PPAR agonist: Difference between revisions
m Journal cites, using AWB (10820) |
m Journal cites, using AWB (10820) |
||
| Line 9: | Line 9: | ||
===PPAR-gamma agonists=== |
===PPAR-gamma agonists=== |
||
[[Peroxisome proliferator-activated receptor gamma|PPARγ (gamma)]] is the main target of the drug class of [[thiazolidinedione]]s (TZDs), used in [[diabetes mellitus]] and other diseases that feature [[insulin resistance]]. It is also mildly activated by certain [[NSAID]]s (such as [[ibuprofen]]) and [[indole]]s, as well as from a number of natural compounds.<ref>Atanasov AG |
[[Peroxisome proliferator-activated receptor gamma|PPARγ (gamma)]] is the main target of the drug class of [[thiazolidinedione]]s (TZDs), used in [[diabetes mellitus]] and other diseases that feature [[insulin resistance]]. It is also mildly activated by certain [[NSAID]]s (such as [[ibuprofen]]) and [[indole]]s, as well as from a number of natural compounds.<ref>{{cite journal | last1 = Atanasov | first1 = AG | last2 = Wang | first2 = JN | last3 = Gu | first3 = SP | last4 = Bu | first4 = J | last5 = Kramer | first5 = MP | last6 = Baumgartner | first6 = L | last7 = Fakhrudin | first7 = N | last8 = Ladurner | first8 = A | last9 = Malainer | first9 = C | last10 = Vuorinen | first10 = A | last11 = Noha | first11 = SM | last12 = Schwaiger | first12 = S | last13 = Rollinger | first13 = JM | last14 = Schuster | first14 = D | last15 = Stuppner | first15 = H | last16 = Dirsch | first16 = VM | last17 = Heiss | first17 = EH | date = Oct 2013 | title = Honokiol: a non-adipogenic PPARγ agonist from nature | url = http://www.sciencedirect.com/science/article/pii/S030441651300281X | journal = Biochim Biophys Acta. | volume = 1830 | issue = 10| pages = 4813–9 | doi = 10.1016/j.bbagen.2013.06.021 | pmid = 23811337 | pmc=3790966}}</ref><ref>{{cite journal | last1 = Atanasov | first1 = AG | last2 = Blunder | first2 = M | last3 = Fakhrudin | first3 = N | last4 = Liu | first4 = X | last5 = Noha | first5 = SM | last6 = Malainer | first6 = C | last7 = Kramer | first7 = MP | last8 = Cocic | first8 = A | last9 = Kunert | first9 = O | last10 = Schinkovitz | first10 = A | last11 = Heiss | first11 = EH | last12 = Schuster | first12 = D | last13 = Dirsch | first13 = VM | last14 = Bauer | first14 = R | date = Apr 2013 | title = Polyacetylenes from Notopterygium incisum--new selective partial agonists of peroxisome proliferator-activated receptor-gamma | url = http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0061755 | journal = PLoS One. | volume = 8 | issue = 4| page = e61755 | doi = 10.1371/journal.pone.0061755 | pmid = 23630612 | pmc=3632601}}</ref> Known inhibitors include the experimental agent GW-9662. |
||
They are also used in treating hyperlipidaemia in [[atherosclerosis]]. Here they act by increasing the expression of [[ABCA1]], which transports extra-hepatic cholesterol into HDL. Increased uptake and excretion from the liver therefore follows. |
They are also used in treating hyperlipidaemia in [[atherosclerosis]]. Here they act by increasing the expression of [[ABCA1]], which transports extra-hepatic cholesterol into HDL. Increased uptake and excretion from the liver therefore follows. |
||
Revision as of 22:29, 4 March 2015
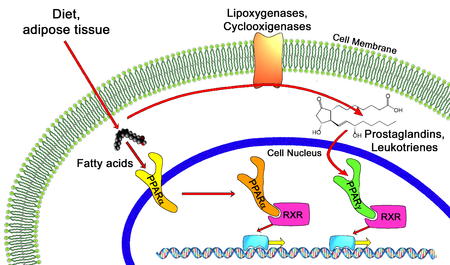
PPAR agonists are drugs which act upon the peroxisome proliferator-activated receptor. They are used for the treatment of symptoms of the metabolic syndrome, mainly for lowering triglycerides and blood sugar.
Classification
PPAR-alpha and PPAR-gamma are the molecular targets of a number of marketed drugs. The main classes of PPAR agonists are:
PPAR-alpha agonists
PPARα (alpha) is the main target of fibrate drugs, a class of amphipathic carboxylic acids (clofibrate, gemfibrozil, ciprofibrate, bezafibrate, and fenofibrate). They were originally indicated for cholesterol disorders and more recently for disorders that feature high triglycerides. Many of them increase the risk of cancer to the point where they eliminate the survival benefit of reduced heart disease.[citation needed]
PPAR-gamma agonists
PPARγ (gamma) is the main target of the drug class of thiazolidinediones (TZDs), used in diabetes mellitus and other diseases that feature insulin resistance. It is also mildly activated by certain NSAIDs (such as ibuprofen) and indoles, as well as from a number of natural compounds.[1][2] Known inhibitors include the experimental agent GW-9662.
They are also used in treating hyperlipidaemia in atherosclerosis. Here they act by increasing the expression of ABCA1, which transports extra-hepatic cholesterol into HDL. Increased uptake and excretion from the liver therefore follows.
Animal studies have shown their possible role in amelioration of pulmonary inflammation, especially in asthma.[3]
They may cause fluid retention and heart failure in those with weak hearts.
PPAR-delta agonists
PPARδ (delta) is the main target of a research chemical named GW501516. It has been shown that agonism of PPARδ changes the body's fuel preference from glucose to lipids,[4] but ironically improves metabolic syndrome (which is characterized by the body being unable to efficiently deal with glucose resulting in insulin resistance and sometimes diabetes).
Dual and pan PPAR agonists
A fourth class of dual PPAR agonists, so-called glitazars, which bind to both the α and γ PPAR isoforms, are currently under active investigation for treatment of a larger subset of the symptoms of the metabolic syndrome.[5][6] These include the experimental compounds aleglitazar, muraglitazar and tesaglitazar. In June 2013, saroglitazar was the first glitazar to be approved for clinical use.[7]
In addition, there is continuing research and development of new dual α/δ and γ/δ PPAR agonists for additional therapeutic indications, as well as "pan" agonists acting on all three isoforms.[8][9]
References
- ^ Atanasov, AG; Wang, JN; Gu, SP; Bu, J; Kramer, MP; Baumgartner, L; Fakhrudin, N; Ladurner, A; Malainer, C; Vuorinen, A; Noha, SM; Schwaiger, S; Rollinger, JM; Schuster, D; Stuppner, H; Dirsch, VM; Heiss, EH (Oct 2013). "Honokiol: a non-adipogenic PPARγ agonist from nature". Biochim Biophys Acta. 1830 (10): 4813–9. doi:10.1016/j.bbagen.2013.06.021. PMC 3790966. PMID 23811337.
- ^ Atanasov, AG; Blunder, M; Fakhrudin, N; Liu, X; Noha, SM; Malainer, C; Kramer, MP; Cocic, A; Kunert, O; Schinkovitz, A; Heiss, EH; Schuster, D; Dirsch, VM; Bauer, R (Apr 2013). "Polyacetylenes from Notopterygium incisum--new selective partial agonists of peroxisome proliferator-activated receptor-gamma". PLoS One. 8 (4): e61755. doi:10.1371/journal.pone.0061755. PMC 3632601. PMID 23630612.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 23866284, please use {{cite journal}} with
|pmid=23866284instead. - ^ B. Brunmair; et al. (2006). "Activation of PPAR-δ in isolated rat skeletal muscle switches fuel preference from glucose to fatty acids". Diabetologia. 49 (11): 2713–22. doi:10.1007/s00125-006-0357-6. PMID 16960684.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Fiévet C, Fruchart JC, Staels B (2006). "PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome". Current Opinion in Pharmacology. 6 (6): 606–14. doi:10.1016/j.coph.2006.06.009. PMID 16973418.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Balakumar P, Rose M, Ganti SS, Krishan P, Singh M (2007). "PPAR dual agonists: are they opening Pandora's Box?". Pharmacol. Res. 56 (2): 91–8. doi:10.1016/j.phrs.2007.03.002. PMID 17428674.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.wallstreet-online.de/nachricht/6228479-zydus-gelingt-durchbruch-lipaglyn-wirkstoff-indien-markt-gelangt Template:De icon
- ^ Staels B, Fruchart JC (2005). "Therapeutic roles of peroxisome proliferator-activated receptor agonists". Diabetes. 54 (8): 2460–70. doi:10.2337/diabetes.54.8.2460. PMID 16046315.
- ^ Nevin DK, Fayne D, Lloyd DG (2011). "Rational targeting of peroxisome proliferating activated receptor subtypes". Current Medicinal Chemistry. 11 (36): 5598–623. PMID 22172067.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
