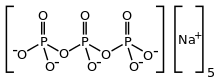
Sodium triphosphate
| |
| Names | |
|---|---|
| IUPAC name
Pentasodium triphosphate
| |
| Other names
sodium tripolyphosphate, polygon, STPP
| |
| Identifiers | |
| ECHA InfoCard | 100.028.944 |
| E number | E451 (thickeners, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| Na5P3O10 | |
| Molar mass | 367.864 g/mol |
| Appearance | white powder |
| Density | 2.52 g/cm3 |
| Melting point | 622 °C (1,152 °F; 895 K) |
| 14.5 g/100 mL (25 °C) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 1469 |
| Related compounds | |
Other anions
|
Trisodium phosphate Tetrasodium pyrophosphate Sodium hexametaphosphate |
Other cations
|
Pentapotassium triphosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium triphosphate (STP), also sodium tripolyphosphate (STPP), or tripolyphosphate (TPP),[1]) is an inorganic compound with formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is produced on a large scale as a component of many domestic and industrial products, especially detergents. Environmental problems associated with eutrophication are attributed to its widespread use.
Preparation and properties
Sodium tripolyphosphate is produced by heating a stoichiometric mixture of disodium phosphate, Na2HPO4, and monosodium phosphate, NaH2PO4, under carefully controlled conditions.[2]
- 2 Na2HPO4 + NaH2PO4 → Na5P3O10 + 2 H2O
In this way, approximately 2 million tons are produced annually.
STPP is a colourless salt, which exists both in anhydrous form and as the hexahydrate. The anion can be described as the pentanionic chain [O3POP(O)2OPO3]5−.[3][4] Many related di-, tri-, and polyphosphates are known including the cyclic triphosphate P3O93−. It binds strongly to metal cations as both a bidentate and tridentate chelating agent.

Uses
In detergents
The majority of STPP is consumed as a component of commercial detergents. It serves as a "builder," industrial jargon for a water softener. In hard water (water that contains high concentrations of Mg2+ and Ca2+), detergents are deactivated. Being a highly charged chelating agent, TPP5− binds to dications tightly and prevents them from interfering with the sulfonate detergent.[5]
Food applications
STPP is a preservative for seafood, meats, poultry, and animal feeds.[5] It is common in food production as E number E451. In foods, STPP is used as an emulsifier and to retain moisture. Many governments regulate the quantities allowed in foods, as it can substantially increase the sale weight of seafood in particular. The United States Food and Drug Administration lists STPP as "generally recognized as safe."
Other uses
Other uses (hundreds of thousands of tons/year) include ceramics (decrease the viscosity of glazes up to a certain limit), leather tanning (as masking agent and synthetic tanning agent - SYNTAN), anticaking, setting retarders, flame retardants, paper, anticorrosion pigments, textiles, rubber manufacture, fermentation, antifreeze."[5] TPP is used as a polyanion crosslinker in polysaccharide based drug delivery.[6]
Health effects
Polyphosphates are hydrolyzed into simpler phosphates, which in moderate amounts are nutritious[citation needed]. For example, ATP, a related derivative of triphosphate, is essential for life. Thus, the toxicity of polyphosphates is low, as the lowest LD50 after oral administration is >1,000 mg/kg body weight.[1] [clarification needed] Similarly, no mutagenic, carcinogenic, or reproductive effects have been noted.[1] Salts of polyphosphate anions are moderately irritating to skin and mucous membranes because they are mildly alkaline.[1]
Environmental effects
In 2000, the worldwide consumption of STPP was estimated to be approximately 2,000,000 tonnes.[5] Because it is very water-soluble, it is not significantly transferred to sewage sludge, and therefore to soil by sludge spreading. No environmental risk related to STPP use in detergents is indicated in soil or air. As an ingredient of household cleaning products, STPP present in domestic waste waters is mainly discharged to the aquatic compartment, directly, via waste water treatment plants, via septic tanks, infiltration or other autonomous waste water systems.
As STPP is an inorganic substance, biodegradation studies are not applicable. However, STPP can be hydrolysed, finally to orthophosphate, which can be assimilated by algae and/or by micro-organisms. STPP thus ends up being assimilated into the natural phosphorus cycle. Reliable published studies confirm biochemical understanding, showing that STPP is progressively hydrolysed by biochemical activity in contact with waste waters (in sewerage pipes and within sewage works) and also in the natural aquatic environment. This information enabled the calculation of “worst case” predicted environmental concentrations using the EUSES model and the HERA detergent scenario. A default regional release of 10% was applied instead of the 7% regional release indicated in the HERA detergent scenario. Reliable acute aquatic ecotoxicity studies are available which show that STPP is not toxic to aquatic organisms: all EC/LC50 values are above 100 mg/l (Daphnia, fish, algae). Because of this, and because of the only temporary presence of STPP in the aquatic environment (due to hydrolysis), no studies have been carried out to date concerning the chronic effects of STPP on these aquatic organisms. Predicted no-effect concentrations were therefore calculated for the aquatic environment and sediments on the basis of the acute aquatic ecotoxicity results.
Effects of wastewater containing phosphorus

Detergents containing phosphorus contribute, together with other sources of phosphorus, to the eutrophication of many fresh waters.[1] Eutrophication is an increase in chemical nutrients—typically compounds containing nitrogen or phosphorus—in an ecosystem. It may occur on land or in water. The term is, however, often used to mean the resultant increase in the ecosystem's primary productivity (excessive plant growth and decay), and further effects including lack of oxygen and severe reductions in water quality and fish and other animal populations.
Phosphorus can theoretically generate its weight 500 times in algae. [7] Whereas the primary production in marine waters is mainly nitrogen-limited, fresh waters are considered to be phosphorus-limited. A large part of the sewage effluents in many countries is released untreated into freshwater recipients, and here the use of phosphorus as complexing agents is still an environmental concern.[1]
See also
References
- ^ a b c d e f Complexing agents, Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products, Danish Environmental Protection Agency, Accessed 2008-07-15
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Corbridge, D. E. C. (1960). "The crystal structure of sodium triphosphate, Na5P3O10, phase I". Acta Crystallographica. 13: 263. doi:10.1107/S0365110X60000583.
- ^ Davies, D. R.; Corbridge, D. E. C. (1958). "The crystal structure of sodium triphosphate, Na5P3O10, phase II". Acta Crystallographica. 11: 315. doi:10.1107/S0365110X58000876.
- ^ a b c d Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann "Phosphoric Acid and Phosphates" in Ullmann’s Encyclopedia of Industrial Chemistry 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_465.pub3
- ^ P. Calvo, C. RemunanLopez, J.L. VilaJato, M.J. Alonso, Novel hydrophilic chitosanpolyethylene oxide nanoparticles as protein carriers, J. Appl. Polym. Sci. 63 (1997) 125–132
- ^ (Wetzel 1983).

