Quantum dot
It has been suggested that Artificial atom be merged into this article. (Discuss) Proposed since March 2015. |
This article may be too technical for most readers to understand. (June 2013) |

Quantum dots (QD) are semiconductor devices that tightly confine electrons or holes in all three spatial dimensions. They can be made via several possible routes including colloidal synthesis, plasma synthesis, or mechanical fabrication. The term “quantum dot” was coined by Mark Reed in 1988;[1] however, they were first discovered in a glass matrix[2] by Alexey Ekimov[3][4][5] in 1981 and in colloidal solutions by Louis E. Brus in 1985.[6] The electronic properties of the quantum dots fall between those of bulk semiconductors and those of discrete molecules of comparable size, and optoelectronic properties such as band gap, can be tuned as a function of particle size and shape for a given composition. For example, the photoluminescence of a QD can be manipulated to specific wavelengths by controlling particle diameter.[7][8][9] Larger QDs (radius of 5-6 nm, for example) emit longer wavelengths resulting in emission colors such as orange or red. Smaller QDs (radius of 2-3 nm, for example) emit shorter wavelengths resulting in colors like blue and green, although the specific colors and sizes vary depending on the exact composition of the QD.[10][11][12]
Because of the high tunability of properties, QDs are of interest in many research applications such as transistors, solar cells, LEDs, and diode lasers. For example, the ability of QDs to precisely convert and tune a spectrum makes them ideal for LCD displays. Previous LCD displays can waste energy converting red-green poor, blue-yellow rich white light into a more balanced lighting. By using QDs, only the necessary colors for ideal images are contained in the screen. The result is a screen that is brighter, clearer, and more energy-efficient. The first commercial application of quantum dots was the Sony XBR X900A series of flat panel televisions released in 2013.[13] QDs are also being researched as possible qubits for quantum computing. Beyond electronic applications, QDs are also being investigated in the medical field for medical imaging. Additionally, their small size allows for QDs to be suspended in solution which leads to possible uses in inkjet printing and spin-coating.[14] These processing techniques result in less-expensive and less time consuming methods of semiconductor fabrication.
| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
Quantum confinement in semiconductors

In a semiconductor crystallite whose size is smaller than twice the size of its exciton Bohr radius, the excitons are squeezed, leading to quantum confinement. The energy levels can then be modeled using the particle in a box model in which the energy of different states is dependent on the length of the box, much like the pitch of a string in a musical instrument is dependent on its length. Comparing the quantum dots size to the Bohr radius of the electron and hole wave functions, 3 regimes can be defined. A 'strong confinement regime' is defined as the quantum dots radius being smaller than both electron and hole Bohr radius, 'weak confinement' is given when the quantum dot is larger than both. For semiconductors in which electron and hole masses are markedly different, an 'intermediate confinement regime' exists, where the quantum dots radius is larger than the Bohr radius of one (typically the hole), but not the other charge carrier.

- Band gap energy
- The band gap can become larger in the strong confinement regime where the size of the quantum dot is smaller than the Exciton Bohr radius ab* as the energy levels split up.
- where ab is the Bohr radius=0.053 nm, m is the mass, μ is the reduced mass, and εr is the size-dependent dielectric constant (Relative permittivity).
- This results in the increase in the total emission energy (the sum of the energy levels in the smaller band gaps in the strong confinement regime is larger than the energy levels in the band gaps of the original levels in the weak confinement regime) and the emission at various wavelengths; which is precisely what happens in the sun, where the quantum confinement effects are completely dominant and the energy levels split up to the degree that the energy spectrum is almost continuous, thus emitting white light[citation needed].
- Confinement energy
- The exciton entity can be modeled using the particle in the box. The electron and the hole can be seen as hydrogen in the Bohr model with the hydrogen nucleus replaced by the hole of positive charge and negative electron mass. Then the energy levels of the exciton can be represented as the solution to the particle in a box at the ground level (n = 1) with the mass replaced by the reduced mass. Thus by varying the size of the quantum dot, the confinement energy of the exciton can be controlled.
- Bound exciton energy
- There is Coulomb attraction between the negatively charged electron and the positively charged hole. The negative energy involved in the attraction is proportional to Rydberg's energy and inversely proportional to square of the size-dependent dielectric constant[15] of the semiconductor. When the size of the semiconductor crystal is smaller than the Exciton Bohr radius, the Coulomb interaction must be modified to fit the situation.
Therefore, the sum of these energies can be represented as:
where μ is the reduced mass, a is the radius, me is the free electron mass, mh is the hole mass, and εr is the size-dependent dielectric constant.
Although the above equations were derived using simplifying assumptions, the implications are clear; the energy of the quantum dots is dependent on their size due to the quantum confinement effects, which dominate below the critical size leading to changes in the optical properties. This effect of quantum confinement on the quantum dots has been experimentally verified[16] and is a key feature of many emerging electronic structures.[17][18]
Besides confinement in all three dimensions (i.e., a quantum dot), other quantum confined semiconductors include:
- Quantum wires, which confine electrons or holes in two spatial dimensions and allow free propagation in the third.
- Quantum wells, which confine electrons or holes in one dimension and allow free propagation in two dimensions.
Production

There are several ways to confine excitons in semiconductors, resulting in different methods to produce quantum-confined semiconductor structures. In general, quantum wires, wells and dots are grown by advanced epitaxial techniques. Nanocrystals may be produced by gas-phase, liquid-phase and solid-phase approaches.[19]
Colloidal synthesis
Colloidal semiconductor nanocrystals are synthesized from precursor compounds dissolved in solutions, much like traditional chemical processes. The synthesis of colloidal quantum dots is done by using precursors,[8] organic surfactants,[20] and solvents. Heating the solution at high temperature, the precursors decompose forming monomers which then nucleate and generate nanocrystals. The temperature during the synthetic process is a critical factor in determining optimal conditions for the nanocrystal growth. It must be high enough to allow for rearrangement and annealing of atoms during the synthesis process while being low enough to promote crystal growth. The concentration of monomers is another critical factor that has to be stringently controlled during nanocrystal growth. The growth process of nanocrystals can occur in two different regimes, "focusing" and "defocusing". At high monomer concentrations, the critical size (the size where nanocrystals neither grow nor shrink) is relatively small, resulting in growth of nearly all particles. In this regime, smaller particles grow faster than large ones (since larger crystals need more atoms to grow than small crystals) resulting in "focusing" of the size distribution to yield nearly monodisperse particles. The size focusing is optimal when the monomer concentration is kept such that the average nanocrystal size present is always slightly larger than the critical size. Over time, the monomer concentration diminishes, the critical size becomes larger than the average size present, and the distribution "defocuses".
There are colloidal methods to produce many different semiconductors. Typical dots are made of binary compounds such as lead sulfide, lead selenide, cadmium selenide, cadmium sulfide, indium arsenide, and indium phosphide. Dots may also be made from ternary compounds such as cadmium selenide sulfide. These quantum dots can contain as few as 100 to 100,000 atoms within the quantum dot volume, with a diameter of ~ 10 to 50 atoms. This corresponds to about 2 to 10 nanometers, and at 10 nm in diameter, nearly 3 million quantum dots could be lined up end to end and fit within the width of a human thumb.
Large batches of quantum dots may be synthesized via colloidal synthesis. Due to this scalability and the convenience of benchtop conditions, colloidal synthetic methods are promising for commercial applications. It is acknowledged[citation needed] to be the least toxic of all the different forms of synthesis.
Plasma synthesis
Plasma synthesis has evolved to be one of the most popular gas-phase approaches for the production of quantum dots, especially those with covalent bonds.[21][22][23] For example, silicon (Si) and germanium (Ge) quantum dots have been synthesized by using nonthermal plasma. The size, shape, surface and composition of quantum dots can all be controlled in nonthermal plasma.[24][25] Doping that seems quite challenging for quantum dots has also been realized in plasma synthesis.[26][27][28] Quantum dots synthesized by plasma are usually in the form of powder, for which surface modification may be carried out. This can lead to excellent dispersion of quantum dots in either organic solvents[29] or water[30] (i. e., colloidal quantum dots).
Fabrication
- Self-assembled quantum dots are typically between 5 and 50 nm in size. Quantum dots defined by lithographically patterned gate electrodes, or by etching on two-dimensional electron gases in semiconductor heterostructures can have lateral dimensions between 20 and 100 nm.
- Some quantum dots are small regions of one material buried in another with a larger band gap. These can be so-called core–shell structures, e.g., with CdSe in the core and ZnS in the shell or from special forms of silica called ormosil.
- Quantum dots sometimes occur spontaneously in quantum well structures due to monolayer fluctuations in the well's thickness.
- Self-assembled quantum dots nucleate spontaneously under certain conditions during molecular beam epitaxy (MBE) and metallorganic vapor phase epitaxy (MOVPE), when a material is grown on a substrate to which it is not lattice matched. The resulting strain produces coherently strained islands on top of a two-dimensional wetting layer. This growth mode is known as Stranski–Krastanov growth. The islands can be subsequently buried to form the quantum dot. This fabrication method has potential for applications in quantum cryptography (i.e. single photon sources) and quantum computation. The main limitations of this method are the cost of fabrication and the lack of control over positioning of individual dots.
- Individual quantum dots can be created from two-dimensional electron or hole gases present in remotely doped quantum wells or semiconductor heterostructures called lateral quantum dots. The sample surface is coated with a thin layer of resist. A lateral pattern is then defined in the resist by electron beam lithography. This pattern can then be transferred to the electron or hole gas by etching, or by depositing metal electrodes (lift-off process) that allow the application of external voltages between the electron gas and the electrodes. Such quantum dots are mainly of interest for experiments and applications involving electron or hole transport, i.e., an electrical current.
- The energy spectrum of a quantum dot can be engineered by controlling the geometrical size, shape, and the strength of the confinement potential. Also, in contrast to atoms, it is relatively easy to connect quantum dots by tunnel barriers to conducting leads, which allows the application of the techniques of tunneling spectroscopy for their investigation.
The quantum dot absorption features correspond to transitions between discrete,three-dimensional particle in a box states of the electron and the hole, both confined to the same nanometer-size box.These discrete transitions are reminiscent of atomic spectra and have resulted in quantum dots also being called artificial atoms.[31]
- Confinement in quantum dots can also arise from electrostatic potentials (generated by external electrodes, doping, strain, or impurities).
- CMOS technology can be employed to fabricate silicon quantum dots. Ultra small (L=20 nm, W=20 nm) CMOS transistors behave as single electron quantum dots when operated at cryogenic temperature over a range of −269 °C (4 K) to about −258 °C (15 K). The transistor displays Coulomb blockade due to progressive charging of electrons one by one. The number of electrons confined in the channel is driven by the gate voltage, starting from an occupation of zero electrons, and it can be set to 1 or many.[32]
Viral assembly
Lee et al. (2002) reported using genetically engineered M13 bacteriophage viruses to create quantum dot biocomposite structures.[33] As a background to this work, it has previously been shown that genetically engineered viruses can recognize specific semiconductor surfaces through the method of selection by combinatorial phage display.[34] Additionally, it is known that liquid crystalline structures of wild-type viruses (Fd, M13, and TMV) are adjustable by controlling the solution concentrations, solution ionic strength, and the external magnetic field applied to the solutions. Consequently, the specific recognition properties of the virus can be used to organize inorganic nanocrystals, forming ordered arrays over the length scale defined by liquid crystal formation. Using this information, Lee et al. (2000) were able to create self-assembled, highly oriented, self-supporting films from a phage and ZnS precursor solution. This system allowed them to vary both the length of bacteriophage and the type of inorganic material through genetic modification and selection.
Electrochemical assembly
Highly ordered arrays of quantum dots may also be self-assembled by electrochemical techniques. A template is created by causing an ionic reaction at an electrolyte-metal interface which results in the spontaneous assembly of nanostructures, including quantum dots, onto the metal which is then used as a mask for mesa-etching these nanostructures on a chosen substrate.
Bulk-manufacture
Quantum dot manufacturing relies on a process called "high temperature dual injection" which has been scaled by multiple companies for commercial applications that require large quantities (hundreds of kilograms to tonnes) of quantum dots. This is a reproducible production method that can be applied to a wide range of quantum dot sizes and compositions.
The bonding in certain cadmium-free quantum dots, such as III-V-based quantum dots, is more covalent than that in II-VI materials, therefore it is more difficult to separate nanoparticle nucleation and growth via a high temperature dual injection synthesis. An alternative method of quantum dot synthesis, the “molecular seeding” process, provides a reproducible route to the production of high quality quantum dots in large volumes. The process utilises identical molecules of a molecular cluster compound as the nucleation sites for nanoparticle growth, thus avoiding the need for a high temperature injection step. Particle growth is maintained by the periodic addition of precursors at moderate temperatures until the desired particle size is reached.[35] The molecular seeding process is not limited to the production of cadmium-free quantum dots; for example, the process can be used to synthesise kilogram batches of high quality II-VI quantum dots in just a few hours.
Another approach for the mass production of colloidal quantum dots can be seen in the transfer of the well-known hot-injection methodology for the synthesis to a technical continuous flow system. The batch-to-batch variations arising from the needs during the mentioned methodology can be overcome by utilizing technical components for mixing and growth as well as transport and temperature adjustments. For the production of CdSe based semiconductor nanoparticles this method has been investigated and tuned to production amounts of kg per month. Since the use of technical components allows for easy interchange in regards of maximum through-put and size, it can be further enhanced to tens or even hundreds of kilograms.[36]
In 2011 a consortium of U.S. and Dutch companies reported a "milestone" in high volume quantum dot manufacturing by applying the traditional high temperature dual injection method to a flow system.[37]
On January 23, 2013 Dow entered into an exclusive licensing agreement with UK-based Nanoco for the use of their low-temperature molecular seeding method for bulk manufacture of cadmium-free quantum dots for electronic displays, and on September 24, 2014 Dow commenced work on the production facility in South Korea capable of producing sufficient quantum dots for "millions of cadmium-free televisions and other devices, such as tablets". Mass production is due to commence in mid-2015.[38] On 24 March 2015 Dow announced a partnership deal with LG Electronics to develop the use of cadmium free quantum dots in displays.[39]
Heavy metal-free quantum dots
In many regions of the world there is now a restriction or ban on the use of heavy metals in many household goods, which means that most cadmium based quantum dots are unusable for consumer-goods applications.
For commercial viability, a range of restricted, heavy metal-free quantum dots has been developed showing bright emissions in the visible and near infra-red region of the spectrum and have similar optical properties to those of CdSe quantum dots. Among these systems are InP/ZnS and CuInS/ZnS, for example.
Peptides are being researched as potential quantum dot material.[40] Since peptides occur naturally in all organisms, such dots would likely be nontoxic and easily biodegraded.
Safety
Environmental impact
The environmental impact of bulk manufacturing and consumption of quantum dots is currently undergoing studies in both private and public labs.[citation needed]
Biological Toxicity
Despite the invaluable potential applications of quantum dots, current literature reveals that some quantum dots pose risks to human health and the environment under certain condition.[41][42][43] Notably, the studies on quantum dots toxicity are focused on cadmium containing particles and has yet to be demonstrated in animal models after physiologically relevant dosing.[43] In vitro studies, based on cell cultures, on quantum dots (QD) toxicity suggests that their toxicity may derive from multiple factors including its physicochemical characteristics (size, shape, composition, surface functional groups, and surface charges) and environment. Assessing their potential toxicity is complex as these factors include properties such as QD size, charge, concentration, chemical composition, capping ligands, and also on their oxidative, mechanical and photolytic stability.[41]
Many research studies have focused on the mechanism of QD cytotoxicity using model cell cultures. It has been demonstrated that after exposure to ultraviolet radiation or oxidized by air CdSe QDs release free cadmium ions causing cell death.[44] Group II-VI QDs also have been reported to induce the formation of reactive oxygen species after exposure to light, which in turn can damage cellular components such as proteins, lipids and DNA.[45] Some studies have also demonstrated that addition of a ZnS shell inhibit the process of reactive oxygen species in CdSe QDs. Another aspect of QD toxicity is the process of their size dependent intracellular pathways that concentrate these particles in cellular organelles that are inaccessible by metal ions, which may result in unique patterns of cytotoxicity compared to their constituent metal ions.[46] The reports of QD localization in the cell nucleus[47] present additional modes of toxicity because they may induce DNA mutation, which in turn will propagate through future generation of cells causing diseases.
Though concentration of QDs in certain organelles have been reported in in vivo studies using animal models, interestingly, no alterations in animal behavior, weight, hematological markers or organ damage has been found through either histological or biochemical analysis.[48] These finding have led scientists to believe that intracellular dose is the most important deterring factor for QD toxicity. Therefore factors determining the QD endocytosis that determine the effective intracellular concentration, such as QD size, shape and surface chemistry determine their toxicity. Excretion of QDs through urine in animal models also have demonstrated via injecting radio-labeled ZnS capped CdSe QDs where the ligand shell was labelled with 99mTc.[49] Though multiple other studies have concluded retention of QDs in cellular levels,[43][50] exocytosis of QDs is still poorly studied in the literature.
While significant research efforts have broadened the understanding of toxicity of QDs, there are large discrepancies in the literature and questions still remains to be answered. Diversity of this class material as compared to normal chemical substances makes the assessment of their toxicity very challenging. As their toxicity may also be dynamic depending on the environmental factors such as pH level, light exposure and cell type, traditional methods of assessing toxicity of chemicals such as LD50 are not applicable for QDs. Therefore researchers are focusing on introducing novel approaches and adapting existing methods to include this unique class of materials.[43] Furthermore, novel strategies to engineer safer QDs are still under exploration by the scientific community.
Optical properties

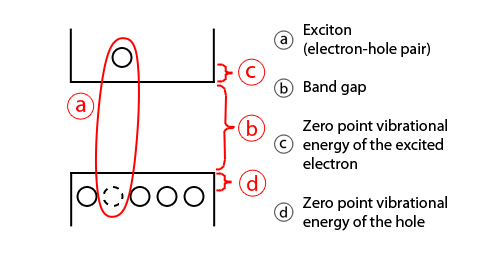
In semiconductors, light absorption generally leads to an electron being excited from the valence to the conduction band, leaving behind a hole. The electron and the hole can bind to each other to form an exciton. When this exciton recombines (i.e. the electron resumes its ground state), the exciton's energy can be emitted as light. This is called Fluorescence. In a simplified model, the energy of the emitted photon can be understood as the sum of the band gap energy between the highest occupied level and the lowest unoccupied energy level, the confinement energies of the hole and the excited electron, and the bound energy of the exciton (the electron-hole pair):
As the confinement energy depends on the quantum dots size, both absorption onset and fluorescence emission can be tuned by changing the size of the quantum dot during its synthesis. The larger the dot, the redder (lower energy) its absorption onset and fluorescence spectrum. Conversely, smaller dots absorb and emit bluer (higher energy) light. Recent articles in Nanotechnology and in other journals have begun to suggest that the shape of the quantum dot may be a factor in the coloration as well, but as yet not enough information is available. Furthermore, it was shown [51] that the lifetime of fluorescence is determined by the size of the quantum dot. Larger dots have more closely spaced energy levels in which the electron-hole pair can be trapped. Therefore, electron-hole pairs in larger dots live longer causing larger dots to show a longer lifetime.
To improve fluorescence quantum yield, quantum dots can be made with "shells" of a larger bandgap semiconductor material around them. The improvement is suggested to be due to the reduced access of electron and hole to non-radiative surface recombination pathways in some cases, but also due to reduced auger recombination in others.
Applications
Quantum dots are particularly significant for optical applications due to their high extinction coefficient.[52] In electronic applications they have been proven to operate like a single electron transistor and show the Coulomb blockade effect. Quantum dots have also been suggested as implementations of qubits for quantum information processing.
The ability to tune the size of quantum dots is advantageous for many applications. For instance, larger quantum dots have a greater spectrum-shift towards red compared to smaller dots, and exhibit less pronounced quantum properties. Conversely, the smaller particles allow one to take advantage of more subtle quantum effects.
Being zero-dimensional, quantum dots have a sharper density of states than higher-dimensional structures. As a result, they have superior transport and optical properties, and are being researched for use in diode lasers, amplifiers, and biological sensors. Quantum dots may be excited within a locally enhanced electromagnetic field produced by gold nanoparticles, which can then be observed from the surface plasmon resonance in the photoluminescent excitation spectrum of (CdSe)ZnS nanocrystals. High-quality quantum dots are well suited for optical encoding and multiplexing applications due to their broad excitation profiles and narrow/symmetric emission spectra. The new generations of quantum dots have far-reaching potential for the study of intracellular processes at the single-molecule level, high-resolution cellular imaging, long-term in vivo observation of cell trafficking, tumor targeting, and diagnostics.
Moreover, a new range of applications involving quantum dots became accessible through the exploitation of CdSe nanocrystals as efficient triplet photosensitizers.[54] Laser excitation of small CdSe nanoparticles enables the extraction of the excited state energy from the Quantum Dots into bulk solution, thus opening the door to a wide range of applications such as photodynamic therapy, photovoltaic devices, molecular electronics, and catalysis.
Computing
Quantum dot technology is one of the most promising candidates for use in solid-state quantum computation. By applying small voltages to the leads, the flow of electrons through the quantum dot can be controlled and thereby precise measurements of the spin and other properties therein can be made. With several entangled quantum dots, or qubits, plus a way of performing operations, quantum calculations and the computers that would perform them might be possible.
Biology
In modern biological analysis, various kinds of organic dyes are used. However, as technology advances, greater flexibility in these dyes is required which traditional dyes are often unable to provide.[55] To this end, quantum dots have quickly filled in the role, being found to be superior to traditional organic dyes on several counts, one of the most immediately obvious being brightness (owing to the high extinction coefficient combined with a comparable quantum yield to fluorescent dyes[56]) as well as their stability (allowing much less photobleaching). It has been estimated that quantum dots are 20 times brighter and 100 times more stable than traditional fluorescent reporters.[55] For single-particle tracking, the irregular blinking of quantum dots is a minor drawback. However, there have been groups which have developed quantum dots which are essentially nonblinking and demonstrated their utility in single molecule tracking experiments.[57][58]
The usage of quantum dots for highly sensitive cellular imaging has seen major advances over the past decade.[59] The improved photostability of quantum dots, for example, allows the acquisition of many consecutive focal-plane images that can be reconstructed into a high-resolution three-dimensional image.[60] Another application that takes advantage of the extraordinary photostability of quantum dot probes is the real-time tracking of molecules and cells over extended periods of time.[61] Antibodies, streptavidin,[62] peptides,[63] DNA,[64] nucleic acid aptamers,[65] or small-molecule ligands [20] can be used to target quantum dots to specific proteins on cells. Researchers were able to observe quantum dots in lymph nodes of mice for more than 4 months.[66]
Semiconductor quantum dots have also been employed for in vitro imaging of pre-labeled cells. The ability to image single-cell migration in real time is expected to be important to several research areas such as embryogenesis, cancer metastasis, stem cell therapeutics, and lymphocyte immunology.
One particular application of Quantum dots in biology is as donor fluorophores in Förster resonance energy transfer, where the large extinction coefficient and spectral purity of these fluorophores make them superior to molecular fluorophores[67] It is also worth noting that the broad absorbance of QDs allows selective excitation of the QD donor and a minimum excitation of a dye acceptor in FRET-based studies.[68] The applicability of the FRET model, which assumes that the Quantum Dot can be approximated as a point dipole, has recently been demonstrated[69]
Scientists have proven that quantum dots are dramatically better than existing methods for delivering a gene-silencing tool, known as siRNA, into cells.[70]
First attempts have been made to use quantum dots for tumor targeting under in vivo conditions. There exist two basic targeting schemes: active targeting and passive targeting. In the case of active targeting, quantum dots are functionalized with tumor-specific binding sites to selectively bind to tumor cells. Passive targeting uses the enhanced permeation and retention of tumor cells for the delivery of quantum dot probes. Fast-growing tumor cells typically have more permeable membranes than healthy cells, allowing the leakage of small nanoparticles into the cell body. Moreover, tumor cells lack an effective lymphatic drainage system, which leads to subsequent nanoparticle-accumulation.
One of the remaining issues with quantum dot probes is their potential in vivo toxicity. For example, CdSe nanocrystals are highly toxic to cultured cells under UV illumination. The energy of UV irradiation is close to that of the covalent chemical bond energy of CdSe nanocrystals. As a result, semiconductor particles can be dissolved, in a process known as photolysis, to release toxic cadmium ions into the culture medium. In the absence of UV irradiation, however, quantum dots with a stable polymer coating have been found to be essentially nontoxic.[66][71] Hydrogel encapsulation of quantum dots allows for quantum dots to be introduced into a stable aqueous solution, reducing the possibility of cadmium leakage.Then again, only little is known about the excretion process of quantum dots from living organisms.[72]
Another potential cutting-edge application of quantum dots is being researched, with quantum dots acting as the inorganic fluorophore for intra-operative detection of tumors using fluorescence spectroscopy.
Delivery of undamaged quantum dots to the cell cytoplasm has been a challenge with existing techniques. Vector-based methods have resulted in aggregation and endosomal sequestration of quantum dots while electroporation can damage the semi-conducting particles and aggregate delivered dots in the cytosol. Cell squeezing – a method invented in 2013 by Armon Sharei, Robert Langer and Klavs Jensen at MIT – has demonstrated efficient cytosolic delivery of quantum dots without inducing aggregation, trapping material in endosomes, or significant loss of cell viability. Moreover, it has shown that individual quantum dots delivered by this approach are detectable in the cell cytosol, thus illustrating the potential of this technique for single molecule tracking studies. These results indicate that Cell squeezing could potentially be implemented as a robust platform for quantum dot based imaging in a variety of applications.[73]
Photovoltaic devices
The tunable absorption spectrum and high extinction coefficients of quantum dots have made them attractive for light harvesting technologies such as photovoltaics. Quantum dots may be able to increase the efficiency and reduce the cost of today's typical silicon photovoltaic cells. According to an experimental proof from 2004,[74] quantum dots of lead selenide can produce more than one exciton from one high energy photon via the process of carrier multiplication or multiple exciton generation (MEG). This compares favorably to today's photovoltaic cells which can only manage one exciton per high-energy photon, with high kinetic energy carriers losing their energy as heat. Quantum dot photovoltaics would theoretically be cheaper to manufacture, as they can be made "using simple chemical reactions."
Quantum Dot only Solar Cells
Research published by Nano Letters in 2015 suggests that aromatic self-assembled monolayers (SAMs) (e.g. 4-nitrobenzoic acid) can be used to improve the band alignment at electrodes for better efficiencies. This technique has provided a record power conversion efficiency (PCE) of 10.7%.[75] The SAM is positioned between ZnO-PbS colloidal quantum dot (CQD) film junction to modify band alignment via the dipole moment of the constituent SAM molecule, and the band tuning may be modified via the density, dipole and the orientation of the SAM molecule.[75]
Quantum Dot in Hybrid Solar Cells
Colloidal quantum dots are also used in inorganic/organic hybrid solar cells. These solar cells are attractive because of potentially their low-cost fabrication and relatively high efficiency.[76] Incorporation of metal oxides, such as ZnO, TiO2, and Nb2O5 nanomaterials into organic photovoltaics have been commercialized using full roll-to-roll processing.[76] In 2015, a publication in Scientific Reports show a 13.2% power conversion efficiency in Si nanowire/PEDOT:PSS hybrid solar cells.[77]
Quantum Dot with Nanowire in Solar Cells
Another use of quantum dots was researched in 2007 when a group from the University of Minnesota created a photovoltaic device that included capping single-crystal ZnO nanowires with CdSe quantum dots, immersed in mercaptopropionic acid as hole transport medium in order to obtain a QD-sensitized solar cell. The morphology of the nanowires allowed the electrons to have a direct pathway to the photoanode. This form of solar cell has been shown to exhibit 50-60% internal quantum efficiencies.[78]
Nanowires with quantum dot coatings have been researched by individuals at University of Science and Technology of China, with silicon nanowires (SiNW) and carbon quantum dots. The use of SiNWs instead of planar silicon enhances the antiflection properties of Si.[79] The SiNW exhibits a light-trapping effect due to light trapping in the SiNW. This use of SiNWs in conjunction with carbon quantum dots resulted in a solar cell that reached 9.10% PCE.[79]
Graphene quantum dots have also been blended with organic electronic materials to improve efficiency and lower cost in photovoltaic devices and organic light emitting diodes (OLEDs) in compared to graphene sheets. These graphene quantum dots were functionalized with organic ligands that experience photoluminescence from UV-Vis absorption.[80]
Light emitting devices
There are several proposed methods for using quantum dots to improve existing light-emitting diode (LED) design, including "Quantum Dot Light Emitting Diode" (QD-LED) displays and "Quantum Dot White Light Emitting Diode" (QD-WLED) displays. Because Quantum dots naturally produce monochromatic light, they can be more efficient than light sources which must be color filtered. QD-LEDs can be fabricated on a silicon substrate, which allows them to be integrated onto standard silicon-based integrated circuits or microelectromechanical systems.[81] Quantum dots are valued for displays, because they emit light in very specific gaussian distributions. This can result in a display with visibly more accurate colors. A conventional color liquid crystal display (LCD) is usually backlit by fluorescent lamps (CCFLs) or conventional white LEDs that are color filtered to produce red, green, and blue pixels. An improvement is using a conventional blue-emitting LED as light source and converting part of the emitted light into pure green and red light by the appropriate quantum dots placed in front of the blue LED. This type of white light as the backlight of an LCD panel allows for the best color gamut at lower cost than a RGB LED combination using three LEDs.
In June 2006, QD Vision announced technical success in making a proof-of-concept quantum dot display and show a bright emission in the visible and near infra-red region of the spectrum. A QD-LED integrated at a scanning microscopy tip was used to demonstrate fluorescence near-field scanning optical microscopy (NSOM) imaging.[82]
Photodetector devices
Quantum dot photodetectors (QDPs) can be fabricated either via solution-processing,[83] or from conventional single-crystalline semiconductors.[84] Conventional single-crystalline semiconductor QDPs are precluded from integration with flexible organic electronics due to the incompatibility of their growth conditions with the process windows required by organic semiconductors. On the other hand, solution-processed QDPs can be readily integrated with an almost infinite variety of substrates, and also postprocessed atop other integrated circuits. Such colloidal QDPs have potential applications in surveillance, machine vision, industrial inspection, spectroscopy, and fluorescent biomedical imaging.
Photocatalysts
Quantum dots also function as photocatalysts for the light driven chemical conversion of water into hydrogen as a pathway to solar fuel. In photocatalysis, electron hole pairs formed in the dot under band gap excitation drive redox reactions in the surrounding liquid. Generally, the photocatalytic activity of the dots is related to the particle size and its degree of quantum confinement.[85] This is because the band gap determines the chemical energy that is stored in the dot in the excited state. An obstacle for the use of quantum dots in photocatalysis is the presence of surfactants on the surface of the dots. These surfactants (or ligands) interfere with the chemical reactivity of the dots by slowing down mass transfer and electron transfer processes. Also, quantum dots made of metal chalcogenides are chemically unstable under oxidizing conditions and undergo photo corrosion reactions.
Theoretical models
A variety of theoretical frameworks exist to model optical, electronic, and structural properties of quantum dots. These may be broadly divided into quantum mechanical, semiclassical, and classical.
Quantum mechanics
Quantum mechanical models and simulations of quantum dots often involve the interaction of electrons with a pseudopotential or random matrix.[86]
Semiclassical
Semiclassical models of quantum dots frequently incorporate a chemical potential. For example, The thermodynamic chemical potential of an N-particle system is given by
whose energy terms may be obtained as solutions of the Schrödinger equation. The definition of capacitance,
- ,
with the potential difference
may be applied to a quantum dot with the addition or removal of individual electrons,
- and .
Then
is the "quantum capacitance" of a quantum dot, where we denoted by I(N) the ionization potential and by A(N) the electron affinity of the N-particle system.[87]
Classical mechanics
Classical models of electrostatic properties of electrons in quantum dots are similar in nature to the Thomson problem of optimally distributing electrons on a unit sphere.
The classical electrostatic treatment of electrons confined to spherical quantum dots is similar to their treatment in the Thomson,[88] or plum pudding model, of the atom.[89]
The classical treatment of both two-dimensional and three-dimensional quantum dots exhibit electron shell-filling behavior. A "periodic table of classical artificial atoms" has been described for two-dimensional quantum dots.[90] As well, several connections have been reported between the three-dimensional Thomson problem and electron shell-filling patterns found in naturally-occurring atoms found throughout the periodic table.[91] This latter work originated in classical electrostatic modeling of electrons in a spherical quantum dot represented by an ideal dielectric sphere.[92]
See also
- Core–shell semiconductor nanocrystal
- Fluorescence
- Nanocrystal solar cell
- Programmable matter
- Quantum dot display
- Quantum dot laser
- Quantum point contact
- Quantum well, carriers confined in one dimension.
- Quantum wire, carriers confined in two dimensions.
- Trojan wave packet
- Superatom
References
- ^ Reed MA, Randall JN, Aggarwal RJ, Matyi RJ, Moore TM, Wetsel AE (1988). "Observation of discrete electronic states in a zero-dimensional semiconductor nanostructure" (PDF). Phys Rev Lett. 60 (6): 535–537. Bibcode:1988PhRvL..60..535R. doi:10.1103/PhysRevLett.60.535. PMID 10038575.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ E. V. Kolobkova, N. V. Nikonorov, V. A. Aseev, "Optical Technologies Silver Nanoclusters Influence on Formation of Quantum Dots in Fluorine Phosphate Glasses", Scientific and Technical Journal of Information Technologies, Mechanics and Optics, Volume 5, Number 12, 2012
- ^ Екимов АИ, Онущенко АА (1981). "Квантовый размерный эффект в трехмерных микрокристаллах полупроводников" (PDF). Письма в ЖЭТФ. 34: 363–366.
- ^ Ekimov AI, Onushchenko AA (1982). "Quantum size effect in the optical-spectra of semiconductor micro-crystals". Soviet Physics Semiconductors-USSR. 16 (7): 775–778.
- ^ Ekimov AI, Efros AL, Onushchenko AA (1985). "Quantum size effect in semiconductor microcrystals". Solid State Communications. 56 (11): 921–924. Bibcode:1985SSCom..56..921E. doi:10.1016/S0038-1098(85)80025-9.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Nanotechnology Timeline". National Nanotechnology Initiative.
- ^ Norris, D.J. (1995). "Measurement and Assignment of the Size-Dependent Optical Spectrum in Cadmium Selenide (CdSe) Quantum Dots, PhD thesis, MIT". hdl:1721.1/11129.
- ^ a b Murray, C. B.; Kagan, C. R.; Bawendi, M. G. (2000). "Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies". Annual Review of Materials Research. 30 (1): 545–610. Bibcode:2000AnRMS..30..545M. doi:10.1146/annurev.matsci.30.1.545.
- ^ Brus, L.E. (2007). "Chemistry and Physics of Semiconductor Nanocrystals" (PDF). Retrieved 7 July 2009.
- ^ "Quantum Dots". Nanosys - Quantum Dot Pioneers. Retrieved 4 December 2015.
- ^ "Nanotechnology Information Center: Properties, Applications, Research, and Safety Guidelines". American Elements.
- ^ Wu, Wei-Yu; Schulman, J. N.; Hsu, T. Y.; Efron, Uzi (7 September 1987). "Effect of size nonuniformity on the absorption spectrum of a semiconductor quantum dot system". Applied Physics Letters. 51 (10): 710–712. doi:10.1063/1.98896. ISSN 0003-6951.
- ^ Bullis, Kevin. (2013-01-11) Quantum Dots Produce More Colorful Sony TVs | MIT Technology Review. Technologyreview.com. Retrieved on 2015-07-19.
- ^ Coe-Sullivan, S.; Steckel, J. S.; Woo, W.-K.; Bawendi, M. G.; Bulović, V. (1 July 2005). "Large-Area Ordered Quantum-Dot Monolayers via Phase Separation During Spin-Casting". Advanced Functional Materials. 15 (7): 1117–1124. doi:10.1002/adfm.200400468. ISSN 1616-3028.
- ^ Brandrup, J.; Immergut, E.H. (1966). Polymer Handbook (2 ed.). New York: Wiley. pp. 240–246.
- ^ Khare, Ankur, Wills, Andrew W., Ammerman, Lauren M., Noris, David J., and Aydil, Eray S. (2011). "Size control and quantum confinement in Cu2ZnSnS4 nanocrystals". Chem. Commun. 47 (42): 47. doi:10.1039/C1CC14687D.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greenemeier, L. (5 February 2008). "New Electronics Promise Wireless at Warp Speed". Scientific American.
- ^ "SCIENCE WATCH; Tiny Lasers Break Speed Record". The New York Times. 31 December 1991.
- ^ Delerue, C. and Lannoo, M. (2004). Nanostructures: Theory and Modelling. Springer. p. 47. ISBN 3-540-20694-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Zherebetskyy D., Scheele M., Zhang Y., Bronstein N., Thompson C., Britt D., Salmeron M., Alivisatos P., Wang L.W. (2014). "Hydroxylation of the surface of PbS nanocrystals passivated with oleic acid". Science. 344 (6190): 1380–1384. Bibcode:2014Sci...344.1380Z. doi:10.1126/science.1252727. PMID 24876347.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mangolini, L.; Thimsen, E.; Kortshagen, U. (2005). "High-yield plasma synthesis of luminescent silicon nanocrystals". Nano Letters. 5: 655–659. doi:10.1021/nl050066y.
- ^ Knipping, J.; Wiggers, H.; Rellinghaus, B.; Roth, P.; Konjhodzic, D.; Meier, C. (2004). "Synthesis of high purity silicon nanoparticles in a low Pressure microwave reactor". Journal of Nanoscience and Technology. 4: 1039–1044. doi:10.1166/jnn.2004.149.
- ^ Sankaran, R. M.; Holunga, D.; Flagan, R. C.; Giapis, K. P. (2005). "Synthesis of blue luminescent Si nanoparticles using atmospheric-pressure microdischarges". Nano Letters. 5: 537–541. doi:10.1021/nl0480060.
- ^ Kortshagen, U (2009). "Nonthermal plasma synthesis of semiconductor nanocrystals". J. Phys. D: Appl. Phys. 42: 113001. doi:10.1088/0022-3727/42/11/113001.
- ^ Pi, X. D.; Kortshagen, U. (2009). "Nonthermal plasma synthesized freestanding silicon–germanium alloy nanocrystals". Nanotechnology. 20: 295602. doi:10.1088/0957-4484/20/29/295602.
- ^ Pi, X. D.; Gresback, R.; Liptak, R. W.; Campbell, S. A.; Kortshagen, U. (2008). "Doping efficiency, dopant location, and oxidation of Si nanocrystals". Applied Physics Letters. 92: 123102.
- ^ Ni, Z. Y.; Pi, X. D.; Ali, M.; Zhou, S.; Nozaki, T.; Yang, D. (2015). "Freestanding doped silicon nanocrystals synthesized by plasma". J. Phys. D: Appl. Phys. 48: 314006. doi:10.1088/0022-3727/48/31/314006.
- ^ Pereira, R. N.; Almeida, A. J. (2015). "Doped semiconductor nanoparticles synthesized in gas-phase plasmas". J. Phys. D: Appl. Phys. 48: 314005. doi:10.1088/0022-3727/48/31/314005.
- ^ Mangolini, L.; Kortshagen, U. (2007). "Plasma-assisted synthesis of silicon nanocrystal inks". Advanced Materials. 19: 2513–2519. doi:10.1002/adma.200700595.
- ^ Pi, X. D.; Yu, T.; Yang, D. (2014). "Water-dispersible silicon-quantum-dot-containing micelles self-assembled from an amphiphilic polymer". Part. Part. Syst. Charact. 31: 751–756. doi:10.1002/ppsc.201300346.
- ^ Silbey, Robert J.; Alberty, Robert A.; Bawendi, Moungi G. (2005). Physical Chemistry, 4th ed. John Wiley &Sons. p. 835.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Prati, Enrico; De Michielis, Marco; Belli, Matteo; Cocco, Simone; Fanciulli, Marco; Kotekar-Patil, Dharmraj; Ruoff, Matthias; Kern, Dieter P; Wharam, David A (2012). "Few electron limit of n-type metal oxide semiconductor single electron transistors". Nanotechnology. 23 (21): 215204. arXiv:1203.4811. Bibcode:2012Nanot..23u5204P. doi:10.1088/0957-4484/23/21/215204. PMID 22552118.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Lee SW, Mao C, Flynn CE, Belcher AM (2002). "Ordering of quantum dots using genetically engineered viruses". Science. 296 (5569): 892–5. Bibcode:2002Sci...296..892L. doi:10.1126/science.1068054. PMID 11988570.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Whaley SR, English DS, Hu EL, Barbara PF, Belcher AM (2000). "Selection of peptides with semiconductor binding specificity for directed nanocrystal assembly". Nature. 405 (6787): 665–8. doi:10.1038/35015043. PMID 10864319.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jawaid A.M., Chattopadhyay S., Wink D.J., Page L.E., Snee P.T. (2013). "A". ACS Nano. 7 (4): 3190–3197. doi:10.1021/nn305697q. PMID 23441602.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Continuous Flow Synthesis Method for Fluorescent Quantum Dots. Azonano.com (2013-06-01). Retrieved on 2015-07-19.
- ^ Quantum Materials Corporation and the Access2Flow Consortium (2011). "Quantum materials corp achieves milestone in High Volume Production of Quantum Dots". Retrieved 7 July 2011.
{{cite news}}: CS1 maint: numeric names: authors list (link) - ^ The Times (25 September 2014). "Nanoco and Dow tune in for sharpest picture yet". Retrieved 9 May 2015.
- ^ MFTTech (24 March 2015). "LG Electronics Partners with Dow to Commercialize LGs New Ultra HD TV with Quantum Dot Technology". Retrieved 9 May 2015.
- ^ Hauser, Charlotte A. E.; Zhang, Shuguang (2010). "Peptides as biological semiconductors". Nature. 468 (7323): 516–517. Bibcode:2010Natur.468..516H. doi:10.1038/468516a. PMID 21107418.
- ^ a b Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors" Environmental Health Perspectives 2006; 114, 165-172.
- ^ Pelley, J. L.; Daar, A. S.; Saner, M. A. (2009). "State of Academic Knowledge on Toxicity and Biological Fate of Quantum Dots". Toxicological Sciences. 112: 276–296. doi:10.1093/toxsci/kfp188. PMC 2777075. PMID 19684286.
- ^ a b c d Tsoi, Kim M.; Dai, Qin; Alman, Benjamin A.; Chan, Warren C. W. (19 March 2013). "Are Quantum Dots Toxic? Exploring the Discrepancy Between Cell Culture and Animal Studies". Accounts of Chemical Research. 46 (3): 662–671. doi:10.1021/ar300040z. ISSN 0001-4842.
- ^ Derfus, Austin M.; Chan, Warren C. W.; Bhatia, Sangeeta N. (1 January 2004). "Probing the Cytotoxicity of Semiconductor Quantum Dots". Nano Letters. 4 (1): 11–18. doi:10.1021/nl0347334. ISSN 1530-6984.
- ^ Liu, Wei; Zhang, Shuping; Wang, Lixin; Qu, Chen; Zhang, Changwen; Hong, Lei; Yuan, Lin; Huang, Zehao; Wang, Zhe (29 September 2011). "CdSe Quantum Dot (QD)-Induced Morphological and Functional Impairments to Liver in Mice". PLoS ONE. 6 (9): e24406. doi:10.1371/journal.pone.0024406. PMC 3182941. PMID 21980346.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Parak, W.j.; Boudreau, R.; Le Gros, M.; Gerion, D.; Zanchet, D.; Micheel, C.m.; Williams, S.c.; Alivisatos, A.p.; Larabell, C. (18 June 2002). "Cell Motility and Metastatic Potential Studies Based on Quantum Dot Imaging of Phagokinetic Tracks". Advanced Materials. 14 (12): 882–885. doi:10.1002/1521-4095(20020618)14:123.0.CO;2-Y. ISSN 1521-4095.
- ^ Green, Mark; Howman, Emily. "Semiconductor quantum dots and free radical induced DNA nicking". Chemical Communications (1): 121. doi:10.1039/b413175d.
- ^ Hauck, T. S.; Anderson, R. E.; Fischer, H. C.; Newbigging, S.; Chan, W. C. W. In vivo Quantum-Dot Toxicity Assessment. Small 2010, 6, 138-144.
- ^ Soo Choi, Hak; Liu, Wenhao; Misra, Preeti; Tanaka, Eiichi; Zimmer, John P.; Itty Ipe, Binil; Bawendi, Moungi G.; Frangioni, John V. (1 October 2007). "Renal clearance of quantum dots". Nature Biotechnology. 25 (10): 1165–1170. doi:10.1038/nbt1340. ISSN 1087-0156. PMC 2702539. PMID 17891134.
- ^ Fischer, Hans C.; Hauck, Tanya S.; Gómez-Aristizábal, Alejandro; Chan, Warren C. W. (18 June 2010). "Exploring Primary Liver Macrophages for Studying Quantum Dot Interactions with Biological Systems". Advanced Materials. 22 (23): 2520–2524. doi:10.1002/adma.200904231. ISSN 1521-4095.
- ^ Van Driel, A. F. (2005). "Frequency-Dependent Spontaneous Emission Rate from CdSe and CdTe Nanocrystals: Influence of Dark States" (PDF). Physical Review Letters. 95 (23): 236804. arXiv:cond-mat/0509565. Bibcode:2005PhRvL..95w6804V. doi:10.1103/PhysRevLett.95.236804. PMID 16384329.
- ^ Leatherdale, C. A.; Woo, W. -K.; Mikulec, F. V.; Bawendi, M. G. (2002). "On the Absorption Cross Section of CdSe Nanocrystal Quantum Dots". The Journal of Physical Chemistry B. 106 (31): 7619–7622. doi:10.1021/jp025698c.
- ^ Achermann, M.; Petruska, M. A.; Smith, D. L.; Koleske, D. D.; Klimov, V. I. (2004). "Energy-transfer pumping of semiconductor nanocrystals using an epitaxial quantum well". Nature. 429 (6992): 642–646. Bibcode:2004Natur.429..642A. doi:10.1038/nature02571. PMID 15190347.
- ^ Mongin C., Garakyaraghi S., Razgoniaeva N., Zamkov M., Castellano F.N. (2016). "Direct observation of triplet energy transfer from semiconductor nanocrystals". Science. 351 (6271): 369–372. doi:10.1126/science.aad6378.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Walling, M. A.; Novak, Shepard (February 2009). "Quantum Dots for Live Cell and In Vivo Imaging". Int. J. Mol. Sci. 10 (2): 441–491. doi:10.3390/ijms10020441. PMC 2660663. PMID 19333416.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Michalet, X.; Pinaud, F. F.; Bentolila, L. A.; Tsay, J. M.; Doose, S.; Li, J. J.; Sundaresan, G.; Wu, A. M.; Gambhir, S. S.; Weiss, S. (2005). "Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics". Science. 307 (5709): 538–44. Bibcode:2005Sci...307..538M. doi:10.1126/science.1104274. PMC 1201471. PMID 15681376.
- ^ Marchuk, K.; Guo, Y.; Sun, W.; Vela, J.; Fang, N. (2012). "High-Precision Tracking with Non-blinking Quantum Dots Resolves Nanoscale Vertical Displacement". Journal of the American Chemical Society. 134 (14): 6108–11. doi:10.1021/ja301332t. PMID 22458433.
- ^ Lane, L. A.; Smith, A. M.; Lian, T.; Nie, S. (2014). "Compact and Blinking-Suppressed Quantum Dots for Single-Particle Tracking in Live Cells". The Journal of Physical Chemistry B. 118 (49): 14140–7. doi:10.1021/jp5064325. PMID 25157589.
- ^ Spie (2014). "Paul Selvin Hot Topics presentation: New Small Quantum Dots for Neuroscience". SPIE Newsroom. doi:10.1117/2.3201403.17.
- ^ Tokumasu, F; Fairhurst, Rm; Ostera, Gr; Brittain, Nj; Hwang, J; Wellems, Te; Dvorak, Ja (2005). "Band 3 modifications in Plasmodium falciparum-infected AA and CC erythrocytes assayed by autocorrelation analysis using quantum dots". Journal of Cell Science (Free full text). 118 (Pt 5): 1091–8. doi:10.1242/jcs.01662. PMID 15731014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dahan, M. (2003). "Diffusion Dynamics of Glycine Receptors Revealed by Single-Quantum Dot Tracking". Science. 302 (5644): 442–5. doi:10.1126/science.1088525. PMID 14564008.
- ^ Howarth, M.; Liu, W.; Puthenveetil, S.; Zheng, Y.; Marshall, L. F.; Schmidt, M. M.; Wittrup, K. D.; Bawendi, M. G.; Ting, A. Y. (2008). "Monovalent, reduced-size quantum dots for imaging receptors on living cells". Nature Methods. 5 (5): 397–9. doi:10.1038/nmeth.1206. PMC 2637151. PMID 18425138.
- ^ Akerman, M. E.; Chan, W. C. W.; Laakkonen, P.; Bhatia, S. N.; Ruoslahti, E. (2002). "Nanocrystal targeting in vivo". Proceedings of the National Academy of Sciences. 99 (20): 12617–21. Bibcode:2002PNAS...9912617A. doi:10.1073/pnas.152463399. PMC 130509. PMID 12235356.
- ^ Farlow, J.; Seo, D.; Broaders, K. E.; Taylor, M. J.; Gartner, Z. J.; Jun, Y. W. (2013). "Formation of targeted monovalent quantum dots by steric exclusion". Nature Methods. 10 (12): 1203–5. doi:10.1038/nmeth.2682. PMID 24122039.
- ^ Dwarakanath, S.; Bruno, J. G.; Shastry, A.; Phillips, T.; John, A.; Kumar, A.; Stephenson, L. D. (2004). "Quantum dot-antibody and aptamer conjugates shift fluorescence upon binding bacteria". Biochemical and Biophysical Research Communications. 325 (3): 739–43. doi:10.1016/j.bbrc.2004.10.099. PMID 15541352.
- ^ a b Ballou, B.; Lagerholm, B. C.; Ernst, L. A.; Bruchez, M. P.; Waggoner, A. S. (2004). "Noninvasive Imaging of Quantum Dots in Mice". Bioconjugate Chemistry. 15 (1): 79–86. doi:10.1021/bc034153y. PMID 14733586.
- ^ Resch-Genger, Ute; Grabolle, Markus; Cavaliere-Jaricot, Sara; Nitschke, Roland; Nann, Thomas (28 August 2008). "Quantum dots versus organic dyes as fluorescent labels". Nature Methods. 5 (9): 763–775. doi:10.1038/nmeth.1248. PMID 18756197.
- ^ Algar, W. Russ; Krull, Ulrich J. (7 November 2007). "Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules". Analytical and Bioanalytical Chemistry. 391 (5): 1609–1618. doi:10.1007/s00216-007-1703-3. PMID 17987281.
- ^ Beane, Gary; Boldt, Klaus; Kirkwood, Nicholas; Mulvaney, Paul (7 August 2014). "Energy Transfer between Quantum Dots and Conjugated Dye Molecules". The Journal of Physical Chemistry C. 118 (31): 18079–18086. doi:10.1021/jp502033d.
- ^ "Gene Silencer and Quantum Dots Reduce Protein Production to a Whisper". Newswise. 23 June 2008.
- ^ Pelley, J. L.; Daar, A. S.; Saner, M. A. (2009). "State of Academic Knowledge on Toxicity and Biological Fate of Quantum Dots". Toxicological Sciences. 112 (2): 276–96. doi:10.1093/toxsci/kfp188. PMC 2777075. PMID 19684286.
- ^ Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J. P.; Itty Ipe, B.; Bawendi, M. G.; Frangioni, J. V. (2007). "Renal clearance of quantum dots". Nature Biotechnology. 25 (10): 1165–70. doi:10.1038/nbt1340. PMC 2702539. PMID 17891134.
- ^ Sharei, A.; Zoldan, J.; Adamo, A.; Sim, W. Y.; Cho, N.; Jackson, E.; Mao, S.; Schneider, S.; Han, M. -J.; Lytton-Jean, A.; Basto, P. A.; Jhunjhunwala, S.; Lee, J.; Heller, D. A.; Kang, J. W.; Hartoularos, G. C.; Kim, K. -S.; Anderson, D. G.; Langer, R.; Jensen, K. F. (2013). "A vector-free microfluidic platform for intracellular delivery". Proceedings of the National Academy of Sciences. 110 (6): 2082–7. Bibcode:2013PNAS..110.2082S. doi:10.1073/pnas.1218705110. PMC 3568376. PMID 23341631.
- ^ Schaller, R.; Klimov, V. (2004). "High Efficiency Carrier Multiplication in PbSe Nanocrystals: Implications for Solar Energy Conversion". Physical Review Letters. 92 (18): 186601. arXiv:cond-mat/0404368. Bibcode:2004PhRvL..92r6601S. doi:10.1103/PhysRevLett.92.186601. PMID 15169518.
- ^ a b Kim, Gi-Hwan; Arquer, F. Pelayo García de; Yoon, Yung Jin; Lan, Xinzheng; Liu, Mengxia; Voznyy, Oleksandr; Yang, Zhenyu; Fan, Fengjia; Ip, Alexander H. (2 November 2015). "High-Efficiency Colloidal Quantum Dot Photovoltaics via Robust Self-Assembled Monolayers". Nano Letters. 15 (11): 7691–7696. doi:10.1021/acs.nanolett.5b03677.
- ^ a b Krebs, Frederik C.; Tromholt, Thomas; Jørgensen, Mikkel (2010). "Upscaling of polymer solar cell fabrication using full roll-to-roll processing". Nanoscale. 2 (6): 873–86. doi:10.1039/b9nr00430k. PMID 20648282.
- ^ Park, Kwang-Tae; Kim, Han-Jung; Park, Min-Joon; Jeong, Jun-Ho; Lee, Jihye; Choi, Dae-Geun; Lee, Jung-Ho; Choi, Jun-Hyuk (15 July 2015). "13.2% efficiency Si nanowire/PEDOT:PSS hybrid solar cell using a transfer-imprinted Au mesh electrode". Scientific Reports. 5: 12093. doi:10.1038/srep12093. PMC 4502511. PMID 26174964.
- ^ Leschkies, Kurtis S.; Divakar, Ramachandran; Basu, Joysurya; Enache-Pommer, Emil; Boercker, Janice E.; Carter, C. Barry; Kortshagen, Uwe R.; Norris, David J.; Aydil, Eray S. (1 June 2007). "Photosensitization of ZnO Nanowires with CdSe Quantum Dots for Photovoltaic Devices". Nano Letters. 7 (6): 1793–1798. doi:10.1021/nl070430o. ISSN 1530-6984.
- ^ a b Xie, Chao; Nie, Biao; Zeng, Longhui; Liang, Feng-Xia; Wang, Ming-Zheng; Luo, Linbao; Feng, Mei; Yu, Yongqiang; Wu, Chun-Yan (22 April 2014). "Core–Shell Heterojunction of Silicon Nanowire Arrays and Carbon Quantum Dots for Photovoltaic Devices and Self-Driven Photodetectors". ACS Nano. 8 (4): 4015–4022. doi:10.1021/nn501001j. ISSN 1936-0851.
- ^ Gupta, Vinay; Chaudhary, Neeraj; Srivastava, Ritu; Sharma, Gauri Datt; Bhardwaj, Ramil; Chand, Suresh (6 July 2011). "Luminscent Graphene Quantum Dots for Organic Photovoltaic Devices". Journal of the American Chemical Society. 133 (26): 9960–9963. doi:10.1021/ja2036749. ISSN 0002-7863.
- ^ "Nano LEDs printed on silicon". nanotechweb.org. 3 July 2009.
- ^ Hoshino, Kazunori; Gopal, Ashwini; Glaz, Micah S.; Vanden Bout, David A.; Zhang, Xiaojing (2012). "Nanoscale fluorescence imaging with quantum dot near-field electroluminescence". Applied Physics Letters. 101 (4): 043118. Bibcode:2012ApPhL.101d3118H. doi:10.1063/1.4739235.
- ^ Konstantatos, G.; Sargent, E. H. (2009). "Solution-Processed Quantum Dot Photodetectors". Proceedings of the IEEE. 97 (10): 1666–1683. doi:10.1109/JPROC.2009.2025612.
- ^ Vaillancourt, J.; Lu, X.-J.; Lu, Xuejun (2011). "A High Operating Temperature (HOT) Middle Wave Infrared (MWIR) Quantum-Dot Photodetector". Optics and Photonics Letters. 4 (2): 1–5. doi:10.1142/S1793528811000196.
- ^ Zhao, Jing; Holmes, Michael A.; Osterloh, Frank E. (2013). "Quantum Confinement Controls Photocatalysis: A Free Energy Analysis for Photocatalytic Proton Reduction at Cd Se Nanocrystals". ACS Nano. 7 (5): 4316–25. doi:10.1021/nn400826h. PMID 23590186.
- ^ Zumbühl DM, Miller JB, Marcus CM, Campman K, Gossard AC (December 2002). "Spin-orbit coupling, antilocalization, and parallel magnetic fields in quantum dots". Phys. Rev. Lett. 89 (27): 276803. arXiv:cond-mat/0208436. Bibcode:2002PhRvL..89A6803Z. doi:10.1103/PhysRevLett.89.276803. PMID 12513231.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
G. J. Iafrate, K. Hess, J. B. Krieger, and M. Macucci (1995). "Capacitive nature of atomic-sized structures". Phys. Rev. B. 52 (15): 10737–10739. Bibcode:1995PhRvB..5210737I. doi:10.1103/physrevb.52.10737.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thomson, J.J. (1904). "On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure" (extract of paper). Philosophical Magazine Series 6. 7 (39): 237–265. doi:10.1080/14786440409463107.
- ^
Bednarek, S.; Szafran, B. and Adamowski, J. (1999). "Many-electron artificial atoms". Phys. Rev. B. 59 (20): 13036–13042. Bibcode:1999PhRvB..5913036B. doi:10.1103/PhysRevB.59.13036.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Bedanov, V. M. and Peeters, F. M. (1994). "Ordering and phase transitions of charged particles in a classical finite two-dimensional system". Physical Review B. 49 (4): 2667–2676. Bibcode:1994PhRvB..49.2667B. doi:10.1103/PhysRevB.49.2667.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ LaFave, T. Jr. (2013). "Correspondences between the classical electrostatic Thomson Problem and atomic electronic structure". Journal of Electrostatics. 71 (6): 1029–1035. doi:10.1016/j.elstat.2013.10.001.
- ^ LaFave, T. Jr. (2011). "The discrete charge dielectric model of electrostatic energy". Journal of Electrostatics. 69 (5): 414–418. doi:10.1016/j.elstat.2013.10.001.