User:Fuse809/sandbox7
| Fuse809/sandbox7 |
|---|
Renal cell carcinoma (RCC, formerly known as hypernephroma) is a kidney cancer that originates in the lining of the proximal convoluted tubule, one type of very small tubes in the kidney that transports waste molecules from the blood to the urine. RCC is the most common type of kidney cancer in adults, responsible for approximately 90-95% of cases.[1] It has been described as one of the deadliest of cancers affecting the genitourinary tract.[2] Initial treatment is most commonly either partial or complete removal of the affected kidney(s) and remains the mainstay of curative treatment.[3] Where the cancer has not metastasised (spread to other organs) or burrowed deeper into the tissues of the kidney the 5-year survival rate is 60-70%, but this is lowered considerably when the cancer has spread. It is relatively resistant to radiation therapy and chemotherapy, although some cases respond to targeted therapies such as sunitinib, temsirolimus, bevacizumab, interferon alfa and sorafenib have improved the outlook for RCC.[4]
The body is remarkably good at hiding the symptoms and as a result people with RCC often have advanced disease by the time it is discovered.[5] The initial symptoms of RCC often include: blood in the urine (occurring in 40% of affected persons at the time they first seek medical attention), flank pain (40%), a mass in the abdomen or flank (25%), weight loss (33%), fever (20%), high blood pressure (20%), night sweats and generally feeling unwell.[1] RCC is also associated with a number of paraneoplastic syndromes (PNS) which are conditions caused by either the hormones produced by the tumour or by the body's attack on the tumour and are present in about 20% of those with RCC.[1] These syndromes most commonly affect tissues which have not been invaded by the cancer.[1] The most common PNSs seen in people with RCC are: anaemia (due to an underproduction of the hormone, erythropoietin), high blood calcium levels, polycythaemia (the opposite to anaemia, due to an overproduction of erythropoietin), thrombocytosis (too many platelets in the blood, leading to an increased tendency for blood clots and bleeds) and secondary amyloidosis.[6] When RCC metastasises it most commonly spreads to the lymph nodes, lungs, liver, adrenal glands, brain or bones.[6]
Signs and symptoms[edit]
Historically, medical practitioners expected a person to present with three findings. This classic triad[7] is 1: haematuria, which is when there is blood present in the urine, 2: flank pain, which is pain on the side of the body between the hip and ribs, and 3: an abdominal mass, similar to bloating but larger. It is now known that this classic triad of symptoms only occurs in 10-15% of cases, and is usually indicative that the renal cell carcinoma (RCC) in an advanced stage.[7] Today, RCC is often asymptomatic (meaning little to no symptoms) and is generally detected incidentally when a person is being examined for other ailments.[8]
Other signs and symptom may include haematuria;[7] loin pain;[7] abdominal mass;[8] malaise, which is a general feeling of feeling unwell;[8] weight loss and/or loss of appetite;[9] anaemia resulting from depression of erythropoietin;[7] erythrocytosis (increased production of red blood cells) due to increased erythropoietin secretion;[7] varicocele, which is seen in males as an enlargement of the tissue at the testicle (more often the left testicle)[8] hypertension (high blood pressure) resulting from secretion of renin by the tumour;[10] hypercalcemia, which is elevation of calcium levels in the blood;[11] sleep disturbance or night sweats;[9] recurrent fevers;[9] and chronic fatigue.[12]
Cause[edit]
Lifestyle[edit]
The greatest risk factors for RCC are lifestyle-related; smoking, obesity and hypertension (high blood pressure) have been estimated to account for up to 50% of cases.[13] Occupational exposure to some chemicals such as asbestos, cadmium, lead, chlorinated solvents, petrochemicals and PAH (polycyclic aromatic hydrocarbon) has been examined by multiple studies with inconclusive results.[14][15][16] Another suspected risk factor is the long term use of non-steroidal anti-inflammatory drugs (NSAIDS).[17]
Finally, studies have found that women who have had a hysterectomy are at more than double the risk of developing RCC than those who have not.[18] The reason for this remains unclear
Genetics[edit]
Hereditary factors have a minor impact on individual susceptibility with immediate relatives of people with RCC having a two to fourfold increased risk of developing the condition.[19] Other genetically linked conditions also increase the risk of RCC, including hereditary papillary renal carcinoma, hereditary leiomyomatosis, Birt-Hogg-Dube syndrome, hyperparathyroidism-jaw tumor syndrome, familial papillary thyroid carcinoma, von Hippel-Lindau disease[20] and sickle cell disease.[21]
The most significant disease affecting risk however is not genetically linked – patients with acquired cystic disease of the kidney requiring dialysis are 30 times greater more likely than the general population to develop RCC.[22]
Pathophysiology[edit]
The tumour arises from the cells of the proximal renal tubular epithelium.[1] It is considered an adenocarcinoma.[6] There are two subtypes: sporadic (that is, non-hereditary) and hereditary.[1] Both such subtypes are associated with mutations in the short-arm of chromosome 3, with the implicated genes being either tumour suppressor genes (VHL and TSC) or oncogenes (like c-Met).[1]
Diagnosis[edit]
The first steps taken in order to diagnose this condition are observing any of the signs and symptoms, and an anamnesis (the detailed medical review of past health state) to evaluate any risk factors. Based on the symptoms presented, a range of biochemical tests (using blood and/or urine samples) are generally also considered as part of screening process to provide sufficient quantitative analysis of any differences in electrolytes, renal and liver function, and blood clotting times.[21] Upon physical examination, palpation of the abdomen may reveal the presence of a mass or an organ enlargement.[23]
Although this disease lacks characterisation in the early stages of tumour development, considerations based on diverse clinical manifestations, as well as resistance to radiation and chemotherapy are important. The main diagnostic tools for detecting renal cell carcinoma are ultrasound, computed tomography (CT) scanning and magnetic resonance imaging (MRI) of the kidneys.[24]
Classification[edit]
Renal cell carcinoma (RCC) is not a single entity, but rather a collection of different types of tumours, each derived from the various parts of the nephron (epithelium or renal tubules) and possessing distinct genetic characteristics, histological features, and, to some extent, clinical phenotypes.
Classification of the Common Histological Subtypes of Renal Cell Carcinoma[21]
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Array-based karyotyping can be used to identify characteristic chromosomal aberrations in renal tumors with challenging morphology.[28][29] Array-based karyotyping performs well on paraffin embedded tumours[30] and is amenable to routine clinical use. See also Virtual Karyotype for CLIA certified laboratories offering array-based karyotyping of solid tumours.
The 2004 World Health Organization (WHO) classification of genitourinary tumours recognizes over 40 subtypes of renal neoplasms. Since the publication of the latest iteration of the WHO classification in 2004, several novel renal tumour subtypes have been described:[31]
- Clear cell papillary renal cell carcinoma and Clear cell renal cell carcinoma with smooth muscle stroma[32]
- Mucinous tubular and spindle cell carcinoma (MTSCC) [31]
- Multilocular cystic clear cell renal cell carcinoma [31]
- Tubulocystic renal cell carcinoma
- Thyroid-like follicular renal cell carcinoma
- Acquired cystic kidney disease-associated renal cell carcinoma
- Renal cell carcinoma with t(6;11) translocation (TFEB)
- Hybrid oncocytoma/chromophobe renal cell carcinoma
- Hereditary leiomyomatosis and renal cell carcinoma(HLRCC)
Laboratory tests[edit]
Laboratory tests are generally conducted when the patient presents signs and symptoms that may be characteristic of kidney impairment. They are not primarily used to diagnose kidney cancer, due to its asymptomatic nature and are generally found incidentally during tests for other illnesses such as gallbladder disease.[33] In other words, these cancers are not detected usually because they do not cause pain or discomfort when they are discovered. Laboratory analysis can provide an assessment on the overall health of the patient and can provide information in determining the staging and degree of metastasis to other parts of the body (if a renal lesion has been identified) before treatment is given.
Urine analysis[edit]
The presence of blood in urine is a common presumptive sign of renal cell carcinoma. The haemoglobin of the blood causes the urine to be rusty, brown or red in colour. Alternatively, urinalysis can test for sugar, protein and bacteria which can also serve as indicators for cancer. A complete blood cell count can also provide additional information regarding the severity and spreading of the cancer.[34]
Complete blood cell count[edit]
The CBC test provides a quantified measure of the different cells in the whole blood sample from the patient. Such cells examined for in this test include red blood cells (erythrocytes), white blood cells (leukocytes) and platelets (thrombocytes). A common sign of renal cell carcinoma is anaemia whereby the patient exhibits deficiency in red blood cells.[35] CBC tests are vital as a screening tool for examination the health of patient prior to surgery. Inconsistencies with platelet counts are also common amongst these cancer patients and further coagulation tests, including Erythrocyte Sedimentation Rate (ESR), Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT) should be considered.
Blood chemistry[edit]
Blood chemistry tests are conducted if renal cell carcinoma is suspected as cancer has the potential to elevate levels of particular chemicals in blood. For example, liver enzymes such as aspartate aminotransferase [AST] and alanine aminotransferase [ALT] are found to be at abnormally high levels.[36] The staging of the cancer can also be determined by abnormal elevated levels of calcium, which suggests that the cancer may have metastasised to the bones.[37] In this case, a doctor should be prompted for a CT scan. Blood chemistry tests also assess the overall function of the kidneys and can allow the doctor to decide upon further radiological tests.
Radiology[edit]
The characteristic appearance of renal cell carcinoma (RCC) is a solid renal lesion which disturbs the renal contour. It will frequently have an irregular or lobulated margin and may be seen as a lump on the lower pelvic or abdomen region. Traditionally, 85 to 90% of solid renal masses will turn out to be RCC but cystic renal mass mass may also be due to RCC.[38] However, the advances of diagnostic modalities are able to incidentally diagnose a great proportion of patients with renal lesions that may appear to be small in size and of benign state. Ten percent of RCC will contain calcifications, and some contain macroscopic fat (likely due to invasion and encasement of the perirenal fat.[39] Deciding on the benign or malignant nature of the renal mass on the basis of its localised size is an issue as renal cell carcinoma may also be cystic. As there are several benign cystic renal lesions (simple renal cyst, haemorrhagic renal cyst, multilocular cystic nephroma, polycystic kidney disease), it may occasionally be difficult for the radiologist to differentiate a benign cystic lesion from a malignant one.[40] The Bosniak classification system for cystic renal lesions that classifies them based specific imaging features into groups that are benign and those that need surgical resection is available.[41]
The main imaging tests performed in order to identify renal cell carcinoma are pelvic and abdominal CT scans, ultrasound tests of the kidneys (ultrasonography), MRI scan, intravenous pyelogram (IVP) or renal angiography.[42] Among these main diagnostic tests, other radiologic tests such as excretory urography, positron-emission tomography (PET) scanning, ultrasonography, arteriography, venography, and bone scanning can also used to aid in the evaluation of staging renal masses and to differentiate non-malignant tumours from malignant tumours.
Computed tomography[edit]
Contrast-enhanced Computed tomography (CT) scanning is a routinely used imaging procedure in determining the stage of the renal cell carcinoma in the abdominal and pelvic regions of the patient. CT scans have the potential to distinguish solid masses from cystic masses and may provide information on the localisation, stage or spread of the cancer to other organs of the patient. Key parts of the human body which are examined for metastatic involvement of renal cell carcinoma may include the renal vein, lymph node and the involvement of the inferior vena cava.[43] According to a study conducted by Sauk et al., multidetector CT imaging characteristics have applications in diagnosing patients with clear renal cell carcinoma by depicting the differences of these cells at the cytogenic level.[44]
Ultrasound[edit]
Ultrasonographic examination can be useful in evaluating questionable asymptomatic kidney tumours and cystic renal lesions if Computed Tomography imaging is inconclusive. This radiologic procedure is a safe and non-invasive which uses high frequency sound waves to generate an interior image of the body on a computer monitor. The image generated by the ultrasound can help diagnose renal cell carcinoma based on the differences of sound reflections on the surface of organs and the abnormal tissue masses. Essentially, ultrasound tests can determine whether the composition of the kidney mass is mainly solid or filled with fluid.[42]
Percutaneous biopsy can be performed by a radiologist using ultrasound or computed tomography to guide sampling of the tumour for the purpose of diagnosis by pathology. However this is not routinely performed because when the typical imaging features of renal cell carcinoma are present, the possibility of an incorrectly negative result together with the risk of a medical complication to the patient may make it unfavourable from a risk-benefit perspective.[11] However, biopsy tests for molecular analysis to distinguish benign from malignant renal tumours is of investigative interest.[11]
Magnetic resonance imaging[edit]
Magnetic Resonance Imaging (MRI) scans provide an image of the soft tissues in the body using radio waves and strong magnets. MRI can be used in replacement of CT if the patient exhibits an allergy to the contrast media cannot be administered.[45][46] Sometimes prior to the MRI scan, an intravenous injection of a contrasting material called gadolinium to allow for a more detailed image. Patients on dialysis or have renal insufficiency should avoid this contrasting material as it may induce a rare, yet severe, side effect known as nephrogenic systemic fibrosis.[47] A bone scan or brain imaging is not routinely performed unless signs or symptoms suggest potential metastatic involvement of these areas. MRI scans should also be considered to evaluate tumour extension which has grown in major blood vessels, including the vena cava, in the abdomen. MRI can be used to observe the possible spread of cancer to the brain or spinal cord should the patient present symptoms that suggest this might be the case.
Intravenous pyelogram[edit]
Intravenous pyelogram (IVP) is a useful procedure in detecting the presence of abnormal renal mass in the urinary tract. This procedure involves the injection of a contrasting dye into the arm of the patient. The dye travels from the blood stream and into the kidneys which in time, passes into the kidneys and bladder. This test is not necessary if a CT or MRI scan has been conducted.[48]
Renal angiography[edit]
Renal angiography uses the same principle as IVP, as this type of X-ray also uses a contrasting dye. This radiologic test serves importance in diagnosing renal cell carcinoma as an aid for examining blood vessels in the kidneys. This diagnostic test relies on the contrasting agent which is injected in the renal artery to be absorbed by the cancerous cells.[49] The contrasting dye provides a clearer outline of abnormally-oriented blood vessels believed to be involved with the tumour. This is imperative for surgeons as it allows the patient’s blood vessels to be mapped prior to operation.[43]
Staging[edit]
The staging of renal cell carcinoma is the most important factor in predicting its prognosis.[50] Staging can follow the TNM staging system, where the size and extent of the tumour (T), involvement of lymph nodes (N) and metastases (M) are classified separately. Also, it can use overall stage grouping into stage I-IV, with the 1997 revision of AJCC described below:[50]
| Stage I | Tumour of a diameter of 7 cm (approx. 23⁄4 inches) or smaller, and limited to the kidney. No lymph node involvement or metastases to distant organs. |
| Stage II | Tumour larger than 7.0 cm but still limited to the kidney. No lymph node involvement or metastases to distant organs. |
| Stage III any of the following |
Tumor of any size with involvement of a nearby lymph node but no metastases to distant organs. Tumour of this stage may be with or without spread to fatty tissue around the kidney, with or without spread into the large veins leading from the kidney to the heart. |
| Tumour with spread to fatty tissue around the kidney and/or spread into the large veins leading from the kidney to the heart, but without spread to any lymph nodes or other organs. | |
| Stage IV any of the following |
Tumour that has spread directly through the fatty tissue and the fascia ligament-like tissue that surrounds the kidney. |
| Involvement of more than one lymph node near the kidney | |
| Involvement of any lymph node not near the kidney | |
| Distant metastases, such as in the lungs, bone, or brain. |
At diagnosis, 30% of renal cell carcinomas have spread to the ipsilateral renal vein, and 5-10% have continued into the inferior vena cava.[51]
Histopathology[edit]
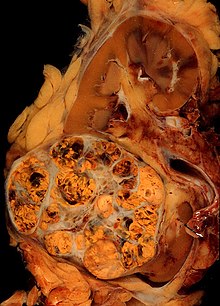

The gross and microscopic appearance of renal cell carcinomas is highly variable. The renal cell carcinoma may present reddened areas where blood vessels have bled, and cysts containing watery fluids.[52] The body of the tumour shows large blood vessels that have walls composed of cancerous cells. Gross examination often shows a yellowish, multilobulated tumor in the renal cortex, which frequently contains zones of necrosis, haemorrhage and scarring. In a microscopic context, there are four major histologic subtypes of renal cell cancer: clear cell (conventional RCC, 75%), papillary (15%), chromophobic (5%), and collecting duct (2%). Sarcomatoid changes (morphology showing spindle cells) can be observed within any RCC subtype. Under light microscopy, these tumour cells can exhibit papillae, tubules or nests, and are quite large, atypical, and polygonal.
Recent studies have brought attention to the close association of the type of cancerous cells to the aggressiveness of the condition. Some studies suggest that these cancerous cells accumulate glycogen and lipids, their cytoplasm appear "clear", the nuclei remain in the middle of the cells, and the cellular membrane is evident.[53] Some cells may be smaller, with eosinophilic cytoplasm, resembling normal tubular cells. The stroma is reduced, but well vascularised. The tumour compresses the surrounding parenchyma, producing a pseudocapsule.[54]
The most common cell type exhibited by renal cell carcinoma is the clear cell, which is named by the dissolving of the cells high lipid content in the cytoplasm. The clear cells are thought to be the least likely to spread and usually respond more favourably to treatment. However, most of the tumours contain a mixture of cells. The most aggressive stage of renal cancer is believed to be the one in which the tumour is mixed, containing both clear and granular cells.[55]
The recommended histologic grading schema for RCC is the Fuhrman system (1982), which is an assessment based on the microscopic morphology of a neoplasm with haematoxylin and eosin (H&E staining). This system categorises renal cell carcinoma with grades 1, 2, 3, 4 based on nuclear characteristics. The details of the Fuhrman grading system for RCC are shown below:[56]
| Grade Level | Nuclear Characteristics |
|---|---|
| Grade I | Nuclei appears round and uniform, 10 μm; nucleoli are inconspicuous or absent. |
| Grade II | Nuclei has an irregular appearance with signs of lobe formation, 15 μm; nucleoli are evident. |
| Grade III | Nuclei appears very irregular, 20 μm; nucleoli are large and prominent. |
| Grade IV | Nuclei appear bizarre and multilobated, 20 μm or more; nucleoli are prominent. |
Nuclear grade is believed to be one of the most imperative prognostic factors in patients with renal cell carcinoma.[21] However, a study by Delahunt et al. (2007) has shown that the Fuhrman grading is ideal for clear cell carcinoma but may not be appropriate chromophobe renal cell carcinomas and that the staging of cancer (accomplished by CT scan) is a more favourable predictor of the prognosis of this disease.[57] In relation to renal cancer staging, the Heidelberg classification system of renal tumours was introduced in 1976 as a means of more completely correlating the histopathological features with the identified genetic defects.[58]
Prognosis[edit]
The prognosis for renal cell carcinoma is largely influenced by a variety of factors, including tumour size, degree of invasion and metastasis, histologic type, and nuclear grade.[21] For metastatic renal cell carcinoma, factors which may present a poor prognosis include a low Karnofsky performance-status score (a standard way of measuring functional impairment in patients with cancer), a low haemoglobin level, a high level of serum lactate dehydrogenase, and a high corrected level of serum calcium.[59][60] For non-metastatic cases, the Leibovich scoring algorithm may be used to predict post-operative disease progression.[61]
Renal cell carcinoma is one of the cancers most strongly associated with paraneoplastic syndromes, most often due to ectopic hormone production by the tumour. The treatment for these complications of RCC is generally limited beyond treating the underlying cancer.
For those that have tumour recurrence after surgery, the prognosis is generally poor. Renal cell carcinoma does not generally respond to chemotherapy or radiation. Immunotherapy, which attempts to induce the body to attack the remaining cancer cells, has shown promise. Recent trials are testing newer agents, though the current complete remission rate with these approaches are still low, around 12-20% in most series. Most recently, treatment with tyrosine kinase inhibitors including nexavar, pazopanib, and rapamycin have shown promise in improving the prognosis for advanced RCC.[62]
Management[edit]
The type of treatment depends on multiple factors and the individual, some of which include:[7][63]
- Which stage the renal cell carcinoma is in
- Which organs and parts of the body are affected or unaffected
- What type of Renal cell carcinoma (RCC)
- Any pre-existing or comorbid conditions the person has
- Overall health and Age of the person
Every form of treatment does have both risks and benefits involved, a health care professional will provide the best options that suit the individual circumstances.
Active surveillance[edit]
Active surveillance or "Watchful waiting" is becoming more common as small renal masses or tumours are being detected and also within the older generation surgery is not always suitable.[64] Active surveillance involves completing various diagnostic procedures, tests and imaging to monitor the progression of the RCC before embarking on a more high risk treatment option like surgery.[64] In the elderly, patients with co-morbidities and in poor surgical candidates, this is especially useful.
Surgery[edit]
Different procedures may be most appropriate, depending on circumstances.
Radical nephrectomy is the removal of the entire affected kidney including Gerota's fascia, the adrenal gland which is on the same side as the affected kidney, and the regional lymph nodes all at the same time.[7] This method although severe is effective. But it is not always appropriate, as it is a major surgery that contains the risk of complication both during and after the surgery and can have a longer recovery time.[65] It is important to note that the other kidney must be fully functional, and this technique is most often used when there is a large tumour present in only one kidney.
Nephron-sparing partial nephrectomy is used when the tumor is small (less than 4 cm in diameter) or when the patient has other medical concerns such as diabetes or hypertension.[7] The partial nephrectomy involves the removal of the affected tissue only, sparing the rest of the kidney, Gerota's fascia and the regional lymph nodes. This allows for more renal preservation as compared to the radical nephrectomy, and this can have positive long term health benefits.[66] Larger and more complex tumors can also be treated with partial nephrectomy by surgeons with a lot of kidney surgery experience.[67]
Laprascopic nephrectomy uses laparoscopic surgery, with minimally invasive surgical techniques. Commonly referred to as key hole surgery, this surgery does not have the large incisions seen in a classically performed radical or partial nephrectomy, but still successfully removes either all or part of the kidney. Laprascopic surgery is associated with shorter stays in hospital and quicker recovery time but there are still risks associated with the surgical procedure.
Surgery for metastatic disease: If metastatic disease is present surgical treatment may still a viable option. Radical and partial nephrectomy can still occur, and in some cases if the metastasis is small this can also be surgically removed.[7] This depends on what stage of growth and how far the disease has spread.
Targeted ablative therapies[edit]
Targeted ablative therapies are also known as percutaneous ablative therapies. Although the development of laprascopic surgical techniques that are used to complete nephrectomies has reduced some of the risks associated with surgery,[68] surgery of any sort in some cases will still not be feasible. For example, the elderly, people already suffering from severe renal dysfunction or people who have several comorbidities surgery of any sort is not warranted.[69] Instead there are targeted therapies which do not involve the removal of any organs or serious surgery. Rather, these therapies involve the ablation of the tumor or the affected area. Ablative treatments use imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) to identify the location of the tumors, which ideally are smaller than 3.5 cm in size and to guide the treatment. However there are some cases where ablation can be used on tumors that are larger.[69]
The two main types of ablation techniques that are used for renal cell carcinoma are radio frequency ablation and cryoablation.[69]
Radio frequency ablation uses an electrode probe which is inserted into the affected tissue, to send radio frequencies to the tissue to generate heat through the friction of water molecules. The heat destroys the tumor tissue.[7] Cell death will generally occur within minutes of being exposed to temperatures of or above 50 °C.
Cryoablation also involves the insertion of a probe in to the affected area.[7] However in this case instead of heat being used to kill the tumor cold is. The probe is cooled with chemical fluids which are very cold. The freezing temperatures cause the tumor cells to die by causing osmotic dehydration, which pulls the water out of the cell destroying the enzyme, organelles, cell membrane and freezing the cytoplasm.[69]
Targeted drug treatments[edit]
Immunotherapy is a method that targets the persons immune system and uses it to their own advantage, this was developed after the observation was made that in some cases there was spontaneous regression.[70] That is, the renal cell carcinoma improved or reverted to a lower phase with no other therapies. Immunotherapy capitalises on this phenomenon and aims to build up a persons immune response to particular cancer cells or agents.[70] Other medications that have been developed target things such as growth factors that have been shown to promote the growth and spread of tumours.[71] They inhibit the growth factor which promotes the growth of tumours to prevent tumours from forming.[72] There have been many different medications developed and most have only been approved in the last 7 or so years.[73]
Some of the most recently developed treatments are listed below:[74]
- Axitinib (been found to be superior to sorafenib and pazopanib in terms of progression-free survival)[75]
- Bevacizumab
- Carfilzomib
- Everolimus
- Interferon-α
- Interleukin-2
- Lenalidomide (research into this treatment is ongoing)[76]
- Pazopanib
- Sirolimus (Rapamycin)
- Sorafenib
- Sunitinib
- Temsirolimus
- Thalidomide (results with this agent have been largely disappointing)[76]
- Tivozanib
Each of the treatments listed above are slightly different, some only work for a little while and others need to be used in conjunction with other therapies.There are also different side effects and risks associated with different forms of medication. As always the advice of a Health Care professional should be sought if considering any of the therapies mentioned.
Chemotherapy[edit]
Chemotherapy and radiotherapy are not as successful in the case of RCC. RCC is resistant in most cases but there is about a 4-5% success rate sometimes, but this is often short lived with more tumours and growths developing later.[7]
Vaccine[edit]
Cancer vaccines are being developed but so far have been found to be effective for only certain forms of the RCC.[7] The vaccines are being designed to "prime" the immune system to provide tumour specific immunity.[70] They are still being developed but the present another treatment possibility.
Adjuvant therapy[edit]
Adjuvant therapy is a form of treatment that is given after a primary treatment has already been administered. In the example of RCC the adjuvant therapies tend to be the medications such as Interleukin-2 which are given after the renal nephrectomy surgery.[72] The idea behind this method is that the adjuvant therapy will prevent the cancer from returning as sometimes cancerous cells can remain in the body after the primary tumour has been removed.[7] A newer form of adjuvant therapy is Neoadjuvant therapy this is similar to adjuvant therapy but in this case the treatment is administered before the intended primary or main treatment. In some cases neoadjuvant therapy has been shown to decrease the size and stage of the RCC to then allow it to be surgically removed.[72] This is a very new form of treatment and the effectiveness is still being assessed. There are not RCC specific Adjuvant or Neoadjuvant therapies, but trials are still ongoing.
Metastatic renal cell carcinoma[edit]
Metastatic renal cell carcinoma (mRCC) is the spread of the primary renal cell carcinoma from the kidney to other organs. 25-30% of people have this metastatic spread by the time they are diagnosed with renal cell carcinoma.[77] This high proportion is explained by the fact that clinical signs are generally mild until the disease progresses to a more severe state.[78] The most common sites for metastasis are the lymph nodes, lung, bones, liver and brain.[8] How this spread affects the staging of the disease and hence prognosis is discussed in the “Diagnosis” and “Prognosis” section.
MRCC has a poor prognosis compared to other cancers although average survival times have increased in the last few years due to treatment advances. Average survival time in 2008 for the metastatic form of the disease was under a year[79] and by 2013 this improved to an average of 22 months.[80] Despite this improvement the 5 year survival rate for mRCC remains under 10%[81] and 20-25% of suffers remain unresponsive to all treatments and in these cases, the disease has a rapid progression.[80]
The available treatments for RCC discussed in the “Treatment” section are also relevant for the metastatic form of the disease. The most relevant of these is Interleukin-2 which is the standard therapy for advanced renal cell carcinoma.[82] In the past six years, seven new treatments have been approved specifically for mRCC (Sunitinib, Temsirolimus, Bevacizumab, Sorafenib, Everolimus, Pazopanib and Axitinib).[4] These new treatments are based on the fact that renal cell carcinomas are very vascular tumors – they contain a large number of blood vessels. The drugs aim to inhibit the growth of new blood vessels in the tumors, hence slowing growth and in some cases reducing the size of the tumors.[83] Side effects unfortunately are quite common with these treatments and include:[84]
- Gastrointestinal effects - nausea, vomiting, diarrhea, anorexia
- Respiratory effects - coughing, dyspnea (difficulty breathing)
- Cardiovascular effects -hypertension(high blood pressure)
- Neurological effects - intracranial hemorrhage (bleeding into the brain), thrombosis (blood clots) in the brain
- Effects on the skin and mucus membranes - rashes, hand-foot syndrome, stomatitis
- Bone marrow suppression -resulting in reduced white blood cells, increasing the risk of infections plus anemia and reduced platelets
- Renal effects - impaired kidney function
- Fatigue.
Radiotherapy and chemotherapy are more commonly used in the metastatic form of RCC to target the secondary tumors in the bones, liver, brain and other organs. While not curative, these treatments do provide relief for suffers from symptoms associated with the spread of tumors.[80] Other potential treatments are still being developed, including tumor vaccines and small molecule inhibitors.[77]
Epidemiology[edit]
The Epidemiology of RCC is complex. It is known that the incidence of the disease varies according to geographic, demographic and, to a lesser extent, hereditary factors. There are some known risk factors, however the significance of other potential risk factors remains more controversial. The incidence of the cancer has been increasing in frequency worldwide at a rate of approximately 2-3% per decade[79] until the last few years where the number of new cases has stabilised.[14]
The incidence of RCC varies between sexes, ages, races and geographic location around the world. Men have a higher incidence than women (approximately 1.6:1)[82] and the vast majority of sufferers are diagnosed after 65 years of age.[82] Asians reportedly have a significantly lower incidence of RCC than whites and while African countries have the lowest reported incidences, African Americans have the highest incidence of the population in the United States.[14] Developed countries have a higher incidence than developing countries, with the highest rates found in North America, Europe and Australia / New Zealand[85]
History[edit]
Daniel Sennert made the first reference suggesting a tumour arising in the kidney in his textPracticae Medicinae, first published in 1613.[86]
Miril published the earliest unequivocal case of renal carcinoma in 1810.[87] He described the case of Françoise Levelly, a 35 year old woman, who presented to Brest Civic Hospital on April 6, 1809, supposedly in the late stages of pregnancy.[86]
Koenig published the first classification of renal tumours based on macroscopic morphology in 1826. Koenig divided the tumors into scirrhous, steatomatous, fungoid and medullary forms.[88]
Hypernephroma controversy[edit]
Following the classification of the tumour, researchers attempted to identify the tissue of origin for renal carcinoma.
The pathogenesis of renal epithelial tumours has provided one of the most enduring controversies of modern surgical pathology. The debate was initiated by Paul Grawitz when in 1883, he published his observations on the morphology of small, yellow renal tumours. Grawitz concluded that only alveolar tumours were of adrenal origin, whereas papillary tumours were derived from renal tissue.[86]
In 1893, Paul Sudeck challenged the theory postulated by Grawitz by publishing descriptions of renal tumours in which he identified atypical features withinrenal tubules and noted a gradation of these atypical features between the tubules and neighboring malignant tumour. In 1894, Otto Lubarsch, who supported the theory postulated by Grawitz coined the term hypernephroid tumor, which was amended tohypernephroma by Felix Victor Birch-Hirschfeld to describe these tumours.[89]
Vigorous criticism of Grawitz was provided by Oskar Stoerk in 1908, who considered the adrenalorigin of renal tumours to be unproved. Despite the compelling arguments against the theory postulated by Grawitz, the term hypernephroma, with its associated adrenal connotation, persisted in the literature.[86]
Foot and Humphreys, and Foote et al. introduced the term Renal Celled Carcinoma to emphasize a renal tubular origin for these tumours. Their designation was slightly altered by Fetter to the now widely accepted term Renal Cell Carcinoma.[90]
Convincing evidence to settle the debate was offered by Oberling et al. in 1959 who studied the ultrastructure of clear cells from eight renal carcinomas. They found that the tumour cellcytoplasm contained numerous mitochondria and deposits of glycogen and fat. They identifiedcytoplasmic membranes inserted perpendicularly onto basement membrane with occasional cells containing microvilli along the free borders. They concluded that these features indicated that the tumours arose from the epithelial cells of the renal convoluted tubule, thus finally settling one of the most debated issues in tumour pathology.[86][91]
See also[edit]
- Stauffer syndrome
- Knudson hypothesis[92]
- Interleukin-2
- Kidney cancer
- Rapamycin
- Vinblastine
- Dysuria
- Interferon
References[edit]
- ^ a b c d e f g Curti, B; Jana, BRP; Javeed, M; Makhoul, I; Sachdeva, K; Hu, W; Perry, M; Talavera, F (26 February 2014). Harris, JE (ed.). "Renal Cell Carcinoma". Medscape Reference. WebMD. Retrieved 7 March 2014.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Ramana, Jayashree (2012). "RCDB: Renal Cancer Gene Database". BMC Research Notes. 5: 246. doi:10.1186/1756-0500-5-246. PMC 3438103. PMID 22608002.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rini BI, Rathmell WK, Godley P (May 2008). "Renal cell carcinoma". Curr Opin Oncol. 20 (3): 300–6. doi:10.1097/CCO.0b013e3282f9782b. PMID 18391630.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Singer, Eric A.; Gupta, Gopal N.; Marchalik, Daniel; Srinivasan, Ramaprasad (2013). "Evolving therapeutic targets in renal cell carcinoma". Current Opinion in Oncology. 25 (3): 273–280. doi:10.1097/CCO.0b013e32835fc857. PMID 23455028. S2CID 11526724.
- ^ Fausto, V; Abbas, A; Fausto, N (2004). Robbins and Cotran Pathologic Basis of disease (7th ed.). Philadelphia, PA: Elsevier/Saunders. ISBN 978-0721601878.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Master, VA (November 2013). "Renal Cell Carcinoma". Merck Manual Professional. Merck Sharp & Dohme Corp. Retrieved 7 March 2014.
- ^ a b c d e f g h i j k l m n o Cohen, Herbert T.; McGovern, Francis J. (2005). "Renal-Cell Carcinoma". New England Journal of Medicine. 353 (23): 2477–90. doi:10.1056/NEJMra043172. PMID 16339096.
- ^ a b c d e Motzer, Robert J.; Bander, Neil H.; Nanus, David M. (1996). "Renal-Cell Carcinoma". New England Journal of Medicine. 335 (12): 865–75. doi:10.1056/NEJM199609193351207. PMID 8778606.
- ^ a b c Kim, Hyung L.; Belldegrun, Arie S.; Freitas, Danielo G.; Bui, Matthew H.T.; Han, KEN-RYU; Dorey, Frederick J.; Figlin, Robert A. (2003). "Paraneoplastic Signs and Symptoms of Renal Cell Carcinoma: Implications for Prognosis". The Journal of Urology. 170 (5): 1742–6. doi:10.1097/01.ju.0000092764.81308.6a. PMID 14532767.
- ^ Birkhauser (2013). "Etiology of Renal Cell Carcinoma: Incidence, Demographics, and Environmental Factors". Renal Cell Carcinoma Clinical Management. Humana Pr Inc. pp. 3–22. ISBN 978-1-62703-061-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Lane, Brian R.; Samplaski, Mary K.; Herts, Brian R.; Zhou, Ming; Novick, Andrew C.; Campbell, Steven C. (2008). "Renal Mass Biopsy—A Renaissance?". The Journal of Urology. 179 (1): 20–7. doi:10.1016/j.juro.2007.08.124. PMID 17997455. Cite error: The named reference "Lane" was defined multiple times with different content (see the help page).
- ^ Metz (2013). "Palliative and Supportive Care for Renal Cancer". Renal Cell Carcinoma Clinical Management. Humana. pp. 339–348. ISBN 978-1-62703-061-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Häggström, Christel; Rapp, Kilian; Stocks, Tanja; Manjer, Jonas; Bjørge, Tone; Ulmer, Hanno; Engeland, Anders; Almqvist, Martin; Concin, Hans; Selmer, R; Ljungberg, B; Tretli, S; Nagel, G; Hallmans, G; Jonsson, H; Stattin, P (2013). Miller, Todd W (ed.). "Metabolic Factors Associated with Risk of Renal Cell Carcinoma". PLOS ONE. 8 (2): e57475. doi:10.1371/journal.pone.0057475. PMC 3585341. PMID 23468995.
- ^ a b c Ljungberg, Börje; Campbell, Steven C.; Choi, Han Yong; Jacqmin, Didier; Lee, Jung Eun; Weikert, Steffen; Kiemeney, Lambertus A. (2011). "The Epidemiology of Renal Cell Carcinoma". European Urology. 60 (4): 615–21. doi:10.1016/j.eururo.2011.06.049. PMID 21741761.
- ^ Dhôte, R.; Pellicer-Coeuret, M.; Thiounn, N.; Debré, B.; Vidal-Trecan, G. (2007). "Risk factors for adult renal cell carcinoma: A systematic review and implications for prevention". BJU International. 86 (1): 20–7. doi:10.1046/j.1464-410x.2000.00708.x. PMID 10886077. S2CID 21271953.
- ^ Boffetta, P.; Fontana, L.; Stewart, P.; Zaridze, D.; Szeszenia-Dabrowska, N.; Janout, V.; Bencko, V.; Foretova, L.; Jinga, V.; Matveev, V.; Kollarova, H.; Ferro, G.; Chow, W.-H.; Rothman, N.; Van Bemmel, D.; Karami, S.; Brennan, P.; Moore, L. E. (2011). "Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: A case-control study from Central and Eastern Europe". Occupational and Environmental Medicine. 68 (10): 723–8. doi:10.1136/oem.2010.056341. PMID 21217163. S2CID 29328889.
- ^ Cho, Eunyoung; Curhan, G; Hankinson, SE; Kantoff, P; Atkins, MB; Stampfer, M; Choueiri, TK (2011). "Prospective Evaluation of Analgesic Use and Risk of Renal Cell Cancer". Archives of Internal Medicine. 171 (16): 1487–93. doi:10.1001/archinternmed.2011.356. PMC 3691864. PMID 21911634.
- ^ Zucchetto, Antonella; Talamini, Renato; Dal Maso, Luigino; Negri, Eva; Polesel, Jerry; Ramazzotti, Valerio; Montella, Maurizio; Canzonieri, Vincenzo; Serraino, Diego; La Vecchia, Carlo; Franceschi, Silvia (2008). "Reproductive, menstrual, and other hormone-related factors and risk of renal cell cancer". International Journal of Cancer. 123 (9): 2213–6. doi:10.1002/ijc.23750. PMID 18711701. S2CID 45289039.
- ^ Lipworth, L; Tarone, RE; Lund, L; McLaughlin, JK (2009). "Epidemiologic characteristics and risk factors for renal cell cancer". Clinical Epidemiology. 1: 33–43. doi:10.2147/clep.s4759. PMC 2943168. PMID 20865085.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pavlovich, Christian P.; Schmidt, Laura S. (2004). "Searching for the hereditary causes of renal-cell carcinoma". Nature Reviews Cancer. 4 (5): 381–93. doi:10.1038/nrc1364. PMID 15122209.
- ^ a b c d e f Rini, Brian I; Campbell, Steven C; Escudier, Bernard (2009). "Renal cell carcinoma". The Lancet. 373 (9669): 1119–1132. doi:10.1016/S0140-6736(09)60229-4. PMID 19269025. S2CID 24535138.
- ^ Baldewijns, Marcella M.L.; Van Vlodrop, Iris J.H.; Schouten, Leo J.; Soetekouw, Patricia M.M.B.; De Bruïne, Adriaan P.; Van Engeland, Manon (2008). "Genetics and epigenetics of renal cell cancer". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1785 (2): 133–155. doi:10.1016/j.bbcan.2007.12.002. PMID 18187049.
- ^ Tjaden, Christin; Werner, Jens; Buechler, Markus W.; Hackert, Thilo (2011). "Reactive Hypertrophy of an Accessory Spleen Mimicking Tumour Recurrence of Metastatic Renal Cell Carcinoma". Asian Journal of Surgery. 34 (1): 50–2. doi:10.1016/S1015-9584(11)60019-5. PMID 21515214.
- ^ Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-7153-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Catto, James W.F.; Shariat, Shahrokh F. (2013). "The Changing Face of Renal Cell Carcinoma: The Impact of Systematic Genetic Sequencing on Our Understanding of This Tumor's Biology". European Urology. 63 (5): 855–7, discussion 857–8. doi:10.1016/j.eururo.2012.09.049. PMID 23026395.
- ^ a b c d e f g Zhou, Ming; He, Huiying (2013). "Pathology of Renal Cell Carcinoma". Renal Cell Carcinoma. pp. 23–41. doi:10.1007/978-1-62703-062-5_2. ISBN 978-1-62703-061-8. S2CID 2101851.
- ^ Lopez-Beltran, Antonio; Scarpelli, Marina; Montironi, Rodolfo; Kirkali, Ziya (2006). "2004 WHO Classification of the Renal Tumors of the Adults". European Urology. 49 (5): 798–805. doi:10.1016/j.eururo.2005.11.035. PMID 16442207.
- ^ Hagenkord, Jill M; Parwani, Anil V; Lyons-Weiler, Maureen A; Alvarez, Karla; Amato, Robert; Gatalica, Zoran; Gonzalez-Berjon, Jose M; Peterson, Leif; Dhir, Rajiv; Monzon, Federico A (2008). "Virtual karyotyping with SNP microarrays reduces uncertainty in the diagnosis of renal epithelial tumors". Diagnostic Pathology. 3: 44. doi:10.1186/1746-1596-3-44. PMC 2588560. PMID 18990225.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Monzon, Federico A; Hagenkord, Jill M; Lyons-Weiler, Maureen A; Balani, Jyoti P; Parwani, Anil V; Sciulli, Christin M; Li, Jia; Chandran, Uma R; Bastacky, Sheldon I; Dhir, Rajiv (2008). "Whole genome SNP arrays as a potential diagnostic tool for the detection of characteristic chromosomal aberrations in renal epithelial tumors". Modern Pathology. 21 (5): 599–608. doi:10.1038/modpathol.2008.20. PMID 18246049. S2CID 24441677.
- ^ Lyons-Weiler M, Hagenkord J, Sciulli C, Dhir R, Monzon FA (March 2008). "Optimization of the Affymetrix GeneChip Mapping 10K 2.0 Assay for routine clinical use on formalin-fixed paraffin-embedded tissues". Diagn. Mol. Pathol. 17 (1): 3–13. doi:10.1097/PDM.0b013e31815aca30. PMID 18303412. S2CID 24420204.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Crumley, S. M.; Divatia, M; Truong, L; Shen, S; Ayala, A. G.; Ro, J. Y. (2013). "Renal cell carcinoma: Evolving and emerging subtypes". World Journal of Clinical Cases. 1 (9): 262–275. doi:10.12998/wjcc.v1.i9.262. PMC 3868710. PMID 24364021.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rohan, S. M.; Xiao, Y; Liang, Y; Dudas, M. E.; Al-Ahmadie, H. A.; Fine, S. W.; Gopalan, A; Reuter, V. E.; Rosenblum, M. K.; Russo, P; Tickoo, S. K. (2011). "Clear-cell papillary renal cell carcinoma: Molecular and immunohistochemical analysis with emphasis on the von Hippel-Lindau gene and hypoxia-inducible factor pathway-related proteins". Modern Pathology. 24 (9): 1207–20. doi:10.1038/modpathol.2011.80. PMID 21602815. S2CID 21904118.
- ^ Wood, Laura S. (30 November 2009). "Renal Cell Carcinoma". Clinical Journal of Oncology Nursing. 13: 3–7. doi:10.1188/09.CJON.S2.3-7. PMID 19948453.
- ^ Bonn, Dorothy (31 Jan 2004). "Urine test for renal-cell carcinoma". The Lancet Oncology. 5 (2): 72. doi:10.1016/S1470-2045(04)01368-3. PMID 14974475.
- ^ Johann, Donald J.; Wei, Bih-Rong; Prieto, Darue A.; Chan, King C.; Ye, Xiaying; Valera, Vladimir A.; Simpson, R. Mark; Rudnick, Paul A.; Xiao, Zhen; Issaq, Haleem J.; Linehan, W. Marston; Stein, Stephen E.; Veenstra, Timothy D.; Blonder, Josip (2010). "Combined Blood/Tissue Analysis for Cancer Biomarker Discovery: Application to Renal Cell Carcinoma". Analytical Chemistry. 82 (5): 1584–8. doi:10.1021/ac902204k. PMC 3251958. PMID 20121140.
- ^ Hatzaras, Ioannis; Gleisner, Ana L.; Pulitano, Carlo; Sandroussi, Charbel; Hirose, Kenzo; Hyder, Omar; Wolfgang, Christopher L.; Aldrighetti, Luca; Crawford, Michael; Choti, Michael A.; Pawlik, Timothy M. (2012). "A multi-institution analysis of outcomes of liver-directed surgery for metastatic renal cell cancer". HPB. 14 (8): 532–8. doi:10.1111/j.1477-2574.2012.00495.x. PMC 3406350. PMID 22762401.
- ^ Motzer, RJ (Apr 1, 2003). "Renal cell carcinoma: a priority malignancy for development and study of novel therapies". Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 21 (7): 1193–4. doi:10.1200/JCO.2003.12.072. PMID 12663704.
- ^ Sahni, V.A. (1 January 2009). "REVIEW: Biopsy of renal masses: when and why". Cancer Imaging. 9 (1): 44–55. doi:10.1102/1470-7330.2009.0005. PMC 2739685. PMID 19602467.
- ^ Nakada, G; Machida, T; Masuda, F; Onishi, T; Yamazaki, H; Kiyota, H; Suzuki, M; Goto, H (1983). "A case of arteriovenous fistulae secondary to renal cell carcinoma accompanied by congestive heart failure". Hinyokika Kiyo. Acta Urologica Japonica. 29 (8): 901–5. PMID 6675440.
- ^ Pattamapaspong, Nuttaya; Muttarak, Malai; Sivasomboon, Chate (2011). "Tuberculosis Arthritis and Tenosynovitis". Seminars in Musculoskeletal Radiology. 15 (5): 459–69. doi:10.1055/s-0031-1293492. PMID 22081281.
- ^ Israel, Gary M.; Bosniak, Morton A. (2005). "How I Do It: Evaluating Renal Masses1". Radiology. 236 (2): 441–50. doi:10.1148/radiol.2362040218. PMID 16040900.
- ^ a b Jubelirer, SJ; Rubin, M (1993). "The use of modern radiologic methods in identifying incidental renal cell carcinoma". The West Virginia Medical Journal. 89 (1): 21–3. PMID 8421912.
- ^ a b Beck, AD (Oct 1997). "Renal cell carcinoma involving the inferior vena cava: radiologic evaluation and surgical management". The Journal of Urology. 118 (4): 533–7. doi:10.1016/s0022-5347(17)58098-2. PMID 916043.
- ^ Sauk, Steven C.; Hsu, Margaret S.; Margolis, Daniel J. A.; Lu, David S. K.; Rao, Nagesh P.; Belldegrun, Arie S.; Pantuck, Allan J.; Raman, Steven S. (2011). "Clear Cell Renal Cell Carcinoma: Multiphasic Multidetector CT Imaging Features Help Predict Genetic Karyotypes". Radiology. 261 (3): 854–62. doi:10.1148/radiol.11101508. PMID 22025734.
- ^ Hricak, H; Demas, BE; Williams, RD; McNamara, MT; Hedgcock, MW; Amparo, EG; Tanagho, EA (1985). "Magnetic resonance imaging in the diagnosis and staging of renal and perirenal neoplasms". Radiology. 154 (3): 709–15. doi:10.1148/radiology.154.3.3969475. PMID 3969475.
- ^ Janus, CL; Mendelson, DS (1991). "Comparison of MRI and CT for study of renal and perirenal masses". Critical Reviews in Diagnostic Imaging. 32 (2): 69–118. PMID 1863349.
- ^ Nishimura, Kazuo; Hida, Shuichi; Okada, Kenichiro; Yoshida, Osamu; Nishimuara, Kazumasa (1988). "Staging and differential diagnosis of renal cell carcinoma: A comparison of magnetic resonance imaging (MRI) and computed tomography (CT)". Acta Urologica Japonica. 34 (8): 1323–31. hdl:2433/119684. PMID 3195400.
- ^ Reznek, RH (Feb 14, 2004). "CT/MRI in staging renal cell carcinoma". Cancer Imaging : The Official Publication of the International Cancer Imaging Society. 4 Spec No A (Spec No A): S25–32. doi:10.1102/1470-7330.2004.0012. PMC 1435344. PMID 18215972.
- ^ Kocak, Mehmet; Sudakoff, Gary S.; Erickson, Scott; Begun, Frank; Datta, Milton (2001). "Using MR Angiography for Surgical Planning in Pelvic Kidney Renal Cell Carcinoma". American Journal of Roentgenology. 177 (3): 659–60. doi:10.2214/ajr.177.3.1770659. PMID 11517066.
- ^ a b Kidney Cancer / General Information at Weill Cornell Medical College, James Buchanan Brady Foundation, Department of Urology
- ^ Oto, A; Herts, B R; Remer, E M; Novick, A C (1998). "Inferior vena cava tumor thrombus in renal cell carcinoma: Staging by MR imaging and impact on surgical treatment". American Journal of Roentgenology. 171 (6): 1619–24. doi:10.2214/ajr.171.6.9843299. PMID 9843299.
- ^ "Clear-cell Carcinoma, Hypernephroid Tumour, or Hypernephroma". Retrieved 2010-03-31.
- ^ Vasil'Eva, NN; Koriakina, RF (1976). "Morphological diagnosis of renal cell carcinoma. Histo-cytological parallels". Arkhiv Patologii. 38 (12): 12–7. PMID 1016084.
- ^ "Renal clear cell carcinoma (Grawitz tumor)". Atlas of Pathology. January 30, 2009.
- ^ López, JI (Mar 2013). "Renal tumors with clear cells. A review". Pathology, Research and Practice. 209 (3): 137–46. doi:10.1016/j.prp.2013.01.007. PMID 23433880.
- ^ Rioux-Leclercq, Nathalie (Nov 2006). "Le grade nucléaire de fuhrman, facteur pronostique du cancer du rein depuis 25 ans" [The Fuhrman grading system for kidney cancer prognosis]. Progrès en urologie (in French). 16 (4): 5–8. PMID 17183964. INIST 18271804.
{{cite journal}}: CS1 maint: date and year (link) - ^ Delahunt, Brett; Sika-Paotonu, Dianne; Bethwaite, Peter B.; McCredie, Margaret R. E.; Martignoni, Guido; Eble, John N.; Jordan, T. (2007). "Fuhrman Grading is not Appropriate for Chromophobe Renal Cell Carcinoma". The American Journal of Surgical Pathology. 31 (6): 957–60. doi:10.1097/01.pas.0000249446.28713.53. PMID 17527087. S2CID 27154220.
- ^ Kovacs, Gyula; Akhtar, Mohammed; Beckwith, Bruce J.; Bugert, Peter; Cooper, Colin S.; Delahunt, Brett; Eble, John N.; Fleming, Stewart; Ljungberg, Börje; Medeiros, L. Jeffrey; Moch, Holger; Reuter, Victor E.; Ritz, Eberhard; Roos, Göran; Schmidt, Dietmar; Srigley, John R.; Störkel, Stephan; Van Den Berg, Eva; Zbar, Bert (1997). "The Heidelberg classification of renal cell tumours". The Journal of Pathology. 183 (2): 131–3. doi:10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. PMID 9390023. S2CID 34796951.
- ^ Motzer, R. J.; Bacik, J; Schwartz, LH; Reuter, V; Russo, P; Marion, S; Mazumdar, M (2003). "Prognostic Factors for Survival in Previously Treated Patients with Metastatic Renal Cell Carcinoma". Journal of Clinical Oncology. 22 (3): 454–63. doi:10.1200/JCO.2004.06.132. PMID 14752067.
- ^ Motzer, RJ; Mazumdar, M; Bacik, J; Berg, W; Amsterdam, A; Ferrara, J (1999). "Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma". Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 17 (8): 2530–40. doi:10.1200/JCO.1999.17.8.2530. PMID 10561319.
- ^ Leibovich, Bradley C.; Blute, Michael L.; Cheville, John C.; Lohse, Christine M.; Frank, Igor; Kwon, Eugene D.; Weaver, Amy L.; Parker, Alexander S.; Zincke, Horst (2003). "Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma". Cancer. 97 (7): 1663–71. doi:10.1002/cncr.11234. PMID 12655523. S2CID 1057155.
- ^ Oosterwijk-Wakka, Jeannette C.; Kats-Ugurlu, Gürsah; Leenders, William P.J.; Kiemeney, Lambertus A.L.M.; Old, Lloyd J.; Mulders, Peter F.A.; Oosterwijk, Egbert (2011). "Effect of tyrosine kinase inhibitor treatment of renal cell carcinoma on the accumulation of carbonic anhydrase IX-specific chimeric monoclonal antibody cG250". BJU International. 107 (1): 118–25. doi:10.1111/j.1464-410X.2010.09314.x. PMID 20346054. S2CID 5780450.
- ^ Simmons (26 September 2012). "Assessment of Oncologic Risk for Clinical Stage T1 Renal Tumours". Renal Cell Carcinoma Clinical Management. Humana Pr Inc. pp. 105–118. ISBN 978-1-62703-061-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Smaldone, Marc C.; Canter, Daniel; Kutikov, Alexander; Uzzo, Robert G. (2013). "Active Surveillance of the Small Renal Mass". Renal Cell Carcinoma. pp. 167–94. doi:10.1007/978-1-62703-062-5_10. ISBN 978-1-62703-061-8.
- ^ Casey, R.G.; Raheem, O.A.; Elmusharaf, E.; Madhavan, P.; Tolan, M.; Lynch, T.H. (2013). "Renal cell carcinoma with IVC and atrial thrombus: A single centre's 10 year surgical experience". The Surgeon. 11 (6): 295–9. doi:10.1016/j.surge.2013.02.007. PMID 23510704.
- ^ Weight, Christopher J.; Larson, Benjamin T.; Fergany, Amr F.; Gao, Tianming; Lane, Brian R.; Campbell, Steven C.; Kaouk, Jihad H.; Klein, Eric A.; Novick, Andrew C. (2010). "Nephrectomy Induced Chronic Renal Insufficiency is Associated with Increased Risk of Cardiovascular Death and Death from Any Cause in Patients with Localized cT1b Renal Masses". The Journal of Urology. 183 (4): 1317–23. doi:10.1016/j.juro.2009.12.030. PMID 20171688.
- ^ Weight, Christopher J.; Crispen, Paul L.; Breau, Rodney H.; Kim, Simon P.; Lohse, Christine M.; Boorjian, Stephen A.; Thompson, R. Houston; Leibovich, Bradley C. (2013). "Practice-setting and surgeon characteristics heavily influence the decision to perform partial nephrectomy among American Urologic Association surgeons". BJU International. 111 (5): 731–8. doi:10.1111/j.1464-410X.2012.11112.x. PMID 22502641. S2CID 829345.
- ^ Russo (2013). "Radical Nephrectomy for Localised Renal Tumours: Oncological and Renal Functional Considerations". Renal Cell Carcinoma Clinical Management. Humana Pr Inc. pp. 119–132. ISBN 978-1-62703-061-8.
- ^ a b c d Matin (2013). "Thermal Ablation". Renal Cell Carcinoma Clinical Management. Humana. pp. 155–166. ISBN 978-1-62703-061-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Davar (2013). "Immunotherapy for Renal Cell Carcinoma". Renal Cell Carcinoma Clinical Management. Humana. pp. 279–302. ISBN 978-1-62703-061-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Santoni, Matteo; De Tursi, Michele; Felici, Alessandra; Lo Re, Giovanni; Ricotta, Riccardo; Ruggeri, Enzo Maria; Sabbatini, Roberto; Santini, Daniele; Vaccaro, Vanja; Milella, Michele (June 2013). "Management of metastatic renal cell carcinoma patients with poor-risk features: current status and future perspectives". Expert Review of Anticancer Therapy. 13 (6): 697–709. doi:10.1586/era.13.52. PMID 23773104. S2CID 36871651.
- ^ a b c Stroup (2013). "Neoadjuvant Targeted Therapy and Concolodative Surgery". Renal Cell Carcinoma Clinical Management. Humana. pp. 219–230. ISBN 978-1-62703-061-8.
- ^ Shoji, Sunao; Nakano, Mayura; Sato, Haruhiro; Tang, Xian Yang; Osamura, Yoshiyuki Robert; Terachi, Toshiro; Uchida, Toyoaki; Takeya, Koichi (January 2014). "The current status of tailor-made medicine with molecular biomarkers for patients with clear cell renal cell carcinoma". Clinical & Experimental Metastasis. 31 (1): 111–34. doi:10.1007/s10585-013-9612-7. PMID 23959576. S2CID 15100003.
- ^ Jonasch, Eric; Futreal, P. Andrew; Davis, Ian J.; Bailey, Sean T.; Kim, William Y.; Brugarolas, James; Giaccia, Amato J.; Kurban, Ghada; Pause, Armin; Frydman, Judith; Zurita, Amado J.; Rini, Brian I.; Sharma, Pam; Atkins, Michael B.; Walker, Cheryl L.; Rathmell, W. Kimryn (July 2012). "State of the science: an update on renal cell carcinoma". Molecular Cancer Research. 10 (7): 859–80. doi:10.1158/1541-7786.MCR-12-0117. PMC 3399969. PMID 22638109.
- ^ Dranitsaris, George; Schmitz, Susanne; Broom, Reuben J. (November 2013). "Small molecule targeted therapies for the second-line treatment for metastatic renal cell carcinoma: a systematic review and indirect comparison of safety and efficacy". Journal of Cancer Research and Clinical Oncology. 139 (11): 1917–26. doi:10.1007/s00432-013-1510-5. PMID 24037486. S2CID 27524034.
- ^ a b Mihaly, Z.; Sztupinszki, Z.; Surowiak, P.; Gyorffy, B. (September 2012). "A comprehensive overview of targeted therapy in metastatic renal cell carcinoma". Current Cancer Drug Targets. 12 (7): 857–72. doi:10.2174/156800912802429265. PMC 3434473. PMID 22515521.
- ^ a b Lam, John S.; Leppert, John T.; Belldegrun, Arie S.; Figlin, Robert A. (2005). "Novel approaches in the therapy of metastatic renal cell carcinoma". World Journal of Urology. 23 (3): 202–12. doi:10.1007/s00345-004-0466-0. PMID 15812574. S2CID 12091991.
- ^ Ljungberg, Börje; Hanbury, Damian C.; Kuczyk, Marcus A.; Merseburger, Axel S.; Mulders, Peter F.A.; Patard, Jean-Jacques; Sinescu, Ioanel C.; European Association of Urology Guideline Group for renal cell carcinoma (2007). "Renal Cell Carcinoma Guideline". European Urology. 51 (6): 1502–10. doi:10.1016/j.eururo.2007.03.035. PMID 17408850.
- ^ a b Gupta, Kiran; Miller, Jeffrey D.; Li, Jim Z.; Russell, Mason W.; Charbonneau, Claudie (2008). "Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review". Cancer Treatment Reviews. 34 (3): 193–205. doi:10.1016/j.ctrv.2007.12.001. PMID 18313224.
- ^ a b c Buti, Sebastiano; Bersanelli, Melissa; Sikokis, Angelica; Maines, Francesca; Facchinetti, Francesco; Bria, Emilio; Ardizzoni, Andrea; Tortora, Giampaolo; Massari, Francesco (2013). "Chemotherapy in metastatic renal cell carcinoma today? A systematic review". Anti-Cancer Drugs. 24 (6): 535–554. doi:10.1097/CAD.0b013e3283609ec1. PMID 23552469. S2CID 205529087.
- ^ Patil, Sujata; Manola, Judith; Elson, Paul; Negrier, Sylvie; Escudier, Bernard; Eisen, Tim; Atkins, Michael; Bukowski, Ronald; Motzer, Robert J. (2012). "Improvement in Overall Survival of Patients with Advanced Renal Cell Carcinoma: Prognostic Factor Trend Analysis from an International Data Set of Clinical Trials". The Journal of Urology. 188 (6): 2095–100. doi:10.1016/j.juro.2012.08.026. PMID 23083849.
- ^ a b c Cohen, Herbert T.; McGovern, Francis J. (2005). "Renal-Cell Carcinoma". New England Journal of Medicine. 353 (23): 2477–90. doi:10.1056/NEJMra043172. PMID 16339096.
- ^ Calvo, Emiliano; Ravaud, Alain; Bellmunt, Joaquim (2013). "What is the optimal therapy for patients with metastatic renal cell carcinoma who progress on an initial VEGFr-TKI?". Cancer Treatment Reviews. 39 (4): 366–74. doi:10.1016/j.ctrv.2012.06.010. PMID 22832091.
- ^ Alasker, Ahmed; Meskawi, Malek; Sun, Maxine; Ismail, Salima; Hanna, Nawar; Hansen, Jens; Tian, Zhe; Bianchi, Marco; Perrotte, Paul; Karakiewicz, Pierre I. (2013). "A contemporary update on rates and management of toxicities of targeted therapies for metastatic renal cell carcinoma". Cancer Treatment Reviews. 39 (4): 388–401. doi:10.1016/j.ctrv.2012.12.006. PMID 23317510.
- ^ Birkhäuser, Frédéric D.; Kroeger, Nils; Pantuck, Allan J. (2013). "Etiology of Renal Cell Carcinoma: Incidence, Demographics, and Environmental Factors". Renal Cell Carcinoma. pp. 3–22. doi:10.1007/978-1-62703-062-5_1. ISBN 978-1-62703-061-8.
- ^ a b c d e Delahunt, Brett (March 8, 2009). "History of Renal Neoplasia" (PDF). United States and Canadian Academy of Pathology 2009 Annual Meeting.[dead link]
- ^ Delahunt, Brett (1996). "Renal cell carcinoma. A historical perspective". J Urol Pathol. 4: 31–49.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Delahunt, Brett; Eble, John N. (2005). "History of the Development of the Classification of Renal Cell Neoplasia". Clinics in Laboratory Medicine. 25 (2): 231–46, v. doi:10.1016/j.cll.2005.01.007. PMID 15848734.
- ^ Judd, E. Starr (1929). "Carcinoma of the Renal Cortex with Factors Bearing on Prognosis". Archives of Internal Medicine. 44 (5): 746. doi:10.1001/archinte.1929.00140050123011.
- ^ Foot, NC; Humphreys, GA; Whitmore, WF (1951). "Renal tumors: Pathology and prognosis in 295 cases". The Journal of Urology. 66 (2): 190–200. doi:10.1016/s0022-5347(17)74326-1. PMID 14861941.
- ^ Oberling, CH.; Rivière, M.; Haguenau, FR. (1960). "Ultrastructure of the Clear Cells in Renal Carcinomas and its Importance for the Demonstration of their Renal Origin". Nature. 186 (4722): 402–403. doi:10.1038/186402a0. PMID 14428164. S2CID 4224899.
- ^ Valladares Ayerbes, Manuel; Aparicio Gallego, Guadalupe; Díaz Prado, Silvia; Jiménez Fonseca, Paula; García Campelo, Rosario; Antón Aparicio, Luis Miguel (2008). "Origin of renal cell carcinomas". Clinical and Translational Oncology. 10 (11): 697–712. doi:10.1007/s12094-008-0276-8. hdl:2183/19413. PMID 19015066.



