Group 7 element: Difference between revisions
| Line 34: | Line 34: | ||
All other elements are either incredibly rare on earth (technetium, rhenium) or completely synthetic (bohrium). While rhenium is naturally occurring, it is one of the rarest metals with approximately 0.001 parts per million of rhenium in the Earth's crust.<ref name=":1" /> In contrast to manganese, only 40 or 50 metric tons of rhenium were mined. Technetium is only found in trace amounts in nature as a product of [[spontaneous fission]]; almost all is produced in laboratories. Bohrium is only produced in nuclear reactors and has never been isolated in pure form. |
All other elements are either incredibly rare on earth (technetium, rhenium) or completely synthetic (bohrium). While rhenium is naturally occurring, it is one of the rarest metals with approximately 0.001 parts per million of rhenium in the Earth's crust.<ref name=":1" /> In contrast to manganese, only 40 or 50 metric tons of rhenium were mined. Technetium is only found in trace amounts in nature as a product of [[spontaneous fission]]; almost all is produced in laboratories. Bohrium is only produced in nuclear reactors and has never been isolated in pure form. |
||
== Occurrence and production == |
|||
==Production== |
|||
{{expand section|date=February 2012}} |
{{expand section|date=February 2012}} |
||
'''Manganese''' |
'''Manganese''' |
||
{{Main article|Manganese# |
{{Main article|Manganese#Production}} |
||
Manganese comprises about 1000 [[Parts per million|ppm]] (0.1%) of the [[Earth's crust]], the 12th most abundant of the crust's elements.<ref name="Emsley2001">{{cite book|title=Nature's Building Blocks: An A-Z Guide to the Elements|last=Emsley|first=John|publisher=Oxford University Press|date=2001|location=Oxford, UK|isbn=978-0-19-850340-8|chapter=Manganese|pages=[https://archive.org/details/naturesbuildingb0000emsl/page/249 249–253]|chapter-url=https://books.google.com/books?id=j-Xu07p3cKwC|url=https://archive.org/details/naturesbuildingb0000emsl/page/249}}</ref> Soil contains 7–9000 ppm of manganese with an average of 440 ppm.<ref name="Emsley2001" /> The atmosphere contains 0.01 μg/m<sup>3</sup>.<ref name="Emsley2001" /> Manganese occurs principally as [[pyrolusite]] ([[manganese(IV) oxide|MnO<sub>2</sub>]]), [[braunite]] (Mn<sup>2+</sup>Mn<sup>3+</sup><sub>6</sub>)(SiO<sub>12</sub>),<ref>{{cite journal|pages=65–71 |journal=Contributions to Mineralogy and Petrology|title=Geochemistry of braunite and associated phases in metamorphosed non-calcareous manganese ores of India|first=P. K.|last=Bhattacharyya|author2=Dasgupta, Somnath |author3=Fukuoka, M. |author4=Roy Supriya |doi=10.1007/BF00371403|date=1984|volume=87|issue=1|bibcode=1984CoMP...87...65B|s2cid=129495326}}</ref> [[psilomelane]] {{chem2|(Ba,H2O)2Mn5O10}}, and to a lesser extent as [[rhodochrosite]] ([[manganese(II) carbonate|MnCO<sub>3</sub>]]). |
|||
{|class="wikitable" |
|||
|[[File:ManganeseOreUSGOV.jpg|center|120px]] |
|||
|[[File:Mineraly.sk - psilomelan.jpg|center|160px]] |
|||
|[[File:Spiegeleisen.jpg|center|185px]] |
|||
|[[File:Dendrites01.jpg|center|144px]] |
|||
|[[File:The Searchlight Rhodochrosite Crystal.jpg|center|152px]] |
|||
|- |
|||
|Manganese ore |
|||
|Psilomelane (manganese ore) |
|||
|[[Spiegeleisen]] is an iron alloy with a manganese content of approximately 15% |
|||
|Manganese oxide dendrites on limestone from [[Solnhofen]], Germany – a kind of [[pseudofossil]]. Scale is in mm |
|||
|Mineral rhodochrosite ([[manganese(II) carbonate]]) |
|||
|} |
|||
[[File:World Manganese Production 2006.svg|thumb|upright=1.6|Percentage of manganese output in 2006 by countries<ref name="USGSMCS2009">USGS Mineral Commodity Summaries 2009</ref>]] |
|||
The most important manganese ore is pyrolusite ([[manganese(IV) oxide|MnO<sub>2</sub>]]). Other economically important manganese ores usually show a close spatial relation to the iron ores, such as [[sphalerite]].<ref name="Holl" /><ref>{{Cite journal|last1=Cook|first1=Nigel J.|last2=Ciobanu|first2=Cristiana L.|last3=Pring|first3=Allan|last4=Skinner|first4=William|last5=Shimizu|first5=Masaaki|last6=Danyushevsky|first6=Leonid|last7=Saini-Eidukat|first7=Bernhardt|last8=Melcher|first8=Frank|date=2009|title=Trace and minor elements in sphalerite: A LA-ICPMS study|url=https://linkinghub.elsevier.com/retrieve/pii/S0016703709003263|journal=Geochimica et Cosmochimica Acta|language=en|volume=73|issue=16|pages=4761–4791|doi=10.1016/j.gca.2009.05.045|bibcode=2009GeCoA..73.4761C}}</ref> Land-based resources are large but irregularly distributed. About 80% of the known world manganese resources are in South Africa; other important manganese deposits are in Ukraine, Australia, India, China, [[Gabon]] and Brazil.<ref name="USGSMCS2009" /> According to 1978 estimate, the [[ocean floor]] has 500 billion tons of [[manganese nodule]]s.<ref>{{cite journal|doi=10.1016/j.micron.2008.10.005|pages=350–358|date=2009|title=Manganese/polymetallic nodules: micro-structural characterization of exolithobiontic- and endolithobiontic microbial biofilms by scanning electron microscopy|volume=40 |issue=3|pmid=19027306|journal=Micron |author1=Wang, X|author2=Schröder, HC|author3=Wiens, M|author4=Schlossmacher, U|author5=Müller, WEG}}</ref> Attempts to find economically viable methods of harvesting manganese nodules were abandoned in the 1970s.<ref>{{cite book |title=Manganese Nodules: Dimensions and Perspectives|journal=Marine Geology|volume=41|issue=3–4|pages=343|publisher=Springer|date=1978|isbn =978-90-277-0500-6|author=United Nations Ocean Economics and Technology Office, Technology Branch, United Nations|bibcode=1981MGeol..41..343C|doi=10.1016/0025-3227(81)90092-X}}</ref> |
|||
In South Africa, most identified deposits are located near [[Hotazel]] in the [[Northern Cape Province]], with a 2011 estimate of 15 billion tons. In 2011 South Africa produced 3.4 million tons, topping all other nations.<ref name="Mbendi">{{cite web |url=http://www.mbendi.com/indy/ming/mang/af/sa/p0005.htm |title=Manganese Mining in South Africa – Overview |publisher=MBendi.com |access-date=4 January 2014 |url-status=bot: unknown |archive-url=https://web.archive.org/web/20160205194737/http://www.mbendi.com/indy/ming/mang/af/sa/p0005.htm |archive-date=5 February 2016}}</ref> |
|||
Manganese is mainly mined in South Africa, Australia, China, Gabon, Brazil, India, Kazakhstan, Ghana, Ukraine and Malaysia.<ref>{{Cite journal|doi = 10.1007/s11837-018-2769-4|title = Review of Manganese Processing for Production of TRIP/TWIP Steels, Part 1: Current Practice and Processing Fundamentals|journal = JOM |volume = 70|issue = 5|pages = 680–690|year = 2018|last1 = Elliott|first1 = R|last2 = Coley|first2 = K|last3 = Mostaghel|first3 = S|last4 = Barati|first4 = M|bibcode = 2018JOM...tmp...63E|s2cid = 139950857}}</ref> |
|||
For the production of [[ferromanganese]], the manganese ore is mixed with iron ore and carbon, and then reduced either in a blast furnace or in an electric arc furnace.<ref name="IndMin">{{cite book|title=Industrial Minerals & Rocks: Commodities, Markets, and Uses |edition=7th|publisher=SME|date=2006|isbn=978-0-87335-233-8|chapter=Manganese|first=L. A.|last=Corathers |author2=Machamer, J. F. |chapter-url=https://books.google.com/books?id=zNicdkuulE4C&pg=PA631|pages=631–636}}</ref> The resulting [[ferromanganese]] has a manganese content of 30 to 80%.<ref name="Holl" /> Pure manganese used for the production of iron-free alloys is produced by [[Leaching (metallurgy)|leaching]] manganese ore with [[sulfuric acid]] and a subsequent [[electrowinning]] process.<ref name="hydrometI">{{cite journal|doi=10.1016/j.hydromet.2007.08.010 |title=Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide|date=2007|last=Zhang|first=Wensheng|author2=Cheng, Chu Yong|journal=Hydrometallurgy|volume=89 |pages=137–159|issue=3–4}}</ref> |
|||
[[File:Manganese Process Flow Diagram.jpg|left|thumb|upright=1.3|alt=Contains reactions and temperatures, as well as showing advanced processes such as the heat exchanger and milling process.|Process flow diagram for a manganese refining circuit.]] |
|||
A more progressive extraction process involves directly reducing (a low grade) manganese ore in a heap leach. This is done by [[Percolation|percolating]] natural gas through the bottom of the heap; the natural gas provides the heat (needs to be at least 850 °C) and the reducing agent (carbon monoxide). This reduces all of the manganese ore to manganese oxide (MnO), which is a leachable form. The ore then travels through a [[Mill (grinding)|grinding]] circuit to reduce the particle size of the ore to between 150 and 250 μm, increasing the surface area to aid leaching. The ore is then added to a leach tank of [[sulfuric acid]] and [[Iron(II)|ferrous iron]] (Fe<sup>2+</sup>) in a 1.6:1 ratio. The iron reacts with the [[manganese dioxide]] (MnO<sub>2</sub>) to form [[iron hydroxide]] (FeO(OH)) and elemental manganese (Mn): |
|||
This process yields approximately 92% recovery of the manganese. For further purification, the manganese can then be sent to an electrowinning facility.<ref name="ManganeseRecovery">{{cite web|url=http://www.americanmanganeseinc.com/wp-content/uploads/2011/08/American-Manganese-Phase-II-August-19-2010-Final-Report-Internet-Version-V2.pdf|title=The Recovery of Manganese from low grade resources: bench scale metallurgical test program completed|date=2010|author=Chow, Norman|author2=Nacu, Anca|author3=Warkentin, Doug|author4=Aksenov, Igor|author5=Teh, Hoe|name-list-style=amp|publisher=Kemetco Research Inc.|url-status=dead|archive-url=https://web.archive.org/web/20120202065633/http://www.americanmanganeseinc.com/wp-content/uploads/2011/08/American-Manganese-Phase-II-August-19-2010-Final-Report-Internet-Version-V2.pdf|archive-date=2 February 2012}}</ref> |
|||
In 1972 the [[Central Intelligence Agency|CIA]]'s [[Project Azorian]], through billionaire [[Howard Hughes]], commissioned the ship ''[[Hughes Glomar Explorer]]'' with the cover story of harvesting manganese nodules from the sea floor.<ref>{{Cite news|url=https://www.bbc.com/news/science-environment-42994812|title=The CIA secret on the ocean floor|date=19 February 2018|work=BBC News|access-date=3 May 2018|language=en-GB}}</ref> That triggered a rush of activity to collect manganese nodules, which was not actually practical. The real mission of ''Hughes Glomar Explorer'' was to raise a sunken [[Union of Soviet Socialist Republics|Soviet]] submarine, the [[Soviet submarine K-129 (1960)|K-129]], with the goal of retrieving Soviet code books.<ref name="azorian">{{cite web |url=http://www2.gwu.edu/~nsarchiv/nukevault/ebb305/index.htm |title=Project Azorian: The CIA's Declassified History of the Glomar Explorer |publisher=National Security Archive at George Washington University |date=12 February 2010 |access-date=18 September 2013}}</ref> |
|||
An abundant resource of manganese in the form of [[Manganese nodule|Mn nodules]] found on the ocean floor.<ref>{{cite book |last1=Hein |first1=James R. |title=Encyclopedia of Marine Geosciences - Manganese Nodules |date=January 2016 |publisher=Springer |pages=408–412 |url=https://www.researchgate.net/publication/306107551 |access-date=2 February 2021}}</ref><ref>{{cite journal |last1=Hoseinpour |first1=Vahid |last2=Ghaemi |first2=Nasser |title=Green synthesis of manganese nanoparticles: Applications and future perspective–A review |journal=Journal of Photochemistry and Photobiology B: Biology |date=1 December 2018 |volume=189 |pages=234–243 |doi=10.1016/j.jphotobiol.2018.10.022 |pmid=30412855 |s2cid=53248245 |url=https://www.sciencedirect.com/science/article/abs/pii/S101113441830959X |access-date=2 February 2021}}</ref> These nodules, which are composed of 29% manganese,<ref>{{cite web |last1=International Seabed Authority |title=Polymetallic Nodules |url=https://isa.org.jm/files/files/documents/eng7.pdf |website=isa.org |publisher=International Seabed Authority |access-date=2 February 2021}}</ref> are located along the [[seabed|ocean floor]] and the potential impact of mining these nodules is being researched. Physical, chemical, and biological environmental impacts can occur due to this nodule mining disturbing the seafloor and causing sediment plumes to form. This suspension includes metals and inorganic nutrients, which can lead to contamination of the near-bottom waters from dissolved toxic compounds. Mn nodules are also the grazing grounds, living space, and protection for endo- and epifaunal systems. When theses nodules are removed, these systems are directly affected. Overall, this can cause species to leave the area or completely die off.<ref>{{Cite journal|last1=Oebius|first1=Horst U|last2=Becker|first2=Hermann J|last3=Rolinski|first3=Susanne|last4=Jankowski|first4=Jacek A|date=January 2001|title=Parametrization and evaluation of marine environmental impacts produced by deep-sea manganese nodule mining|url=http://dx.doi.org/10.1016/s0967-0645(01)00052-2|journal=Deep Sea Research Part II: Topical Studies in Oceanography|volume=48|issue=17–18|pages=3453–3467|doi=10.1016/s0967-0645(01)00052-2|bibcode=2001DSRII..48.3453O|issn=0967-0645}}</ref> Prior to the commencement of the mining itself, research is being conducted by [[United Nations]] affiliated bodies and state-sponsored companies in an attempt to fully understand [[environmental issues|environmental impacts]] in the hopes of mitigating these impacts.<ref>{{cite journal |last1=Thompson |first1=Kirsten F. |last2=Miller |first2=Kathryn A. |last3=Currie |first3=Duncan |last4=Johnston |first4=Paul |last5=Santillo |first5=David |title=Seabed Mining and Approaches to Governance of the Deep Seabed |journal=Frontiers in Marine Science |date=2018 |volume=5 |doi=10.3389/fmars.2018.00480 |s2cid=54465407 |doi-access=free }}</ref> |
|||
'''Technetium''' |
'''Technetium''' |
||
{{Main article|Technetium#Occurrence and Production}} |
{{Main article|Technetium#Occurrence and Production}} |
||
Technetium was created by bombarding [[molybdenum]] atoms with [[deuteron]]s that had been accelerated by a device called a [[cyclotron]]. Technetium occurs naturally in the Earth's [[Crust (geology)|crust]] in minute concentrations of about 0.003 parts per trillion. Technetium is so rare because the [[half-life|half-lives]] of <sup>97</sup>Tc and <sup>98</sup>Tc are only 4.2 million years. More than a thousand of such periods have passed since the formation of the [[Earth]], so the probability of survival of even one atom of [[primordial nuclide|primordial]] technetium is effectively zero. However, small amounts exist as spontaneous [[fission product]]s in [[uranium ore]]s. A kilogram of uranium contains an estimated 1 [[Orders of magnitude (mass)|nanogram]] (10<sup>−9</sup> g) equivalent to ten trillion atoms of technetium.<ref name="blocks" /><ref>{{cite journal|doi=10.1021/ac961159q |title=Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials|date=1997 |last1=Dixon|first1=P.|last2=Curtis|first2=David B. |last3=Musgrave|first3=John |last4=Roensch|first4=Fred|last5=Roach|first5=Jeff|last6=Rokop|first6=Don|journal=Analytical Chemistry |volume=69|issue=9|pages=1692–1699|pmid=21639292}}</ref><ref>{{cite journal |doi=10.1016/S0016-7037(98)00282-8 |title=Nature's uncommon elements: plutonium and technetium|last1=Curtis|first1=D. |last2=Fabryka-Martin|first2=June|last3=Dixon|first3=Paul|last4=Cramer|first4=Jan|date=1999 |journal=Geochimica et Cosmochimica Acta |volume=63|issue=2|pages=275|bibcode=1999GeCoA..63..275C |url=https://digital.library.unt.edu/ark:/67531/metadc704244/}}</ref> Some [[red giant]] stars with the spectral types S-, M-, and N contain a spectral absorption line indicating the presence of technetium.{{sfn|Hammond|2004|p={{page needed|date=June 2021}}}}<ref>{{cite journal|doi=10.1126/science.114.2951.59|date=1951 |last1=Moore|first1=C. E.|title=Technetium in the Sun|journal=Science |volume=114 |issue=2951 |pages=59–61 |pmid=17782983|bibcode=1951Sci...114...59M}}</ref><!--Technetium in Red Giant Stars P Merrill — Science, 1952--> These red giants are known informally as [[technetium star]]s. |
|||
Technetium was created by bombarding [[molybdenum]] atoms with [[deuteron]]s that had been accelerated by a device called a [[cyclotron]]. It sometimes can be found in nature but not in large quantity. |
|||
'''Rhenium''' |
'''Rhenium''' |
||
{{Main article|Rhenium#Production}} |
{{Main article|Rhenium#Production}} |
||
Most of the rhenium extracted comes from [[Porphyry (geology)|porphyry]] [[molybdenum]] deposits.<ref>{{cite book|chapter=Chapter 7: By-Products of Porphyry Copper and Molybdenum Deposits|first1=D. A.|last1=John|first2=R. D.|last2=Taylor|title=Rare earth and critical elements in ore deposits|year=2016|volume=18|pages=137–164|url=https://pubs.er.usgs.gov/publication/70048652|editor=Philip L. Verplanck and Murray W. Hitzman}}</ref> These ores typically contain 0.001% to 0.2% rhenium.<ref name="G&W">{{Greenwood&Earnshaw2nd}}</ref> |
|||
[[Image:Molybdenit 1.jpg|thumb|left|Molybdenite]] |
|||
Rhenium is one of the rarest elements in [[Earth's crust]] with an average concentration of 1 ppb;<ref name="G&W" /> other sources quote the number of 0.5 ppb making it the 77th most abundant element in Earth's crust.<ref name="Emsley2001p358">{{cite book|title=Nature's Building Blocks: An A-Z Guide to the Elements|last=Emsley|first=John|publisher=Oxford University Press|date=2001|location=Oxford, England, UK|isbn=978-0-19-850340-8|chapter=Rhenium|pages=[https://archive.org/details/naturesbuildingb0000emsl/page/358 358–360]|chapter-url=https://books.google.com/books?id=j-Xu07p3cKwC|url=https://archive.org/details/naturesbuildingb0000emsl/page/358}}</ref> Rhenium is probably not found free in nature (its possible natural occurrence is uncertain), but occurs in amounts up to 0.2%<ref name="G&W" /> in the mineral [[molybdenite]] (which is primarily [[molybdenum disulfide]]), the major commercial source, although single molybdenite samples with up to 1.88% have been found.<ref name="Rousch" /> [[Chile]] has the world's largest rhenium reserves, part of the copper ore deposits, and was the leading producer as of 2005.<ref>{{cite web|url=http://minerals.usgs.gov/minerals/pubs/country/2005/cimyb05.pdf |first=Steve T.|last=Anderson| publisher=[[United States Geological Survey]]|title=2005 Minerals Yearbook: Chile|access-date=2008-10-26}}</ref> It was only recently that the first rhenium [[mineral]] was found and described (in 1994), a rhenium [[sulfide mineral]] (ReS<sub>2</sub>) condensing from a [[fumarole]] on [[Kudriavy]] volcano, [[Iturup]] island, in the [[Kuril Islands]].<ref>{{cite journal|last=Korzhinsky|first=M. A.|author2=Tkachenko, S. I. |author3=Shmulovich, K. I. |author4=Taran Y. A. |author5= Steinberg, G. S. | date=2004-05-05|title=Discovery of a pure rhenium mineral at Kudriavy volcano|journal=[[Nature (journal)|Nature]]|volume=369|pages=51–52|doi=10.1038/369051a0|issue=6475|bibcode = 1994Natur.369...51K |s2cid=4344624}}</ref> Kudriavy discharges up to 20–60 kg rhenium per year mostly in the form of rhenium disulfide.<ref>{{cite journal| last1 = Kremenetsky| first1 = A. A.| last2 = Chaplygin| first2 = I. V.| title = Concentration of rhenium and other rare metals in gases of the Kudryavy Volcano (Iturup Island, Kurile Islands)| journal = Doklady Earth Sciences| volume = 430| issue = 1| page = 114| date = 2010| doi = 10.1134/S1028334X10010253|bibcode = 2010DokES.430..114K | s2cid = 140632604}}</ref><ref>{{cite journal | last1 = Tessalina | first1 = S. | last2 = Yudovskaya | first2 = M. | last3 = Chaplygin | first3 = I. | last4 = Birck | first4 = J. | last5 = Capmas | first5 = F. | title = Sources of unique rhenium enrichment in fumaroles and sulphides at Kudryavy volcano | journal = Geochimica et Cosmochimica Acta | volume = 72 | page = 889 | date = 2008 | doi = 10.1016/j.gca.2007.11.015 | bibcode=2008GeCoA..72..889T | issue = 3}}</ref> Named [[rheniite]], this rare mineral commands high prices among collectors.<ref>{{cite web|url=http://www.galleries.com/minerals/sulfides/rheniite/rheniite.htm|publisher=Amethyst Galleries|title=The Mineral Rheniite}}</ref> <!--Dr. Kremenetsky from [[Russian Academy of Sciences|RAS]] Mineralogy Institute argues that this source could be commercially exploited,<ref>[http://www.nkj.ru/archive/articles/5340/ Завод на вулкане] // Наука и жизнь, № 11, 2000, in Russian.</ref> but currently there is no active attempts to extract it.--> |
|||
[[Image:Ammonium perrhenate.jpg|thumb|right|Ammonium perrhenate]] |
|||
Most of the rhenium extracted comes from [[Porphyry (geology)|porphyry]] [[molybdenum]] deposits.<ref>{{cite book|chapter=Chapter 7: By-Products of Porphyry Copper and Molybdenum Deposits|first1=D. A.|last1=John|first2=R. D.|last2=Taylor|title=Rare earth and critical elements in ore deposits|year=2016|volume=18|pages=137–164|url=https://pubs.er.usgs.gov/publication/70048652|editor=Philip L. Verplanck and Murray W. Hitzman}}</ref> These ores typically contain 0.001% to 0.2% rhenium.<ref name="G&W">{{Greenwood&Earnshaw2nd}}</ref> Roasting the ore volatilizes rhenium oxides.<ref name="Rousch">{{cite journal|doi = 10.1021/cr60291a002|title = Recent advances in the chemistry of rhenium|date = 1974|author = Rouschias, George|journal = Chemical Reviews|volume = 74|page = 531|issue = 5}}</ref> [[Rhenium(VII) oxide]] and [[perrhenic acid]] readily dissolve in water; they are leached from flue dusts and gasses and extracted by precipitating with [[potassium chloride|potassium]] or [[ammonium chloride]] as the [[perrhenate]] salts, and purified by [[Recrystallization (chemistry)|recrystallization]].<ref name="G&W" /> Total world production is between 40 and 50 tons/year; the main producers are in Chile, the United States, Peru, and Poland.<ref name="USGS_2012_summary">{{cite web|title=Rhenium|work=Mineral Commodity Summaries |publisher=U.S. Geological Survey|date=January 2012|url=http://minerals.usgs.gov/minerals/pubs/commodity/rhenium/mcs-2012-rheni.pdf|first=Michael J.|last=Magyar|access-date=2013-09-04}}</ref> Recycling of used Pt-Re catalyst and special alloys allow the recovery of another 10 tons per year. Prices for the metal rose rapidly in early 2008, from $1000–$2000 per [[kilogram|kg]] in 2003–2006 to over $10,000 in February 2008.<ref name="minormetals">{{cite web|title=MinorMetal prices|publisher=minormetals.com|url=http://www.minormetals.com/|access-date=2008-02-17}}</ref><ref>{{cite web|url=http://in.reuters.com/article/oilRpt/idINL1037587920080710|first=Jan|last=Harvey|title=Analysis: Super hot metal rhenium may reach "platinum prices"|date=2008-07-10|access-date=2008-10-26|publisher=Reuters India}}</ref> The metal form is prepared by reducing [[ammonium perrhenate]] with [[hydrogen]] at high temperatures:<ref name="Brauer" /> |
|||
:2 NH<sub>4</sub>ReO<sub>4</sub> + 7 H<sub>2</sub> → 2 Re + 8 H<sub>2</sub>O + 2 NH<sub>3</sub> |
|||
:There are technologies for the associated extraction of rhenium from productive solutions of underground leaching of uranium ores.<ref>{{Cite journal |last1=Rudenko |first1=A.A. |last2=Troshkina |first2=I.D. |last3=Danileyko |first3=V.V. |last4=Barabanov |first4=O.S. |last5=Vatsura |first5=F.Y. |title=Prospects for selective-and-advanced recovery of rhenium from pregnant solutions of in-situ leaching of uranium ores at Dobrovolnoye deposit |url=https://mst.misis.ru/jour/article/view/287 |journal=Gornye Nauki I Tekhnologii = Mining Science and Technology (Russia) |year=2021 |volume=6 |issue=3 |pages=158–169|doi=10.17073/2500-0632-2021-3-158-169 |s2cid=241476783 }}</ref> |
|||
'''Bohrium''' |
'''Bohrium''' |
||
Revision as of 15:47, 19 October 2022
This article needs additional citations for verification. (December 2009) |
Template:Periodic table (group 7) Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. They are manganese (Mn), technetium (Tc), rhenium (Re), and bohrium (Bh). All known elements of group 7 are transition metals.
Like other groups, the members of this family show patterns in their electron configurations, especially the outermost shells resulting in trends in chemical behavior.
Chemistry
| Z | Element | No. of electrons/shell |
|---|---|---|
| 25 | manganese | 2, 8, 13, 2 |
| 43 | technetium | 2, 8, 18, 13, 2 |
| 75 | rhenium | 2, 8, 18, 32, 13, 2 |
| 107 | bohrium | 2, 8, 18, 32, 32, 13, 2 |
Bohrium has not been isolated in pure form.
History
Manganese was discovered much earlier than the other group 7 elements owing to its much larger abundance in nature. While Johan Gottlieb Gahn is credited with the isolation of manganese in 1774, Ignatius Kaim reported his production of manganese in his dissertation in 1771.[1]
Group 7 contains the two naturally occurring transition metals discovered last: technetium and rhenium. Rhenium was discovered when Masataka Ogawa found what he thought was element 43 in thorianite, but this was dismissed; recent[when?] studies by H. K. Yoshihara suggest that he discovered rhenium instead, a fact not realized at the time. Walter Noddack, Otto Berg, and Ida Tacke were the first to conclusively identify rhenium;[2] it was thought they discovered element 43 as well, but as the experiment could not be replicated, it was dismissed. Technetium was formally discovered in December 1936 by Carlo Perrier and Emilio Segré, who discovered technetium-95 and technetium-97. Bohrium was discovered in 1981 by a team led by Peter Armbruster and Gottfried Münzenburg by bombarding bismuth-209 with chromium-54.
Occurrence
Manganese is the only common group 7 element, with the fifth largest abundance in the Earth's crust of any metal. It is most commonly found as manganese dioxide or manganese carbonate.[1] In 2007, 11 million metric tons of manganese were mined.
All other elements are either incredibly rare on earth (technetium, rhenium) or completely synthetic (bohrium). While rhenium is naturally occurring, it is one of the rarest metals with approximately 0.001 parts per million of rhenium in the Earth's crust.[2] In contrast to manganese, only 40 or 50 metric tons of rhenium were mined. Technetium is only found in trace amounts in nature as a product of spontaneous fission; almost all is produced in laboratories. Bohrium is only produced in nuclear reactors and has never been isolated in pure form.
Occurrence and production
This section needs expansion. You can help by adding to it. (February 2012) |
Manganese
Manganese comprises about 1000 ppm (0.1%) of the Earth's crust, the 12th most abundant of the crust's elements.[3] Soil contains 7–9000 ppm of manganese with an average of 440 ppm.[3] The atmosphere contains 0.01 μg/m3.[3] Manganese occurs principally as pyrolusite (MnO2), braunite (Mn2+Mn3+6)(SiO12),[4] psilomelane (Ba,H2O)2Mn5O10, and to a lesser extent as rhodochrosite (MnCO3).
 |
 |
 |
 |
 |
| Manganese ore | Psilomelane (manganese ore) | Spiegeleisen is an iron alloy with a manganese content of approximately 15% | Manganese oxide dendrites on limestone from Solnhofen, Germany – a kind of pseudofossil. Scale is in mm | Mineral rhodochrosite (manganese(II) carbonate) |

The most important manganese ore is pyrolusite (MnO2). Other economically important manganese ores usually show a close spatial relation to the iron ores, such as sphalerite.[6][7] Land-based resources are large but irregularly distributed. About 80% of the known world manganese resources are in South Africa; other important manganese deposits are in Ukraine, Australia, India, China, Gabon and Brazil.[5] According to 1978 estimate, the ocean floor has 500 billion tons of manganese nodules.[8] Attempts to find economically viable methods of harvesting manganese nodules were abandoned in the 1970s.[9]
In South Africa, most identified deposits are located near Hotazel in the Northern Cape Province, with a 2011 estimate of 15 billion tons. In 2011 South Africa produced 3.4 million tons, topping all other nations.[10]
Manganese is mainly mined in South Africa, Australia, China, Gabon, Brazil, India, Kazakhstan, Ghana, Ukraine and Malaysia.[11]
For the production of ferromanganese, the manganese ore is mixed with iron ore and carbon, and then reduced either in a blast furnace or in an electric arc furnace.[12] The resulting ferromanganese has a manganese content of 30 to 80%.[6] Pure manganese used for the production of iron-free alloys is produced by leaching manganese ore with sulfuric acid and a subsequent electrowinning process.[13]
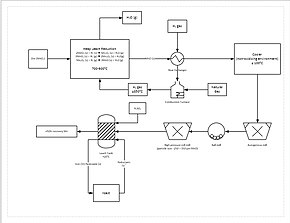
A more progressive extraction process involves directly reducing (a low grade) manganese ore in a heap leach. This is done by percolating natural gas through the bottom of the heap; the natural gas provides the heat (needs to be at least 850 °C) and the reducing agent (carbon monoxide). This reduces all of the manganese ore to manganese oxide (MnO), which is a leachable form. The ore then travels through a grinding circuit to reduce the particle size of the ore to between 150 and 250 μm, increasing the surface area to aid leaching. The ore is then added to a leach tank of sulfuric acid and ferrous iron (Fe2+) in a 1.6:1 ratio. The iron reacts with the manganese dioxide (MnO2) to form iron hydroxide (FeO(OH)) and elemental manganese (Mn):
This process yields approximately 92% recovery of the manganese. For further purification, the manganese can then be sent to an electrowinning facility.[14]
In 1972 the CIA's Project Azorian, through billionaire Howard Hughes, commissioned the ship Hughes Glomar Explorer with the cover story of harvesting manganese nodules from the sea floor.[15] That triggered a rush of activity to collect manganese nodules, which was not actually practical. The real mission of Hughes Glomar Explorer was to raise a sunken Soviet submarine, the K-129, with the goal of retrieving Soviet code books.[16]
An abundant resource of manganese in the form of Mn nodules found on the ocean floor.[17][18] These nodules, which are composed of 29% manganese,[19] are located along the ocean floor and the potential impact of mining these nodules is being researched. Physical, chemical, and biological environmental impacts can occur due to this nodule mining disturbing the seafloor and causing sediment plumes to form. This suspension includes metals and inorganic nutrients, which can lead to contamination of the near-bottom waters from dissolved toxic compounds. Mn nodules are also the grazing grounds, living space, and protection for endo- and epifaunal systems. When theses nodules are removed, these systems are directly affected. Overall, this can cause species to leave the area or completely die off.[20] Prior to the commencement of the mining itself, research is being conducted by United Nations affiliated bodies and state-sponsored companies in an attempt to fully understand environmental impacts in the hopes of mitigating these impacts.[21]
Technetium
Technetium was created by bombarding molybdenum atoms with deuterons that had been accelerated by a device called a cyclotron. Technetium occurs naturally in the Earth's crust in minute concentrations of about 0.003 parts per trillion. Technetium is so rare because the half-lives of 97Tc and 98Tc are only 4.2 million years. More than a thousand of such periods have passed since the formation of the Earth, so the probability of survival of even one atom of primordial technetium is effectively zero. However, small amounts exist as spontaneous fission products in uranium ores. A kilogram of uranium contains an estimated 1 nanogram (10−9 g) equivalent to ten trillion atoms of technetium.[22][23][24] Some red giant stars with the spectral types S-, M-, and N contain a spectral absorption line indicating the presence of technetium.[25][26] These red giants are known informally as technetium stars.
Rhenium

Rhenium is one of the rarest elements in Earth's crust with an average concentration of 1 ppb;[27] other sources quote the number of 0.5 ppb making it the 77th most abundant element in Earth's crust.[28] Rhenium is probably not found free in nature (its possible natural occurrence is uncertain), but occurs in amounts up to 0.2%[27] in the mineral molybdenite (which is primarily molybdenum disulfide), the major commercial source, although single molybdenite samples with up to 1.88% have been found.[29] Chile has the world's largest rhenium reserves, part of the copper ore deposits, and was the leading producer as of 2005.[30] It was only recently that the first rhenium mineral was found and described (in 1994), a rhenium sulfide mineral (ReS2) condensing from a fumarole on Kudriavy volcano, Iturup island, in the Kuril Islands.[31] Kudriavy discharges up to 20–60 kg rhenium per year mostly in the form of rhenium disulfide.[32][33] Named rheniite, this rare mineral commands high prices among collectors.[34]

Most of the rhenium extracted comes from porphyry molybdenum deposits.[35] These ores typically contain 0.001% to 0.2% rhenium.[27] Roasting the ore volatilizes rhenium oxides.[29] Rhenium(VII) oxide and perrhenic acid readily dissolve in water; they are leached from flue dusts and gasses and extracted by precipitating with potassium or ammonium chloride as the perrhenate salts, and purified by recrystallization.[27] Total world production is between 40 and 50 tons/year; the main producers are in Chile, the United States, Peru, and Poland.[36] Recycling of used Pt-Re catalyst and special alloys allow the recovery of another 10 tons per year. Prices for the metal rose rapidly in early 2008, from $1000–$2000 per kg in 2003–2006 to over $10,000 in February 2008.[37][38] The metal form is prepared by reducing ammonium perrhenate with hydrogen at high temperatures:[39]
- 2 NH4ReO4 + 7 H2 → 2 Re + 8 H2O + 2 NH3
- There are technologies for the associated extraction of rhenium from productive solutions of underground leaching of uranium ores.[40]
Bohrium
Bohrium is a synthetic element that does not occur in nature. Very few atoms have been synthesized, and also due to its radioactivity, only limited research has been conducted.
Applications
This section needs expansion. You can help by adding to it. (February 2012) |

The facial isomer of both rhenium and manganese 2,2'-bipyridyl tricarbonyl halide complexes have been extensively researched as catalysts for electrochemical carbon dioxide reduction due to their high selectivity and stability. They are commonly abbreviated as M(R-bpy)(CO)3X where M = Mn, Re; R-bpy = 4,4'-disubstituted 2,2'-bipyridine; and X = Cl, Br.
Rhenium
The catalytic activity of Re(bpy)(CO)3Cl for carbon dioxide reduction was first studied by Lehn et al.[41] and Meyer et al.[42] in 1984 and 1985, respectively. Re(R-bpy)(CO)3X complexes exclusively produce CO from CO2 reduction with Faradaic efficiencies of close to 100% even in solutions with high concentrations of water or Brønsted acids.[43]
The catalytic mechanism of Re(R-bpy)(CO)3X involves reduction of the complex twice and loss of the X ligand to generate a five-coordinate active species which binds CO2. These complexes will reduce CO2 both with and without an additional acid present; however, the presence of an acid increases catalytic activity.[43] The high selectivity of these complexes to CO2 reduction over the competing hydrogen evolution reaction has been shown by density functional theory studies to be related to the faster kinetics of CO2 binding compared to H+ binding.[44]
Manganese
The rarity of rhenium has shifted research toward the manganese version of these catalysts as a more sustainable alternative.[43] The first reports of catalytic activity of Mn(R-bpy)(CO)3Br towards CO2 reduction came from Chardon-Noblat and coworkers in 2011.[45] Compared to Re analogs, Mn(R-bpy)(CO)3Br shows catalytic activity at lower overpotentials.[44]
The catalytic mechanism for Mn(R-bpy)(CO)3X is complex and depends on the steric profile of the bipyridine ligand. When R is not bulky, the catalyst dimerizes to form [Mn(R-bpy)(CO)3]2 before forming the active species. When R is bulky, however, the complex forms the active species without dimerizing, reducing the overpotential of CO2 reduction by 200-300 mV. Unlike Re(R-bpy)(CO)3X, Mn(R-bpy)(CO)3X only reduces CO2 in the presence of an acid.[44]
Technetium
Technetium-99m is used in radioimaging.[46]
Bohrium
Bohrium is a synthetic element and is too radioactive to be used in anything.
Precautions
Although being an essential trace element in the human body, manganese can be somewhat toxic if ingested in higher amounts than normal.[citation needed] Technetium should be handled with care due to its radioactivity.
Biological role and precautions
Only manganese has a role in the human body. It is an essential trace nutrient, with the body containing approximately 10 milligrams at any given time, being mainly in the liver and kidneys. Many enzymes contain manganese, making it essential for life, and is also found in chloroplasts. Technetium, rhenium, and bohrium have no known biological roles. Technetium is, however, used in radioimaging.
References
- ^ a b "Manganese - Element information, properties and uses | Periodic Table". www.rsc.org. Retrieved 2019-12-02.
- ^ a b "Rhenium - Element information, properties and uses | Periodic Table". www.rsc.org. Retrieved 2019-12-02.
- ^ a b c Emsley, John (2001). "Manganese". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, UK: Oxford University Press. pp. 249–253. ISBN 978-0-19-850340-8.
- ^ Bhattacharyya, P. K.; Dasgupta, Somnath; Fukuoka, M.; Roy Supriya (1984). "Geochemistry of braunite and associated phases in metamorphosed non-calcareous manganese ores of India". Contributions to Mineralogy and Petrology. 87 (1): 65–71. Bibcode:1984CoMP...87...65B. doi:10.1007/BF00371403. S2CID 129495326.
- ^ a b USGS Mineral Commodity Summaries 2009
- ^ a b Cite error: The named reference
Hollwas invoked but never defined (see the help page). - ^ Cook, Nigel J.; Ciobanu, Cristiana L.; Pring, Allan; Skinner, William; Shimizu, Masaaki; Danyushevsky, Leonid; Saini-Eidukat, Bernhardt; Melcher, Frank (2009). "Trace and minor elements in sphalerite: A LA-ICPMS study". Geochimica et Cosmochimica Acta. 73 (16): 4761–4791. Bibcode:2009GeCoA..73.4761C. doi:10.1016/j.gca.2009.05.045.
- ^ Wang, X; Schröder, HC; Wiens, M; Schlossmacher, U; Müller, WEG (2009). "Manganese/polymetallic nodules: micro-structural characterization of exolithobiontic- and endolithobiontic microbial biofilms by scanning electron microscopy". Micron. 40 (3): 350–358. doi:10.1016/j.micron.2008.10.005. PMID 19027306.
- ^ United Nations Ocean Economics and Technology Office, Technology Branch, United Nations (1978). Manganese Nodules: Dimensions and Perspectives. Vol. 41. Springer. p. 343. Bibcode:1981MGeol..41..343C. doi:10.1016/0025-3227(81)90092-X. ISBN 978-90-277-0500-6.
{{cite book}}:|journal=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Manganese Mining in South Africa – Overview". MBendi.com. Archived from the original on 5 February 2016. Retrieved 4 January 2014.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ Elliott, R; Coley, K; Mostaghel, S; Barati, M (2018). "Review of Manganese Processing for Production of TRIP/TWIP Steels, Part 1: Current Practice and Processing Fundamentals". JOM. 70 (5): 680–690. Bibcode:2018JOM...tmp...63E. doi:10.1007/s11837-018-2769-4. S2CID 139950857.
{{cite journal}}: CS1 maint: bibcode (link) - ^ Corathers, L. A.; Machamer, J. F. (2006). "Manganese". Industrial Minerals & Rocks: Commodities, Markets, and Uses (7th ed.). SME. pp. 631–636. ISBN 978-0-87335-233-8.
- ^ Zhang, Wensheng; Cheng, Chu Yong (2007). "Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide". Hydrometallurgy. 89 (3–4): 137–159. doi:10.1016/j.hydromet.2007.08.010.
- ^ Chow, Norman; Nacu, Anca; Warkentin, Doug; Aksenov, Igor & Teh, Hoe (2010). "The Recovery of Manganese from low grade resources: bench scale metallurgical test program completed" (PDF). Kemetco Research Inc. Archived from the original (PDF) on 2 February 2012.
- ^ "The CIA secret on the ocean floor". BBC News. 19 February 2018. Retrieved 3 May 2018.
- ^ "Project Azorian: The CIA's Declassified History of the Glomar Explorer". National Security Archive at George Washington University. 12 February 2010. Retrieved 18 September 2013.
- ^ Hein, James R. (January 2016). Encyclopedia of Marine Geosciences - Manganese Nodules. Springer. pp. 408–412. Retrieved 2 February 2021.
- ^ Hoseinpour, Vahid; Ghaemi, Nasser (1 December 2018). "Green synthesis of manganese nanoparticles: Applications and future perspective–A review". Journal of Photochemistry and Photobiology B: Biology. 189: 234–243. doi:10.1016/j.jphotobiol.2018.10.022. PMID 30412855. S2CID 53248245. Retrieved 2 February 2021.
- ^ International Seabed Authority. "Polymetallic Nodules" (PDF). isa.org. International Seabed Authority. Retrieved 2 February 2021.
- ^ Oebius, Horst U; Becker, Hermann J; Rolinski, Susanne; Jankowski, Jacek A (January 2001). "Parametrization and evaluation of marine environmental impacts produced by deep-sea manganese nodule mining". Deep Sea Research Part II: Topical Studies in Oceanography. 48 (17–18): 3453–3467. Bibcode:2001DSRII..48.3453O. doi:10.1016/s0967-0645(01)00052-2. ISSN 0967-0645.
- ^ Thompson, Kirsten F.; Miller, Kathryn A.; Currie, Duncan; Johnston, Paul; Santillo, David (2018). "Seabed Mining and Approaches to Governance of the Deep Seabed". Frontiers in Marine Science. 5. doi:10.3389/fmars.2018.00480. S2CID 54465407.
- ^ Cite error: The named reference
blockswas invoked but never defined (see the help page). - ^ Dixon, P.; Curtis, David B.; Musgrave, John; Roensch, Fred; Roach, Jeff; Rokop, Don (1997). "Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials". Analytical Chemistry. 69 (9): 1692–1699. doi:10.1021/ac961159q. PMID 21639292.
- ^ Curtis, D.; Fabryka-Martin, June; Dixon, Paul; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta. 63 (2): 275. Bibcode:1999GeCoA..63..275C. doi:10.1016/S0016-7037(98)00282-8.
- ^ Hammond 2004, p. [page needed].
- ^ Moore, C. E. (1951). "Technetium in the Sun". Science. 114 (2951): 59–61. Bibcode:1951Sci...114...59M. doi:10.1126/science.114.2951.59. PMID 17782983.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Emsley, John (2001). "Rhenium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 358–360. ISBN 978-0-19-850340-8.
- ^ a b Rouschias, George (1974). "Recent advances in the chemistry of rhenium". Chemical Reviews. 74 (5): 531. doi:10.1021/cr60291a002.
- ^ Anderson, Steve T. "2005 Minerals Yearbook: Chile" (PDF). United States Geological Survey. Retrieved 2008-10-26.
- ^ Korzhinsky, M. A.; Tkachenko, S. I.; Shmulovich, K. I.; Taran Y. A.; Steinberg, G. S. (2004-05-05). "Discovery of a pure rhenium mineral at Kudriavy volcano". Nature. 369 (6475): 51–52. Bibcode:1994Natur.369...51K. doi:10.1038/369051a0. S2CID 4344624.
- ^ Kremenetsky, A. A.; Chaplygin, I. V. (2010). "Concentration of rhenium and other rare metals in gases of the Kudryavy Volcano (Iturup Island, Kurile Islands)". Doklady Earth Sciences. 430 (1): 114. Bibcode:2010DokES.430..114K. doi:10.1134/S1028334X10010253. S2CID 140632604.
- ^ Tessalina, S.; Yudovskaya, M.; Chaplygin, I.; Birck, J.; Capmas, F. (2008). "Sources of unique rhenium enrichment in fumaroles and sulphides at Kudryavy volcano". Geochimica et Cosmochimica Acta. 72 (3): 889. Bibcode:2008GeCoA..72..889T. doi:10.1016/j.gca.2007.11.015.
- ^ "The Mineral Rheniite". Amethyst Galleries.
- ^ John, D. A.; Taylor, R. D. (2016). "Chapter 7: By-Products of Porphyry Copper and Molybdenum Deposits". In Philip L. Verplanck and Murray W. Hitzman (ed.). Rare earth and critical elements in ore deposits. Vol. 18. pp. 137–164.
- ^ Magyar, Michael J. (January 2012). "Rhenium" (PDF). Mineral Commodity Summaries. U.S. Geological Survey. Retrieved 2013-09-04.
- ^ "MinorMetal prices". minormetals.com. Retrieved 2008-02-17.
- ^ Harvey, Jan (2008-07-10). "Analysis: Super hot metal rhenium may reach "platinum prices"". Reuters India. Retrieved 2008-10-26.
- ^ Cite error: The named reference
Brauerwas invoked but never defined (see the help page). - ^ Rudenko, A.A.; Troshkina, I.D.; Danileyko, V.V.; Barabanov, O.S.; Vatsura, F.Y. (2021). "Prospects for selective-and-advanced recovery of rhenium from pregnant solutions of in-situ leaching of uranium ores at Dobrovolnoye deposit". Gornye Nauki I Tekhnologii = Mining Science and Technology (Russia). 6 (3): 158–169. doi:10.17073/2500-0632-2021-3-158-169. S2CID 241476783.
- ^ Hawecker, Jeannot (1984). "Electrocatalytic Reduction of Carbon Dioxide Mediated by Re(bipy)(CO)3Cl (bipy = 2,2'-bipyridine)". J. Chem. Soc., Chem. Commun.: 328–330. doi:10.1039/C39840000328.
- ^ Sullivan, B. Patrick (1985). "One- and Two-electron Pathways in the Electrocatalytic Reduction of CO2 by fac-Re(bpy)(CO)3Cl (bpy = 2,2'-bipyridine)". J. Chem. Soc., Chem. Commun.: 1414–1416. doi:10.1039/C39850001414.
- ^ a b c Grice, Kyle (2014). "Recent Studies of Rhenium and Manganese Bipyridine Carbonyl Catalysts for the Electrochemical Reduction of CO2". Advances in Inorganic Chemistry. 66: 163–188. doi:10.1016/B978-0-12-420221-4.00005-6. ISBN 9780124202214.
- ^ a b c Francke, Robert (2018). "Homogeneously Catalyzed Electroreduction of Carbon Dioxide -- Methods, Mechanisms, and Catalysts". Chemical Reviews. 118 (9): 4631–4701. doi:10.1021/acs.chemrev.7b00459. PMID 29319300.
- ^ Bourrez, Marc (2011). "[Mn(bipyridyl)(CO)3Br]: an abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction". Angewandte Chemie International Edition in English. 50 (42): 9903–9906. doi:10.1002/anie.201103616. PMID 21922614.
- ^ Alberto, Roger; Nadeem, Qaisar (2021). "Chapter 7. 99m Technetium-Based Imaging Agents and Developments in 99Tc Chemistry". Metal Ions in Bio-Imaging Techniques. Springer. pp. 195–238. doi:10.1515/9783110685701-013. S2CID 233684677.
