Sciences. 1057 (1): 393–402. doi:10.1196/annals.1356.030. PMID 16399909.
- ^ The Woman's Health Program Monash University, Oestrogen and Progestin as Hormone Therapy
- ^ "Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society". Menopause (New York, N.Y.). 17 (2): 242–55. March 2010. doi:10.1097/gme.0b013e3181d0f6b9. PMID 20154637.
- ^ a b Main, C; Knight, B; Moxham, T; Gabriel Sanchez, R; Sanchez Gomez, LM; Roqué i Figuls, M; Bonfill Cosp, X (30 April 2013). "Hormone therapy for preventing cardiovascular disease in post-menopausal women". Cochrane Database of Systematic Reviews. 4: CD002229. doi:10.1002/14651858.CD002229.pub3. PMID 23633307.
- ^ Maryam Darabi; Mohsen Ani; Mojtaba Panjehpour; Mohammed Rabbani; Ahmad Movahedian; Elahe Zarean (January–February 2011). "Effect of estrogen receptor beta A1730G polymorphism on ABCA1 gene expression response to postmenopausal hormone replacement therapy". Genetic Testing and Molecular Biomarkers. 15 (1–2): 11–15. doi:10.1089/gtmb.2010.0106. PMID 21117950.
- ^ de Villiers, TJ; Stevenson, JC (June 2012). "The WHI: the effect of hormone replacement therapy on fracture prevention". Climacteric. 15 (3): 263–6. doi:10.3109/13697137.2012.659975. PMID 22612613.
- ^ Marjoribanks, J; Farquhar, C; Roberts, H; Lethaby, A (11 July 2012). "Long term hormone therapy for perimenopausal and postmenopausal women". Cochrane Database of Systematic Reviews. 7: CD004143. doi:10.1002/14651858.CD004143.pub4. PMID 22786488.
- ^ Chlebowski, R. T.; Anderson, G. L. (2015). "Menopausal hormone therapy and breast cancer mortality: clinical implications". Therapeutic Advances in Drug Safety. 6 (2): 45–56. doi:10.1177/2042098614568300. ISSN 2042-0986.
- ^ Davis, SR; Dinatale, I; Rivera-Woll, L; Davison, S (2005). "Postmenopausal hormone therapy: from monkey glands to transdermal patches". J Endocrinol. 185 (2): 207–22. doi:10.1677/joe.1.05847. PMID 15845914.
- ^ Bevers, TB (2007). "The STAR Trial: Evidence for Raloxifene as a Breast Cancer Risk Reduction Agent for Postmenopausal Women". J Natl Compr Canc Netw. 5 (8): 817–22.
- ^ Orleans, RJ; Li, L; Kim, MJ; Guo, J; Sobhan, M; Soule, L; Joffe, HV (2014). "FDA approval of paroxetine for menopausal hot flushes". The New England Journal of Medicine. 370 (19): 1777–9. doi:10.1056/NEJMp1402080. PMID 24806158.
- ^ Nedrow, A; Miller, J; Walker, M; Nygren, P; Huffman, LH; Nelson, HD (2006). "Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review". Arch Intern Med. 166 (14): 1453–65. doi:10.1001/archinte.166.14.1453.
- ^ Bolaños, R; Del Castillo, A; Francia, J (2010). "Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: systematic review and meta-analysis". Menopause. 17 (3): 660–6. doi:10.1097/gme.0b013e3181cb4fb5. PMID 20464785.
- ^ Lethaby, A; Marjoribanks, J; Kronenberg, F; Roberts, H; Eden, J; Brown, J (10 December 2013). "Phytoestrogens for menopausal vasomotor symptoms". Cochrane Database of Systematic Reviews. 12: CD001395. doi:10.1002/14651858.CD001395.pub4. PMID 24323914.
- ^ EFSA Femarelle® and bone mineral density Scientific substantiation of a health claim related to "Femarelle®" and "induces bone formation and increases bone mineral density reducing the risk for osteoporosis and other bone disorders" pursuant to Article 14 of the Regulation (EC) No 1924/20061 Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies. The EFSA Journal (2008) 785, pp. 1–10]
- ^ Leach, MJ; Moore, V (12 September 2012). "Black cohosh (Cimicifuga spp.) for menopausal symptoms". Cochrane Database of Systematic Reviews. 9: CD007244. doi:10.1002/14651858.CD007244.pub2. PMID 22972105.
- ^ Dodin, S; Blanchet, C; Marc, I; Ernst, E; Wu, T; Vaillancourt, C; Paquette, J; Maunsell, E (30 July 2013). "Acupuncture for menopausal hot flushes". Cochrane Database of Systematic Reviews. 7: CD007410. doi:10.1002/14651858.CD007410.pub2. PMID 23897589.
- ^ Clement, YN; Onakpoya, I; Hung, SK; Ernst, E (March 2011). "Effects of herbal and dietary supplements on cognition in menopause: a systematic review". Maturitas. 68 (3): 256–63. doi:10.1016/j.maturitas.2010.12.005. PMID 21237589.
- ^ Zhu, X; Liew, Y; Liu, ZL (15 March 2016). "Chinese herbal medicine for menopausal symptoms". The Cochrane database of systematic reviews. 3: CD009023. doi:10.1002/14651858.CD009023.pub2. PMID 26976671. Retrieved 18 March 2016.
- ^ Wells, GA; Cranney, A; Peterson, J; Boucher, M; Shea, B; Robinson, V; Coyle, D; Tugwell, P (23 January 2008). "Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women". Cochrane Database of Systematic Reviews (1): CD001155. doi:10.1002/14651858.CD001155.pub2. PMID 18253985.
- ^ Winterich, J. (August 2008). "Gender, medicine, and the menopausal body: How biology and culture influence women's experiences with menopause". Paper presented at the annual meeting of the American Sociological Association, New York. Retrieved 11 November 2008 from Allacademic.com
- ^ Gannon, L; Ekstrom, B (1993). "Attitudes toward menopause: The influence of sociocultural paradigms". Psychology of Women Quarterly. 17: 275–288. doi:10.1111/j.1471-6402.1993.tb00487.x.
- ^ Avis, N.; Stellato, R. Crawford; Bromberger, J.; Gan, P.; Cain, V.; Kagawa-Singer, M (2001). "Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic group". Social Science & Medicine. 52 (3): 345–356. doi:10.1016/S0277-9536(00)00147-7.
- ^ a b Lock, M (1998). "Menopause: lessons from anthropology". Psychosomatic Medicine. 60 (4): 410–9. doi:10.1097/00006842-199807000-00005. PMID 9710286.
- ^ Melby, MK (2005). "Factor analysis of climacteric symptoms in Japan". Maturitas. 52 (3–4): 205–22. doi:10.1016/j.maturitas.2005.02.002. PMID 16154301.
- ^ a b Lock, M. & Nguyen, V. (2010) An Anthropology of Biomedicine, Chapter 4 "Local Biologies and Human Difference" (pp. 84–89), West Sussex, Wiley-Blackwell
- ^ Gold, EB; Block, G; Crawford, S; Lachance, L; FitzGerald, G; Miracle, H; Sherman, S (2004). "Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women's Health Across the Nation". American Journal of Epidemiology. 159 (12): 1189–99. doi:10.1093/aje/kwh168. PMID 15191936.
- ^ Maoz, B.; Dowty, N.; Antonovsky, A.; Wisjenbeck, H. (1970). "Female attitudes to menopause". Social Psychiatry. 5: 35–40. doi:10.1007/BF01539794.
- ^ Stotland, N. L. (2002). "Menopause: Social expectations, women's realities". Archives of Women's Mental Health. 5: 5–8. doi:10.1007/s007370200016.
- ^ Lock, Margaret M., and Vinh-Kim Nguyen. "Chapter 2". An Anthropology of Biomedicine. Chichester, West Sussex: Wiley-Blackwell, 2010. pp. 32–56. Print.
- ^ a b c Walker, ML; Herndon, JG (2008). "Menopause in nonhuman primates?". Biology of Reproduction. 79 (3): 398–406. doi:10.1095/biolreprod.108.068536. PMC 2553520. PMID 18495681.
- ^ Gaulin, SJ (1980). "Sexual Dimorphism in the Human Post-reproductive Life-span: Possible Causes". Journal of Human Evolution. 9 (3): 227–232. doi:10.1016/0047-2484(80)90024-X.
- ^ Holmberg, I. (1970), "Fecundity, Fertility and Family Planning". Demography Institute University of Gothenburg Reports. 10: pp. 1–109
- ^ Washburn, S.L. (1981). "Longevity in Primates". In: Aging: Biology and Behavior by McGaugh, J.L. and S. B. Kiesler, S.B. (eds). pp. 11–29. Academic Press.
- ^ Hawkes, K (2004). "Human longevity: The grandmother effect". Nature. 428 (6979): 128–129. doi:10.1038/428128a. PMID 15014476.
- ^ Ricklefs, RE; Wikelski, M (2002). "The Physiology/Life-history Nexus". Trends in Ecology & Evolution. 17 (10): 462–468. doi:10.1016/S0169-5347(02)02578-8.
- ^ Darwin, Charles. Origin of Species. Retrieved 24 September 2013.
- ^ Peccei, JS (2001). "Menopause: Adaptation or Epiphenomenon?". Evolutionary Anthropology. 10 (2): 43–57. doi:10.1002/evan.1013.
- ^ Lahdenperä, M; Lummaa, V; Helle, S; Tremblay, M; Russell, AF (2004). "Fitness benefits of prolonged post-reproductive lifespan in women". Nature. 428 (6979): 178–181. doi:10.1038/nature02367. PMID 15014499.
- ^ Voland, E. and Beise, J. (2002). "Opposite Effects of Maternal and Paternal Grandmothers on Infant Survival in Historical Krummörn". MPIDR WP 2001–026.
- ^ Mace, R and Sear, R. (2004). "Are Humans Communal Breeders?" In: Voland, E., Chasiotis, A. and Schiefenhoevel, W. (eds). Grandmotherhood—the Evolutionary Significance of the Second Half of Female Life. Rutgers University Press.
- ^ Peccei, JS (2001). "A critique of the grandmother hypotheses: Old and new". American Journal of Human Biology. 13 (4): 434–452. doi:10.1002/ajhb.1076. PMID 11400215.
- ^ "Engels was Right: Early Human Kinship was Matrilineal".
- ^ 10. Finch, C.E. 1990. Longevity senescence and the genome. University of Chicago Press. London.
- ^ Pavard, S; Sibert, A; Heyer, E (2007). "The effect of maternal care on child survival: a demographic, genetic, and evolutionary perspective". Evolution. 61 (5): 1153–61. doi:10.1111/j.1558-5646.2007.00086.x. PMID 17492968.
- ^ Walker R, S; Flinn, MV; Hill, KR (2010). "Evolutionary history of partible paternity in lowland South America". Proceedings of the National Academy of Sciences of the United States of America. 107 (45): 19195–200. doi:10.1073/pnas.1002598107. PMC 2984172. PMID 20974947.
- ^ Massart, F; Reginster, JY; Brandi, ML (2001). "Genetics of Menopause-Associatred Diseases". Maturitas. 40 (2): 103–116. doi:10.1016/S0378-5122(01)00283-3. PMID 11716989.
- ^ Leidy, L. "Menopause in evolutionary perspective". in: Trevathan WR, McKenna JJ, Smith EO (Eds.) Evolutionary Medicine. Oxford University Press, pp. 407–427
- ^ Clinical Obstetrics and Gynaecology (3 ed.). Elsevier Health Sciences. 2014. p. 69. ISBN 9780702054105.
- ^ Walker, ML (1995). "Menopause in female rhesus monkeys". Am J Primatol. 35: 59–71. doi:10.1002/ajp.1350350106.
- ^ Bowden, D. M. and Williams, D. D. (1985). Aging. Adv.Vet.Sci.Comp.Med. 28: 306–341
- ^ The Asian Elephant.
- ^ Marsh, H and Kasuya, T. (1986). Evidence for Reproductive Senescence in Female Cetaceans. Report of the International Whaling Commission. 8: pp. 57–74.
- ^ Brent L.J.N., Franks D.W.; et al. (2015). "Ecological Knowledge, Leadership, and the Evolution of Menopause in Killer Whales". Current Biology. 25 (6): 746–750. doi:10.1016/j.cub.2015.01.037.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ McAuliffe, K; Whitehead, H (2005). "Eusociality, menopause and information in matrilineal whales". Trends Ecol Evolution. 20 (12): 650. doi:10.1016/j.tree.2005.09.003. PMID 16701451.
- ^ DAL.ca
- ^ Reznick, D; Bryant, M; Holmes, D (January 2006). "The evolution of senescence and post-reproductive lifespan in guppies (Poecilia reticulata)". PLoS Biology. 4 (1): e7. doi:10.1371/journal.pbio.0040007. PMC 1318473. PMID 16363919.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Canine reproduction
- ^ dead link Archived 10 June 2015 at the Wayback Machine
