Rhenium(III) iodide
Appearance
(Redirected from Rhenium triiodide)
| |
| Names | |
|---|---|
| Other names
Rhenium(III) iodide, triiodorhenium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.036.074 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| I3Re | |
| Molar mass | 566.920 g·mol−1 |
| Appearance | violet-black crystals |
| Density | 6.37 g/cm3 |
| Melting point | 800 °C (1,470 °F; 1,070 K) |
| poorly soluble | |
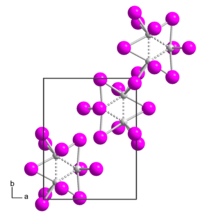
| Structure | |
| monoclinic | |
| Related compounds | |
Related compounds
|
Iridium triiodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rhenium(III) iodide is a binary chemical compound of rhenium and iodide with the chemical formula ReI
3.[1][2]
Synthesis
[edit]Rhenium(III) iodide can be synthesized by the decomposition of rhenium(IV) iodide:[3][4]
- 2ReI4 → 2ReI3 + I2
Another way to make it is by introduction of ethanol into a mixture of perrhenic acid and hydroiodic acid.
- HReO4 + 3HI + 2C2H5OH → ReI3 + 4H2O + 2CH3CHO
Physical properties
[edit]Rhenium(III) iodide forms violet-black crystals. It is poorly soluble in water, acetone, ethanol, ether, and dilute acid solutions.
Chemical properties
[edit]When heated in vacuum up to 170 °C, the compound decomposes to rhenium(II) iodide, and at 380 °C — to rhenium(I) iodide:
- 2ReI3 → 2ReI2 + I2
- ReI3 → ReI + I2
References
[edit]- ^ "Rhenium(III) Iodide". American Elements. Retrieved 6 May 2023.
- ^ "Rhenium(III) iodide". Alfa Chemistry. Retrieved 6 May 2023.
- ^ Kemmitt, R. D. W.; Peacock, R. D. (26 January 2016). The Chemistry of Manganese, Technetium and Rhenium: Pergamon Texts in Inorganic Chemistry. Elsevier. p. 918. ISBN 978-1-4831-8762-4. Retrieved 6 May 2023.
- ^ Inorganic Syntheses, Volume 7. John Wiley & Sons. 22 September 2009. p. 185. ISBN 978-0-470-13270-8. Retrieved 6 May 2023.
