Brachytherapy: Difference between revisions
No edit summary |
m Bot: Deprecating Template:Cite pmid and some minor fixations |
||
| Line 10: | Line 10: | ||
}} |
}} |
||
'''Brachytherapy''' (from the [[Ancient Greek|Greek]] word βραχύς ''brachys'', meaning "short-distance"), also known as '''internal radiotherapy''', '''sealed source radiotherapy''', '''curietherapy''' or '''endocurietherapy''', is a form of [[radiotherapy]] where a sealed [[radiation|radiation source]] is placed inside or next to the area requiring treatment. Brachytherapy is commonly used as an effective treatment for [[cervical cancer|cervical]], [[Prostate cancer|prostate]], [[Breast cancer|breast]], and [[skin cancer]] and can also be used to treat tumours in many other body sites.<ref name="GEC-ESTRO"> |
'''Brachytherapy''' (from the [[Ancient Greek|Greek]] word βραχύς ''brachys'', meaning "short-distance"), also known as '''internal radiotherapy''', '''sealed source radiotherapy''', '''curietherapy''' or '''endocurietherapy''', is a form of [[radiotherapy]] where a sealed [[radiation|radiation source]] is placed inside or next to the area requiring treatment. Brachytherapy is commonly used as an effective treatment for [[cervical cancer|cervical]], [[Prostate cancer|prostate]], [[Breast cancer|breast]], and [[skin cancer]] and can also be used to treat tumours in many other body sites.<ref name="GEC-ESTRO"> |
||
{{Cite book | editor1-last = Gerbaulet | editor1-first = Alain | editor2-last = Pötter | editor2-first = Richard | editor3-last = Mazeron | editor3-first = Jean-Jacques | editor4-last = Meertens | editor4-first = Harm | editor5-last = Limbergen | editor5-first = Erik Van | year = 2002 | title = The GEC ESTRO handbook of brachytherapy | publisher = European Society for Therapeutic Radiology and Oncology | location = Leuven, Belgium | url=http://www.estro-education.org/publications/Documents/GEC%20ESTRO%20Handbook%20of%20Brachytherapy.html | accessdate = | isbn=978-90-804532-6-5}}</ref> |
|||
{{Cite book |
|||
| editor1-last = Gerbaulet |
|||
| editor1-first = Alain |
|||
| editor2-last = Pötter |
|||
| editor2-first = Richard |
|||
| editor3-last = Mazeron |
|||
| editor3-first = Jean-Jacques |
|||
| editor4-last = Meertens |
|||
| editor4-first = Harm |
|||
| editor5-last = Limbergen |
|||
| editor5-first = Erik Van |
|||
| year = 2002 |
|||
| title = The GEC ESTRO handbook of brachytherapy |
|||
| publisher = European Society for Therapeutic Radiology and Oncology |
|||
| location = Leuven, Belgium |
|||
| url=http://www.estro-education.org/publications/Documents/GEC%20ESTRO%20Handbook%20of%20Brachytherapy.html |
|||
| accessdate = |
|||
| isbn=978-90-804532-6-5 |
|||
}}</ref> |
|||
Brachytherapy can be used alone or in combination with other therapies such as surgery, [[external beam radiotherapy]] (EBRT) and [[chemotherapy]]. |
Brachytherapy can be used alone or in combination with other therapies such as surgery, [[external beam radiotherapy]] (EBRT) and [[chemotherapy]]. |
||
Brachytherapy contrasts with [[unsealed source radiotherapy]] in which a therapeutic [[radionuclide]] (radioisotope) is injected into the body to chemically localize to the tissue requiring destruction. It also contrasts to EBRT, in which high-energy x-rays (or occasionally gamma-rays from a radioisotope like [[cobalt-60]]) are directed at the tumour from outside the body. Brachytherapy instead involves the precise placement of short-range radiation-sources (radioisotopes) directly at the site of the cancerous tumour. These are enclosed in a protective capsule or wire, which allows the ionizing radiation to escape to treat and kill surrounding tissue but prevents the charge of radioisotope from moving or dissolving in body fluids. The capsule may be removed later, or (with some radioisotopes) it may be allowed to remain in place.<ref name="GEC-ESTRO" />{{rp|Ch. 1}}<ref name="Stewart 2007"> |
Brachytherapy contrasts with [[unsealed source radiotherapy]] in which a therapeutic [[radionuclide]] (radioisotope) is injected into the body to chemically localize to the tissue requiring destruction. It also contrasts to EBRT, in which high-energy x-rays (or occasionally gamma-rays from a radioisotope like [[cobalt-60]]) are directed at the tumour from outside the body. Brachytherapy instead involves the precise placement of short-range radiation-sources (radioisotopes) directly at the site of the cancerous tumour. These are enclosed in a protective capsule or wire, which allows the ionizing radiation to escape to treat and kill surrounding tissue but prevents the charge of radioisotope from moving or dissolving in body fluids. The capsule may be removed later, or (with some radioisotopes) it may be allowed to remain in place.<ref name="GEC-ESTRO" />{{rp|Ch. 1}}<ref name="Stewart 2007"> |
||
{{Cite book | last = Stewart AJ | authorlink = | editor = Devlin P | year = 2007 | title = Brachytherapy. Applications and Techniques | chapter = Radiobiological concepts for brachytherapy | publisher = LWW | location = Philadelphia | id =| author2 = |
|||
{{Cite book |
|||
| last = Stewart AJ |
|||
| authorlink = |
|||
| editor = Devlin P |
|||
| year = 2007 |
|||
| title = Brachytherapy. Applications and Techniques |
|||
| chapter = Radiobiological concepts for brachytherapy |
|||
| publisher = LWW |
|||
| location = Philadelphia |
|||
| id = |
|||
| author2 = |
|||
and others| displayauthors = |
and others| displayauthors = |
||
1}}</ref> |
1}}</ref> |
||
| Line 49: | Line 21: | ||
A course of brachytherapy can be completed in less time than other radiotherapy techniques. This can help reduce the chance for surviving cancer cells to divide and grow in the intervals between each radiotherapy dose.<ref name="Stewart 2007" /> Patients typically have to make fewer visits to the radiotherapy clinic compared with EBRT, and the treatment is often performed on an outpatient basis. This makes treatment accessible and convenient for many patients.<ref name="BMJGroup-2009" /><ref name="Kelley 2007"> |
A course of brachytherapy can be completed in less time than other radiotherapy techniques. This can help reduce the chance for surviving cancer cells to divide and grow in the intervals between each radiotherapy dose.<ref name="Stewart 2007" /> Patients typically have to make fewer visits to the radiotherapy clinic compared with EBRT, and the treatment is often performed on an outpatient basis. This makes treatment accessible and convenient for many patients.<ref name="BMJGroup-2009" /><ref name="Kelley 2007"> |
||
{{Cite book | last = Kelley JR | authorlink = | editor = Devlin P | year = 2007 | title = Brachytherapy. Applications and Techniques | chapter = Breast brachytherapy | publisher = LWW | location = Philadelphia | id =| author2 = |
|||
{{Cite book |
|||
| last = Kelley JR |
|||
| authorlink = |
|||
| editor = Devlin P |
|||
| year = 2007 |
|||
| title = Brachytherapy. Applications and Techniques |
|||
| chapter = Breast brachytherapy |
|||
| publisher = LWW |
|||
| location = Philadelphia |
|||
| id = |
|||
| author2 = |
|||
and others| displayauthors = |
and others| displayauthors = |
||
1}}</ref> These features of brachytherapy mean that most patients are able to tolerate the brachytherapy procedure very well. |
1}}</ref> These features of brachytherapy mean that most patients are able to tolerate the brachytherapy procedure very well. |
||
Brachytherapy represents an effective treatment option for many types of cancer. Treatment results have demonstrated that the cancer cure rates of brachytherapy are either comparable to surgery and EBRT or are improved when used in combination with these techniques.<ref name="Viswanathan 2007"> |
Brachytherapy represents an effective treatment option for many types of cancer. Treatment results have demonstrated that the cancer cure rates of brachytherapy are either comparable to surgery and EBRT or are improved when used in combination with these techniques.<ref name="Viswanathan 2007"> |
||
{{Cite book | last = Viswanathan AN | authorlink = | editor = Devlin P | year = 2007 | title = Brachytherapy. Applications and Techniques | chapter = Gynecologic brachytherapy | publisher = LWW | location = Philadelphia | id =| author2 = |
|||
{{Cite book |
|||
| last = Viswanathan AN |
|||
| authorlink = |
|||
| editor = Devlin P |
|||
| year = 2007 |
|||
| title = Brachytherapy. Applications and Techniques |
|||
| chapter = Gynecologic brachytherapy |
|||
| publisher = LWW |
|||
| location = Philadelphia |
|||
| id = |
|||
| author2 = |
|||
and others| displayauthors = |
and others| displayauthors = |
||
1}}</ref><ref name="Pickles-2009"> |
1}}</ref><ref name="Pickles-2009"> |
||
{{Cite journal| first1 = T.| last1 = Pickles | first2 = M.| first3 = W. J.| title = Brachytherapy or Conformal External Radiotherapy for Prostate Cancer: A Single-Institution Matched-Pair Analysis| journal = International Journal of Radiation OncologyBiologyPhysics| year = 2009| pmid = 19570619 | doi = 10.1016/j.ijrobp.2009.01.081| last2 = Keyes| last3 = Morris| volume = 76| issue = 1| pages = 43–49}}</ref><ref name="Haie-Meder-2009"> |
|||
{{Cite pmid|19570619}}</ref><ref name="Haie-Meder-2009"> |
|||
{{Cite journal| first1 = C.| last1 = Haie-meder | first2 = C.| first3 = A.| first4 = I.| first5 = P.| first6 = N.| title = DVH parameters and outcome for patients with early-stage cervical cancer treated with preoperative MRI-based low dose rate brachytherapy followed by surgery| journal = Radiotherapy and Oncology| volume = 93| pages = 316–321| year = 2009 | doi = 10.1016/j.radonc.2009.05.004| pmid = 19586673| last2 = Chargari| last3 = Rey| last4 = Dumas| last5 = Morice| last6 = Magné| issue = 2}}</ref><ref name="Batterman-2004"> |
|||
{{Cite pmid|19586673}}</ref><ref name="Batterman-2004"> |
|||
{{Cite journal| first1 = J.| title = Results of permanent prostate brachytherapy, 13 years of experience at a single institution| last1 = Battermann| journal = Radiotherapy and Oncology| volume = 71| pages = 23–28| year = 2004 | doi = 10.1016/j.radonc.2004.01.020| pmid = 15066292| last2 = Boon | first2 = T.| last3 = Moerland | first3 = M.| issue = 1 }}</ref><ref name="Galalae-2004"> |
|||
{{Cite pmid|15066292}}</ref><ref name="Galalae-2004"> |
|||
{{Cite journal| first1 = R.| last1 = Galalae | first2 = A.| first3 = T.| first4 = C.| first5 = G.| first6 = N.| first7 = S.| first8 = G.| first9 = M.| last10 = Kovács | first10 = G.| title = Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer| journal = International Journal of Radiation OncologyBiologyPhysics| volume = 58| issue = 4| pages = 1048–1055| year = 2004| pmid = 15001244 | doi = 10.1016/j.ijrobp.2003.08.003| last2 = Martinez| last3 = Mate| last4 = Mitchell| last5 = Edmundson| last6 = Nuernberg| last7 = Eulau| last8 = Gustafson| last9 = Gribble}}</ref><ref name="Hoskin-2007"> |
|||
{{Cite pmid|15001244}}</ref><ref name="Hoskin-2007"> |
|||
{{Cite journal| first1 = P. J.| last1 = Hoskin | first2 = K.| first3 = P.| first4 = L.| first5 = P.| title = High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomised phase three trial| journal = Radiotherapy and Oncology| volume = 84| pages = 114–120| year = 2007 | doi = 10.1016/j.radonc.2007.04.011| pmid = 17531335| last2 = Motohashi| last3 = Bownes| last4 = Bryant| last5 = Ostler| issue = 2}}</ref><ref name="Pieters -2009"> |
|||
{{Cite pmid|17531335}}</ref><ref name="Pieters -2009"> |
|||
{{Cite journal| first1 = B. R. | first2 = D. Z.| first3 = C. C. E.| last1 = Pieters| first4 = A. H.| title = Comparison of three radiotherapy modalities on biochemical control and overall survival for the treatment of prostate cancer: A systematic review| journal = Radiotherapy and Oncology| volume = 93| pages = 168–173| year = 2009 | doi = 10.1016/j.radonc.2009.08.033| pmid = 19748692| last2 = De Back| last3 = Koning| last4 = Zwinderman| issue = 2}}</ref><ref name="Nelson-2009"> |
|||
{{Cite pmid|19748692}}</ref><ref name="Nelson-2009"> |
|||
{{Cite journal| first1 = J. C.| last1 = Nelson | first2 = P. D.| first3 = F. A.| first4 = C. A.| first5 = D.| first6 = H. C.| first7 = M. A.| first8 = V. J.| first9 = P. W.| last10 = Fine | first10 = R. E.| last11 = Keleher | first11 = A. J.| last12 = Kuerer | first12 = H. M.| title = Four-year clinical update from the American Society of Breast Surgeons MammoSite brachytherapy trial| journal = The American Journal of Surgery| volume = 198| issue = 1| pages = 83–91| year = 2009| pmid = 19268900 | doi = 10.1016/j.amjsurg.2008.09.016| last2 = Beitsch| last3 = Vicini| last4 = Quiet| last5 = Garcia| last6 = Snider| last7 = Gittleman| last8 = Zannis| last9 = Whitworth}}</ref> In addition, brachytherapy is associated with a low risk of serious adverse side effects.<ref name="Ferrer-2008"> |
|||
{{Cite pmid|19268900}}</ref> In addition, brachytherapy is associated with a low risk of serious adverse side effects.<ref name="Ferrer-2008"> |
|||
{{Cite journal| first1 = M.| last1 = Ferrer | first2 = J.| first3 = F.| first4 = P.| first5 = V.| first6 = A.| first7 = A.| first8 = I.| first9 = M.| last10 = Villavicencio | first10 = H.| last11 = Craven-Bratle | first11 = J.| last12 = Garin | first12 = O.| last13 = Aguiló | first13 = F.| author14 = Multicentric Spanish Group of Clinically Localized Prostate Cancer| title = Health-Related Quality of Life 2 Years After Treatment with Radical Prostatectomy, Prostate Brachytherapy, or External Beam Radiotherapy in Patients with Clinically Localized Prostate Cancer| journal = International Journal of Radiation OncologyBiologyPhysics| volume = 72| issue = 2| pages = 421–432| year = 2008| pmid = 18325680 | doi = 10.1016/j.ijrobp.2007.12.024| last2 = Suárez| last3 = Guedea| last4 = Fernández| last5 = MacÍas| last6 = Mariño| last7 = Hervas| last8 = Herruzo| last9 = Ortiz}}</ref><ref name="Frank-2007"> |
|||
{{Cite pmid|18325680}}</ref><ref name="Frank-2007"> |
|||
{{Cite journal| first1 = S.| last1 = Frank | first2 = L.| first3 = J.| first4 = A.| first5 = R.| first6 = D.| title = An Assessment of Quality of Life Following Radical Prostatectomy, High Dose External Beam Radiation Therapy and Brachytherapy Iodine Implantation as Monotherapies for Localized Prostate Cancer| journal = The Journal of Urology| volume = 177| pages = 2151–2156| year = 2007 | doi = 10.1016/j.juro.2007.01.134| pmid = 17509305| last2 = Pisters| last3 = Davis| last4 = Lee| last5 = Bassett| last6 = Kuban| issue = 6}}</ref> |
|||
{{Cite pmid|17509305}}</ref> |
|||
The global market for brachytherapy reached US$680 million in 2013, of which the High-Dose Rate (HDR) and LDR segments accounted for 70%. Microspheres and electronic brachytherapy commanded the remaining 30%. The brachytherapy market is expected to reach over US$2.4 billion in 2030, growing by 8% annually, mainly driven by the microspheres market as well as electronic brachytherapy, which is gaining significant interest worldwide as a user-friendly technology.<ref>http://www.prlog.org/12390829-brachytherapy-market-recovery-to-reach-us-2-4-billion.html</ref> |
The global market for brachytherapy reached US$680 million in 2013, of which the High-Dose Rate (HDR) and LDR segments accounted for 70%. Microspheres and electronic brachytherapy commanded the remaining 30%. The brachytherapy market is expected to reach over US$2.4 billion in 2030, growing by 8% annually, mainly driven by the microspheres market as well as electronic brachytherapy, which is gaining significant interest worldwide as a user-friendly technology.<ref>http://www.prlog.org/12390829-brachytherapy-market-recovery-to-reach-us-2-4-billion.html</ref> |
||
| Line 92: | Line 44: | ||
Brachytherapy dates back to 1901 (shortly after the discovery of radioactivity by [[Henri Becquerel]] in 1896) when [[Pierre Curie]] suggested to [[Henri-Alexandre Danlos]] that a radioactive source could be inserted into a tumour.<ref name="Gupta-1995"> |
Brachytherapy dates back to 1901 (shortly after the discovery of radioactivity by [[Henri Becquerel]] in 1896) when [[Pierre Curie]] suggested to [[Henri-Alexandre Danlos]] that a radioactive source could be inserted into a tumour.<ref name="Gupta-1995"> |
||
{{Cite journal | author = Gupta VK. | year = 1995 | title = Brachytherapy – past, present and future | journal = Journal of Medical Physics | volume = 20 | issue = | pages = 31–38 | pmid = | url = }}</ref><ref name="Nag 2009"> |
|||
{{Cite journal |
|||
{{cite web | url = http://www.americanbrachytherapy.org/aboutBrachytherapy/history.cfm | title = A brief history of brachytherapy | accessdate = 25 September 2009 | author = Nag S | authorlink = | work = |publisher = | pages = |language = |quote = | archiveurl = |archivedate =}}</ref> |
|||
| author = Gupta VK. |
|||
| year = 1995 |
|||
| title = Brachytherapy – past, present and future |
|||
| journal = Journal of Medical Physics |
|||
| volume = 20 |
|||
| issue = |
|||
| pages = 31–38 |
|||
| pmid = |
|||
| url = |
|||
}}</ref><ref name="Nag 2009"> |
|||
{{cite web |
|||
| url = http://www.americanbrachytherapy.org/aboutBrachytherapy/history.cfm |
|||
| title = A brief history of brachytherapy |
|||
| accessdate = 25 September 2009 |
|||
| author = Nag S |
|||
| authorlink = | work = |publisher = |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
}}</ref> |
|||
It was found that the radiation caused the tumour to shrink.<ref name="Nag 2009" /> Independently, [[Alexander Graham Bell]] also suggested the use of radiation in this way.<ref name="Nag 2009"/> In the early twentieth century, techniques for the application of brachytherapy were pioneered at the Curie institute in Paris by Danlos and at St Luke's and Memorial Hospital in New York by [[Robert Abbe]].<ref name="GEC-ESTRO" />{{rp|Ch. 1}}<ref name="Nag 2009" /> |
It was found that the radiation caused the tumour to shrink.<ref name="Nag 2009" /> Independently, [[Alexander Graham Bell]] also suggested the use of radiation in this way.<ref name="Nag 2009"/> In the early twentieth century, techniques for the application of brachytherapy were pioneered at the Curie institute in Paris by Danlos and at St Luke's and Memorial Hospital in New York by [[Robert Abbe]].<ref name="GEC-ESTRO" />{{rp|Ch. 1}}<ref name="Nag 2009" /> |
||
Interstitial radium therapy was common in the 1930s.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} Gold seeds filled with [[radon]] were used as early as 1942<ref>{{ |
Interstitial radium therapy was common in the 1930s.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} Gold seeds filled with [[radon]] were used as early as 1942<ref>{{Cite journal | doi = 10.1001/archderm.1975.01630180085013 | last1 = Goldstein | first1 = N. | title = Radon seed implants. Residual radioactivity after 33 years | journal = Archives of dermatology | volume = 111 | issue = 6 | pages = 757–759 | year = 1975 | pmid = 1137421}}</ref> until at least 1958.<ref>{{cite journal|last=Winston|first=P.|title=Carcinoma of the Trachea Treated by Radon Seed Implantation|journal=The Journal of Laryngology & Otology|date=June 1958|volume=72|issue=6|pages=496–499|doi=10.1017/S0022215100054232}}</ref> [[Gold]] shells were selected by Gino Failla around 1920 to shield [[beta ray]]s while passing [[gamma ray]]s.<ref>{{cite web|last=Oak Ridge Associated Universitie|title=Seeds (ca. 1940s - 1960s)|url=http://www.orau.org/ptp/collection/brachytherapy/seeds.htm|work=Health Physics Historical Instrumentation Collection|accessdate=12 November 2012}}</ref> [[Cobalt]] needles were also used briefly after world war II.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} Radon and cobalt were replaced by radioactive [[tantalum]] and gold, before [[iridium]] rose in prominence.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} First used in 1958, iridium is the most commonly used artificial source for brachytherapy today.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} |
||
Following initial interest in brachytherapy in Europe and the US, its use declined in the middle of the twentieth century due to the problem of radiation exposure to operators from the manual application of the radioactive sources.<ref name="Nag 2009" /><ref name="Aronowitz-2008"> |
Following initial interest in brachytherapy in Europe and the US, its use declined in the middle of the twentieth century due to the problem of radiation exposure to operators from the manual application of the radioactive sources.<ref name="Nag 2009" /><ref name="Aronowitz-2008"> |
||
{{Cite |
{{Cite journal| first1 = J.| title = The "Golden Age" of prostate brachytherapy: A cautionary tale| last1 = Aronowitz| journal = Brachytherapy| volume = 7| pages = 55–59| year = 2008 | doi = 10.1016/j.brachy.2007.12.004| pmid = 18299114| issue = 1}}</ref> However, the development of [[brachytherapy#Treatment delivery|remote afterloading systems]], which allow the radiation to be delivered from a shielded safe, and the use of new radioactive sources in the 1950s and 1960s, reduced the risk of unnecessary radiation exposure to the operator and patients.<ref name="Gupta-1995" /> This, together with more recent advancements in three-dimensional imaging modalities, computerised treatment planning systems and delivery equipment has made brachytherapy a safe and effective treatment for many types of cancer today.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} |
||
==Types== |
==Types== |
||
| Line 128: | Line 62: | ||
* Contact brachytherapy involves placement of the radiation source in a space next to the target tissue.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} This space may be a body cavity ('''intracavitary''' brachytherapy) such as the [[cervix]], [[uterus]] or [[vagina]]; a body lumen ('''intraluminal''' brachytherapy) such as the [[Vertebrate trachea|trachea]] or [[oesophagus]]; or externally ('''surface''' brachytherapy) such as the [[skin]].<ref name="GEC-ESTRO" />{{rp|Ch. 1}} A radiation source can also be placed in blood vessels ('''intravascular''' brachytherapy) for the treatment of [[Coronary stent#Restenosis|coronary in-stent restenosis]].<ref name="Giap 2007"> |
* Contact brachytherapy involves placement of the radiation source in a space next to the target tissue.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} This space may be a body cavity ('''intracavitary''' brachytherapy) such as the [[cervix]], [[uterus]] or [[vagina]]; a body lumen ('''intraluminal''' brachytherapy) such as the [[Vertebrate trachea|trachea]] or [[oesophagus]]; or externally ('''surface''' brachytherapy) such as the [[skin]].<ref name="GEC-ESTRO" />{{rp|Ch. 1}} A radiation source can also be placed in blood vessels ('''intravascular''' brachytherapy) for the treatment of [[Coronary stent#Restenosis|coronary in-stent restenosis]].<ref name="Giap 2007"> |
||
{{Cite book | last = Giap H and Tripuraneni P. | authorlink = | editor = Devlin P | year = 2007 | title = Brachytherapy. Applications and Techniques | chapter = Vascular brachytherapy | publisher = LWW | location = Philadelphia | id =}}</ref> |
|||
{{Cite book |
|||
| last = Giap H and Tripuraneni P. |
|||
| authorlink = |
|||
| editor = Devlin P |
|||
| year = 2007 |
|||
| title = Brachytherapy. Applications and Techniques |
|||
| chapter = Vascular brachytherapy |
|||
| publisher = LWW |
|||
| location = Philadelphia |
|||
| id = |
|||
}}</ref> |
|||
===Dose rate=== |
===Dose rate=== |
||
| Line 145: | Line 69: | ||
* '''Low-dose rate(LDR)''' brachytherapy involves implanting radiation sources that emit radiation at a rate of up to 2 Gy·h<sup>−1</sup>.<ref name="Thomadsen 2005"> |
* '''Low-dose rate(LDR)''' brachytherapy involves implanting radiation sources that emit radiation at a rate of up to 2 Gy·h<sup>−1</sup>.<ref name="Thomadsen 2005"> |
||
{{Cite book | last = Thomadsen BR | authorlink = | year = 2005 | title = Brachytherapy Physics | chapter = | publisher = Medical Physics Publishing | location = | id =| author2 = |
|||
{{Cite book |
|||
| last = Thomadsen BR |
|||
| authorlink = |
|||
| year = 2005 |
|||
| title = Brachytherapy Physics |
|||
| chapter = |
|||
| publisher = Medical Physics Publishing |
|||
| location = |
|||
| id = |
|||
| author2 = |
|||
and others| displayauthors = |
and others| displayauthors = |
||
1}}</ref> LDR brachytherapy is commonly used for cancers of the oral cavity,<ref name="Mazaron-2009"> |
1}}</ref> LDR brachytherapy is commonly used for cancers of the oral cavity,<ref name="Mazaron-2009"> |
||
{{Cite |
{{Cite journal | last1 = Mazeron | first1 = J. J. | last2 = Ardiet | first2 = J. M. | last3 = Haie-Méder | first3 = C. | last4 = Kovács | first4 = G. R. | last5 = Levendag | first5 = P. | last6 = Peiffert | first6 = D. | last7 = Polo | first7 = A. | last8 = Rovirosa | first8 = A. | last9 = Strnad | first9 = V. | doi = 10.1016/j.radonc.2009.01.005 | title = GEC-ESTRO recommendations for brachytherapy for head and neck squamous cell carcinomas | journal = Radiotherapy and Oncology | volume = 91 | issue = 2 | pages = 150–156 | year = 2009 | pmid = 19329209 | pmc = }}</ref> [[oropharynx]],<ref name="Mazaron-2009" /> [[sarcomas]]<ref name="GEC-ESTRO" />{{rp|Ch. 27}} and [[prostate cancer]]<ref name="GEC-ESTRO" />{{rp|Ch. 20}}<ref name="Koukourakis-2009" > |
||
{{Cite journal | author = Koukourakis G | year = 2009 | title = Brachytherapy for prostate cancer: A systematic review | journal = Adv Urol | volume = 26 | issue = 1 | pages =63–8 | pmid = 2735748 | url = | author2 = and others | displayauthors = 1 }}</ref> |
|||
{{Cite journal |
|||
| author = Koukourakis G |
|||
| year = 2009 |
|||
| title = Brachytherapy for prostate cancer: A systematic review |
|||
| journal = Adv Urol |
|||
| volume = 26 |
|||
| issue = 1 |
|||
| pages =63–8 |
|||
| pmid = 2735748 |
|||
| url = |
|||
| author2 = and others |
|||
| displayauthors = 1 |
|||
}}</ref> |
|||
* '''Medium-dose rate (MDR)''' brachytherapy is characterized by a medium rate of dose delivery, ranging between 2 Gy·h<sup>−1</sup> to 12 Gy·h<sup>−1</sup>.<ref name="Thomadsen 2005" /> |
* '''Medium-dose rate (MDR)''' brachytherapy is characterized by a medium rate of dose delivery, ranging between 2 Gy·h<sup>−1</sup> to 12 Gy·h<sup>−1</sup>.<ref name="Thomadsen 2005" /> |
||
* '''High-dose rate (HDR)''' brachytherapy is when the rate of dose delivery exceeds 12 Gy·h<sup>−1</sup>.<ref name="Thomadsen 2005" /> The most common applications of HDR brachytherapy are in tumours of the [[cervix]], [[esophagus]], [[lungs]], [[breasts]] and [[prostate]].<ref name="GEC-ESTRO" /> Most HDR treatments are performed on an outpatient basis, but this is dependent on the treatment site.<ref name="Nag-2004"> |
* '''High-dose rate (HDR)''' brachytherapy is when the rate of dose delivery exceeds 12 Gy·h<sup>−1</sup>.<ref name="Thomadsen 2005" /> The most common applications of HDR brachytherapy are in tumours of the [[cervix]], [[esophagus]], [[lungs]], [[breasts]] and [[prostate]].<ref name="GEC-ESTRO" /> Most HDR treatments are performed on an outpatient basis, but this is dependent on the treatment site.<ref name="Nag-2004"> |
||
{{Cite journal | author = Nag S. | year = 2004 | title = High dose rate brachytherapy: its clinical applications and treatment guidelines | journal = Technology in Cancer Research and Treatment | volume = 3 | issue = 3 | pages = 269–87 | pmid = 15161320 | url = }}</ref> |
|||
{{Cite journal |
|||
| author = Nag S. |
|||
| year = 2004 |
|||
| title = High dose rate brachytherapy: its clinical applications and treatment guidelines |
|||
| journal = Technology in Cancer Research and Treatment |
|||
| volume = 3 |
|||
| issue = 3 |
|||
| pages = 269–87 |
|||
| pmid = 15161320 |
|||
| url = |
|||
}}</ref> |
|||
* '''Pulsed-dose rate (PDR)''' brachytherapy involves short pulses of radiation, typically once an hour, to simulate the overall rate and effectiveness of LDR treatment. Typical tumour sites treated by PDR brachytherapy are gynaecological<ref name="GEC-ESTRO" />{{rp|Ch. 14}} and head and neck cancers.<ref name="Mazaron-2009" /> |
* '''Pulsed-dose rate (PDR)''' brachytherapy involves short pulses of radiation, typically once an hour, to simulate the overall rate and effectiveness of LDR treatment. Typical tumour sites treated by PDR brachytherapy are gynaecological<ref name="GEC-ESTRO" />{{rp|Ch. 14}} and head and neck cancers.<ref name="Mazaron-2009" /> |
||
| Line 193: | Line 86: | ||
The placement of radiation sources in the target area can be temporary or permanent. |
The placement of radiation sources in the target area can be temporary or permanent. |
||
* Temporary brachytherapy involves placement of radiation sources for a set duration (usually a number of minutes or hours) before being withdrawn.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} The specific treatment duration will depend on many different factors, including the required rate of dose delivery and the type, size and location of the cancer. In LDR and PDR brachytherapy, the source typically stays in place up to 24 hours before being removed, while in HDR brachytherapy this time is typically a few minutes.<ref name="Flynn 2005">{{Cite book |
* Temporary brachytherapy involves placement of radiation sources for a set duration (usually a number of minutes or hours) before being withdrawn.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} The specific treatment duration will depend on many different factors, including the required rate of dose delivery and the type, size and location of the cancer. In LDR and PDR brachytherapy, the source typically stays in place up to 24 hours before being removed, while in HDR brachytherapy this time is typically a few minutes.<ref name="Flynn 2005">{{Cite book | last = Flynn A | authorlink = | editor = Hoskin P, Coyle C | year = 2005 | title = Radiotherapy in practice: brachytherapy | chapter = Isotopes and delivery systems for brachytherapy | publisher = Oxford University Press | location = New York | id =| author2 = |
||
| last = Flynn A |
|||
| authorlink = |
|||
| editor = Hoskin P, Coyle C |
|||
| year = 2005 |
|||
| title = Radiotherapy in practice: brachytherapy |
|||
| chapter = Isotopes and delivery systems for brachytherapy |
|||
| publisher = Oxford University Press |
|||
| location = New York |
|||
| id = |
|||
| author2 = |
|||
and others| displayauthors = |
and others| displayauthors = |
||
1}}</ref> |
1}}</ref> |
||
* Permanent brachytherapy, also known as seed implantation, involves placing small LDR radioactive seeds or pellets (about the size of a grain of rice) in the tumour or treatment site and leaving them there permanently to gradually decay. Over a period of weeks or months, the level of radiation emitted by the sources will decline to almost zero. The inactive seeds then remain in the treatment site with no lasting effect.<ref name="Moule-2009"> |
* Permanent brachytherapy, also known as seed implantation, involves placing small LDR radioactive seeds or pellets (about the size of a grain of rice) in the tumour or treatment site and leaving them there permanently to gradually decay. Over a period of weeks or months, the level of radiation emitted by the sources will decline to almost zero. The inactive seeds then remain in the treatment site with no lasting effect.<ref name="Moule-2009"> |
||
{{Cite |
{{Cite journal| first1 = R. N.| last1 = Moule | first2 = P. J.| title = Non-surgical treatment of localised prostate cancer| journal = Surgical Oncology| volume = 18| pages = 255–267| year = 2009 | doi = 10.1016/j.suronc.2009.03.006| pmid = 19442516| last2 = Hoskin| issue = 3}}</ref> Permanent brachytherapy is most commonly used in the treatment of [[prostate cancer]].<ref name="Koukourakis-2009" /> |
||
{{-}} |
{{-}} |
||
| Line 215: | Line 98: | ||
Brachytherapy is commonly used to treat cancers of the [[cervix]], [[prostate]], [[breast]], and [[skin]].<ref name="GEC-ESTRO" /> |
Brachytherapy is commonly used to treat cancers of the [[cervix]], [[prostate]], [[breast]], and [[skin]].<ref name="GEC-ESTRO" /> |
||
Brachytherapy can also be used in the treatment of tumours of the [[Human brain|brain]], [[Human eye|eye]], head and neck region (lip, [[floor of mouth]], tongue, [[nasopharynx]] and [[oropharynx]]),<ref name="Mazaron-2009" /> respiratory tract ([[Vertebrate trachea|trachea]] and [[bronchi]]), digestive tract ([[oesophagus]], [[gall bladder]], [[bile duct|bile-ducts]], [[rectum]], [[anus]]),<ref name="Dvorák-2002">{{Cite |
Brachytherapy can also be used in the treatment of tumours of the [[Human brain|brain]], [[Human eye|eye]], head and neck region (lip, [[floor of mouth]], tongue, [[nasopharynx]] and [[oropharynx]]),<ref name="Mazaron-2009" /> respiratory tract ([[Vertebrate trachea|trachea]] and [[bronchi]]), digestive tract ([[oesophagus]], [[gall bladder]], [[bile duct|bile-ducts]], [[rectum]], [[anus]]),<ref name="Dvorák-2002">{{Cite journal| pmid = 12143240| year = 2002| author1 = Dvorák | first2 = P. | first3 = B. | first4 = B. | first5 = J. | first6 = Z. | first7 = Z. | first8 = J.| title = Intraluminal high dose rate brachytherapy in the treatment of bile duct and gallbladder carcinomas| volume = 49| issue = 46| pages = 916–917| journal = Hepato-gastroenterology| last2 = Jandík| last3 = Melichar| last4 = Jon| last5 = Mergancová| last6 = Zoul| last7 = Vacek| last8 = Petera}}</ref> urinary tract ([[bladder]], [[urethra]], [[penis]]), female reproductive tract ([[uterus]], [[vagina]], [[vulva]]), and soft tissues.<ref name="GEC-ESTRO" /> |
||
As the radiation sources can be precisely positioned at the tumour treatment site, brachytherapy enables a high dose of radiation to be applied to a small area. Furthermore, because the radiation sources are placed in or next to the target tumour, the sources maintain their position in relation to the tumour when the patient moves or if there is any movement of the tumour within the body. Therefore, the radiation sources remain accurately targeted. This enables clinicians to achieve a high level of dose conformity – i.e. ensuring the whole of the tumour receives an optimal level of radiation. It also reduces the risk of damage to healthy tissue, organs or structures around the tumour,<ref name="Nag-2004" /> thus enhancing the chance of cure and preservation of organ function. |
As the radiation sources can be precisely positioned at the tumour treatment site, brachytherapy enables a high dose of radiation to be applied to a small area. Furthermore, because the radiation sources are placed in or next to the target tumour, the sources maintain their position in relation to the tumour when the patient moves or if there is any movement of the tumour within the body. Therefore, the radiation sources remain accurately targeted. This enables clinicians to achieve a high level of dose conformity – i.e. ensuring the whole of the tumour receives an optimal level of radiation. It also reduces the risk of damage to healthy tissue, organs or structures around the tumour,<ref name="Nag-2004" /> thus enhancing the chance of cure and preservation of organ function. |
||
The use of HDR brachytherapy enables overall treatment times to be reduced compared with EBRT.<ref name="Joseph-2008"> |
The use of HDR brachytherapy enables overall treatment times to be reduced compared with EBRT.<ref name="Joseph-2008"> |
||
{{Cite journal| first1 = K. J.| last1 = Joseph | first2 = R.| first3 = D.| first4 = J.| first5 = N.| first6 = C.| last2 = Alvi| last6 = Small| last3 = Skarsgard| last5 = Pervez| last4 = Tonita| last7 = Tai | first7 = P.| title = Analysis of health related quality of life (HRQoL) of patients with clinically localized prostate cancer, one year after treatment with external beam radiotherapy (EBRT) alone versus EBRT and high dose rate brachytherapy (HDRBT)| journal = Radiation Oncology| volume = 3| pages = 20| year = 2008 | doi = 10.1186/1748-717X-3-20| pmid = 18627617| pmc = 2494997}}</ref><ref name="Holmboe-2000"> |
|||
{{Cite pmid|18627617}}</ref><ref name="Holmboe-2000"> |
|||
{{Cite journal | pmid = 11089712 | year = 2000 | author1 = Holmboe | first2 = J. | title = Treatment decisions for localized prostate cancer: asking men what's important | volume = 15 | issue = 10 | pages = 694–701 | pmc = 1495597 | journal = Journal of general internal medicine | doi = 10.1046/j.1525-1497.2000.90842.x | last2 = Concato}}</ref> |
|||
{{Cite pmid|11089712}}</ref> |
|||
Patients receiving brachytherapy generally have to make fewer visits for radiotherapy compared with EBRT, and overall radiotherapy treatment plans can be completed in less time.<ref name="Hoskin 2005 book"> |
Patients receiving brachytherapy generally have to make fewer visits for radiotherapy compared with EBRT, and overall radiotherapy treatment plans can be completed in less time.<ref name="Hoskin 2005 book"> |
||
{{Cite book | authorlink = | editor = Hoskin P, Coyle C | year = 2005 | title = Radiotherapy in practice: brachytherapy | chapter = | publisher = Oxford University Press | location = New York | id = | isbn =0-19-852940-6}}</ref> |
|||
{{Cite book |
|||
| authorlink = |
|||
| editor = Hoskin P, Coyle C |
|||
| year = 2005 |
|||
| title = Radiotherapy in practice: brachytherapy |
|||
| chapter = |
|||
| publisher = Oxford University Press |
|||
| location = New York |
|||
| id = |
|||
| isbn =0-19-852940-6 |
|||
}}</ref> |
|||
Many brachytherapy procedures are performed on an outpatient basis. This convenience may be particularly relevant for patients who have to work, older patients, or patients who live some distance from treatment centres, to ensure that they have access to radiotherapy treatment and adhere to treatment plans. Shorter treatment times and outpatient procedures can also help improve the efficiency of radiotherapy clinics.<ref name="Guedea-2008"> |
Many brachytherapy procedures are performed on an outpatient basis. This convenience may be particularly relevant for patients who have to work, older patients, or patients who live some distance from treatment centres, to ensure that they have access to radiotherapy treatment and adhere to treatment plans. Shorter treatment times and outpatient procedures can also help improve the efficiency of radiotherapy clinics.<ref name="Guedea-2008"> |
||
{{Cite journal| first1 = F.| last1 = Guedea | first2 = M.| first3 = J.| first4 = J.| first5 = P.| first6 = J.| title = Patterns of Care for Brachytherapy in Europe: Facilities and resources in brachytherapy in the European area| journal = Brachytherapy| volume = 7| pages = 223–230| year = 2008 | doi = 10.1016/j.brachy.2008.03.001| pmid = 18579448| last2 = Ventura| last3 = Mazeron| last4 = Torrecilla| last5 = Bilbao| last6 = Borràs| issue = 3}}</ref><ref name="Quang-2007"> |
|||
{{Cite pmid|18579448}}</ref><ref name="Quang-2007"> |
|||
{{Cite journal | author = Quang TS | year = 2007 | title = Technological evolution in the treatment of prostate cancer | journal = Oncology | volume = 21 | issue = | pages = | pmid = | url = http://www.cancernetwork.com/display/article/10165/62318 | author2 = and others | displayauthors = 1 }}</ref> |
|||
{{Cite journal |
|||
| author = Quang TS |
|||
| year = 2007 |
|||
| title = Technological evolution in the treatment of prostate cancer |
|||
| journal = Oncology |
|||
| volume = 21 |
|||
| issue = |
|||
| pages = |
|||
| pmid = |
|||
| url = http://www.cancernetwork.com/display/article/10165/62318 |
|||
| author2 = and others |
|||
| displayauthors = 1 |
|||
}}</ref> |
|||
Brachytherapy can be used with the aim of curing the cancer in cases of small or locally advanced tumours, provided the cancer has not metastasized (spread to other parts of the body). In appropriately selected cases, brachytherapy for primary tumours often represents a comparable approach to surgery, achieving the same probability of cure and with similar side effects.<ref name="Guedea-2009"> |
Brachytherapy can be used with the aim of curing the cancer in cases of small or locally advanced tumours, provided the cancer has not metastasized (spread to other parts of the body). In appropriately selected cases, brachytherapy for primary tumours often represents a comparable approach to surgery, achieving the same probability of cure and with similar side effects.<ref name="Guedea-2009"> |
||
{{Cite journal | doi = 10.1007/s12094-009-0387-x | last1 = Guedea | first1 = F. | last2 = Ferrer | first2 = M. | last3 = Pera | first3 = J. | last4 = Aguiló | first4 = F. | last5 = Boladeras | first5 = A. | last6 = Suárez | first6 = J. F. | last7 = Cunillera | first7 = O. | last8 = Ferrer | first8 = F. | last9 = Pardo | first9 = Y. | last10 = Martínez | first10 = E. | last11 = Ventura | first11 = M. | title = Quality of life two years after radical prostatectomy, prostate brachytherapy or external beam radiotherapy for clinically localised prostate cancer: The Catalan Institute of Oncology/Bellvitge Hospital experience | journal = Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico | volume = 11 | issue = 7 | pages = 470–478 | year = 2009 | pmid = 19574206}}</ref><ref name="Litwin-2007"> |
|||
{{Cite pmid|19574206}}</ref><ref name="Litwin-2007"> |
|||
{{Cite journal| first1 = M. S.| last1 = Litwin | first2 = J. L.| first3 = L.| first4 = J. M.| first5 = S. P.| first6 = H. R.| first7 = R. E.| title = Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer| journal = Cancer| volume = 109| pages = 2239–2247| year = 2007 | doi = 10.1002/cncr.22676| pmid = 17455209| last2 = Gore| last3 = Kwan| last4 = Brandeis| last5 = Lee| last6 = Withers| last7 = Reiter| issue = 11}}</ref> |
|||
{{Cite pmid|17455209}}</ref> |
|||
However, in locally advanced tumours, surgery may not routinely provide the best chance of cure and is often not technically feasible to perform. In these cases radiotherapy, including brachytherapy, offers the only chance of cure.<ref name="Pistis -2009"> |
However, in locally advanced tumours, surgery may not routinely provide the best chance of cure and is often not technically feasible to perform. In these cases radiotherapy, including brachytherapy, offers the only chance of cure.<ref name="Pistis -2009"> |
||
{{Cite journal| first1 = F.| first2 = F.| first3 = J.| last1 = Pistis| first4 = C.| first5 = M.| first6 = A.| first7 = E.| first8 = A.| first9 = F.| last10 = Gabriele | first10 = P.| last11 = Linares | first11 = L.| title = External beam radiotherapy plus high-dose-rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology| journal = Brachytherapy| year = 2009 | doi = 10.1016/j.brachy.2009.05.001| last2 = Guedea| last3 = Pera| last4 = Gutierrez| last5 = Ventura| last6 = Polo| last7 = Martinez| last8 = Boladeras| last9 = Ferrer| volume = 9| pages = 15–22| pmid = 19734106| issue = 1}}</ref><ref name="Lertsanguansinchai-2004"> |
|||
{{Cite pmid|19734106}}</ref><ref name="Lertsanguansinchai-2004"> |
|||
{{Cite journal| first1 = P.| last1 = Lertsanguansinchai | first2 = C.| first3 = K.| first4 = C.| first5 = P.| first6 = T.| first7 = A.| first8 = S.| first9 = C.| last10 = Tresukosol | first10 = D.| last11 = Charoonsantikul | first11 = C.| title = Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma| journal = International Journal of Radiation OncologyBiologyPhysics| volume = 59| issue = 5| pages = 1424–1431| year = 2004| pmid = 15275728 | doi = 10.1016/j.ijrobp.2004.01.034| last2 = Lertbutsayanukul| last3 = Shotelersuk| last4 = Khorprasert| last5 = Rojpornpradit| last6 = Chottetanaprasith| last7 = Srisuthep| last8 = Suriyapee| last9 = Jumpangern}}</ref> |
|||
{{Cite pmid|15275728}}</ref> |
|||
In more advanced disease stages, brachytherapy can be used as palliative treatment for symptom relief from pain and bleeding. |
In more advanced disease stages, brachytherapy can be used as palliative treatment for symptom relief from pain and bleeding. |
||
In cases where the tumour is not easily accessible or is too large to ensure an optimal distribution of irradiation to the treatment area, brachytherapy can be combined with other treatments, such as EBRT and/or surgery.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} Combination therapy of brachytherapy exclusively with chemotherapy is rare.<ref name="Roddiger-2006"> |
In cases where the tumour is not easily accessible or is too large to ensure an optimal distribution of irradiation to the treatment area, brachytherapy can be combined with other treatments, such as EBRT and/or surgery.<ref name="GEC-ESTRO" />{{rp|Ch. 1}} Combination therapy of brachytherapy exclusively with chemotherapy is rare.<ref name="Roddiger-2006"> |
||
{{Cite journal | author = Roddiger SJ | year = 2006 | title = Neoadjuvant interstitial high-dose-rate (HDR) brachytherapy combined with systemic chemotherapy in patients with breast cancer | journal = Strahlenther Onkol | volume = 182 | issue = 1 | pages = 22–9. | pmid =16404517 | url = | doi = 10.1007/s00066-006-1454-7 | author2 = and others | displayauthors = 1 }}</ref> |
|||
{{Cite journal |
|||
| author = Roddiger SJ |
|||
| year = 2006 |
|||
| title = Neoadjuvant interstitial high-dose-rate (HDR) brachytherapy combined with systemic chemotherapy in patients with breast cancer |
|||
| journal = Strahlenther Onkol |
|||
| volume = 182 |
|||
| issue = 1 |
|||
| pages = 22–9. |
|||
| pmid =16404517 |
|||
| url = |
|||
| doi = 10.1007/s00066-006-1454-7 |
|||
| author2 = and others |
|||
| displayauthors = 1 |
|||
}}</ref> |
|||
===Cervical cancer=== |
===Cervical cancer=== |
||
Brachytherapy is commonly used in the treatment of early or locally confined [[cervical cancer]] and is a standard of care in many countries.<ref name="GEC-ESTRO" />{{rp|Ch. 14}}<ref name="Gaffney-2007"> |
Brachytherapy is commonly used in the treatment of early or locally confined [[cervical cancer]] and is a standard of care in many countries.<ref name="GEC-ESTRO" />{{rp|Ch. 14}}<ref name="Gaffney-2007"> |
||
{{Cite journal| first1 = D.| last1 = Gaffney | first2 = A.| first3 = K.| first4 = N.| first5 = T.| first6 = S.| first7 = P.| first8 = L.| first9 = A.| last10 = Pötter | first10 = R.| last11 = Colombo | first11 = A.| last12 = Randall | first12 = M.| last13 = Mirza | first13 = M. R.| last14 = Trimble | first14 = E. L.| title = Practice Patterns of Radiotherapy in Cervical Cancer Among Member Groups of the Gynecologic Cancer Intergroup (GCIG)| journal = International Journal of Radiation OncologyBiologyPhysics| volume = 68| issue = 2| pages = 485–490| year = 2007| pmid = 17336465 | doi = 10.1016/j.ijrobp.2006.12.013| last2 = Du Bois| last3 = Narayan| last4 = Reed| last5 = Toita| last6 = Pignata| last7 = Blake| last8 = Portelance| last9 = Sadoyze}}</ref><ref name="NICE-IPG160"> |
|||
{{Cite pmid|17336465}}</ref><ref name="NICE-IPG160"> |
|||
{{cite web | url = http://www.nice.org.uk/IPG160 | title = High dose rate brachytherapy for carcinoma of the cervix | accessdate = 25 September 2009 | author = National Institute for Health and Clinical Excellence | authorlink = |date= March 2006 | work = |publisher = NICE | pages = |language = |quote = | archiveurl = |archivedate =}}</ref><ref name="Viswanathan-ABS"> |
|||
{{cite web |
|||
{{cite web | url = http://www.americanbrachytherapy.org/guidelines/cervical_cancer_taskgroup.pdf | title = American Brachytherapy Society cervical cancer brachytherapy task group | accessdate = 25 September 2009 | author = Viswanathan AN | authorlink = | work = |publisher = American Brachytherapy Society | pages = |language = |quote = | archiveurl = |archivedate =|display-authors=etal}}</ref><ref name="Viswanathan-2009"> |
|||
| url = http://www.nice.org.uk/IPG160 |
|||
{{Cite journal| first1 = A. N.| last1 = Viswanathan | first2 = B. A.| title = Three-Dimensional Imaging in Gynecologic Brachytherapy: A Survey of the American Brachytherapy Society| journal = International Journal of Radiation OncologyBiologyPhysics| year = 2009| pmid = 19619956 | doi = 10.1016/j.ijrobp.2009.01.043| last2 = Erickson| volume = 76| issue = 1| pages = 104–109}}</ref> |
|||
| title = High dose rate brachytherapy for carcinoma of the cervix |
|||
| accessdate = 25 September 2009 |
|||
| author = National Institute for Health and Clinical Excellence |
|||
| authorlink = |date= March 2006 |
|||
| work = |publisher = NICE |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
}}</ref><ref name="Viswanathan-ABS"> |
|||
{{cite web |
|||
| url = http://www.americanbrachytherapy.org/guidelines/cervical_cancer_taskgroup.pdf |
|||
| title = American Brachytherapy Society cervical cancer brachytherapy task group |
|||
| accessdate = 25 September 2009 |
|||
| author = Viswanathan AN |
|||
| authorlink = | work = |publisher = American Brachytherapy Society |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
|display-authors=etal}}</ref><ref name="Viswanathan-2009"> |
|||
{{Cite pmid|19619956}}</ref> |
|||
Cervical cancer can be treated with either LDR, PDR or HDR brachytherapy.<ref name="Haie-Meder-2009" /><ref name="Viswanathan-ABS" /><ref name="Kim-2009"> |
Cervical cancer can be treated with either LDR, PDR or HDR brachytherapy.<ref name="Haie-Meder-2009" /><ref name="Viswanathan-ABS" /><ref name="Kim-2009"> |
||
{{Cite journal| first1 = D. H.| last1 = Kim | first2 = A. .| first3 = V. .| first4 = J. .| first5 = L. M.| first6 = J. .| first7 = R. .| first8 = I. C.| title = High–Dose Rate Brachytherapy Using Inverse Planning Simulated Annealing for Locoregionally Advanced Cervical Cancer: A Clinical Report with 2-Year Follow-Up| journal = International Journal of Radiation OncologyBiologyPhysics| year = 2009| pmid = 19409728 | doi = 10.1016/j.ijrobp.2009.01.002| last2 = Wang-Chesebro| last3 = Weinberg| last4 = Pouliot| last5 = Chen| last6 = Speight| last7 = Littell| last8 = Hsu| volume = 75| issue = 5| pages = 1329–1334}}</ref> |
|||
{{Cite pmid|19409728}}</ref> |
|||
Used in combination with EBRT, brachytherapy can provide better outcomes than EBRT alone.<ref name="Viswanathan 2007"/> |
Used in combination with EBRT, brachytherapy can provide better outcomes than EBRT alone.<ref name="Viswanathan 2007"/> |
||
The precision of brachytherapy enables a high dose of targeted radiation to be delivered to the cervix, while minimising radiation exposure to adjacent tissues and organs.<ref name="NICE-IPG160" /><ref name="Viswanathan-ABS" /><ref name="Pötter-2008"> |
The precision of brachytherapy enables a high dose of targeted radiation to be delivered to the cervix, while minimising radiation exposure to adjacent tissues and organs.<ref name="NICE-IPG160" /><ref name="Viswanathan-ABS" /><ref name="Pötter-2008"> |
||
{{Cite journal| first1 = R.| last1 = Potter | first2 = C.| first3 = E.| first4 = J.| first5 = D.| first6 = K.| first7 = J.| title = Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma| journal = Acta Oncologica| volume = 47| pages = 1325–1336| year = 2008 | doi = 10.1080/02841860802282794| pmid = 18661430| last2 = Kirisits| last3 = Fidarova| last4 = Dimopoulos| last5 = Berger| last6 = Tanderup| last7 = Lindegaard| issue = 7}}</ref><ref name="Pötter-2006"> |
|||
{{Cite pmid|18661430}}</ref><ref name="Pötter-2006"> |
|||
{{Cite journal| first1 = R.| last1 = Pötter | first2 = C.| first3 = E. V.| first4 = I.| first5 = M. D.| first6 = J.| first7 = I.| first8 = B.| first9 = S.| last10 = Nulens | first10 = A.| last11 = Petrow | first11 = P.| last12 = Rownd | first12 = J.| last13 = Kirisits | first13 = C.| last14 = Gec Estro Working | first14 = G.| title = Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology| journal = Radiotherapy and Oncology| volume = 78| pages = 67–77| year = 2006 | doi = 10.1016/j.radonc.2005.11.014| pmid = 16403584| last2 = Haie-Meder| last3 = Van Limbergen| last4 = Barillot| last5 = De Brabandere| last6 = Dimopoulos| last7 = Dumas| last8 = Erickson| last9 = Lang| issue = 1}}</ref> |
|||
{{Cite pmid|16403584}}</ref> |
|||
The chances of staying free of disease (disease-free survival) and of staying alive (overall survival) are similar for LDR, PDR and HDR treatments.<ref name="Lertsanguansinchai-2004" /><ref name="Hareyama-2002"> |
The chances of staying free of disease (disease-free survival) and of staying alive (overall survival) are similar for LDR, PDR and HDR treatments.<ref name="Lertsanguansinchai-2004" /><ref name="Hareyama-2002"> |
||
{{Cite journal | doi = 10.1002/cncr.10207 | title = High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix | pmid = 11815967 | year = 2002 | last1 = Hareyama | first1 = M. . | last2 = Sakata | first2 = K. I. | last3 = Oouchi | first3 = A. . | last4 = Nagakura | first4 = H. . | last5 = Shido | first5 = M. . | last6 = Someya | first6 = M. . | last7 = Koito | first7 = K. . | journal = Cancer | volume = 94 | issue = 1 | pages = 117–124}}</ref> |
|||
{{Cite pmid|11815967}}</ref> |
|||
However, a key advantage of HDR treatment is that each dose can be delivered on an outpatient basis with a short administration time<ref name="Viswanathan 2007" /> providing greater convenience for many patients. |
However, a key advantage of HDR treatment is that each dose can be delivered on an outpatient basis with a short administration time<ref name="Viswanathan 2007" /> providing greater convenience for many patients. |
||
===Prostate cancer=== |
===Prostate cancer=== |
||
{{Main|Prostate brachytherapy}}Brachytherapy to treat [[prostate cancer]] can be given either as permanent LDR seed implantation or as temporary HDR brachytherapy.<ref name="GEC-ESTRO" />{{rp|Ch. 20}}<ref name="Merrick-ABS"> |
{{Main|Prostate brachytherapy}}Brachytherapy to treat [[prostate cancer]] can be given either as permanent LDR seed implantation or as temporary HDR brachytherapy.<ref name="GEC-ESTRO" />{{rp|Ch. 20}}<ref name="Merrick-ABS"> |
||
{{cite web | url = http://www.americanbrachytherapy.org/guidelines/prostate_low-doseratetaskgroup.pdf | title = American Brachytherapy Society prostate low-dose rate task group | accessdate = 25 September 2009 | author = Merrick GS | authorlink = | work = |publisher = American Brachytherapy Society | pages = |language = |quote = | archiveurl = |archivedate =|display-authors=etal}}</ref><ref name="Hsu-ABS"> |
|||
{{cite web |
|||
{{cite web | url = http://www.americanbrachytherapy.org/guidelines/HDRTaskGroup.pdf | title = American Brachytherapy Society prostate high-dose rate task group | accessdate = 25 September 2009 | author = Hsu I-C | authorlink = | work = |publisher = American Brachytherapy Society | pages = |language = |quote = | archiveurl = |archivedate =|display-authors=etal}}</ref> |
|||
| url = http://www.americanbrachytherapy.org/guidelines/prostate_low-doseratetaskgroup.pdf |
|||
| title = American Brachytherapy Society prostate low-dose rate task group |
|||
| accessdate = 25 September 2009 |
|||
| author = Merrick GS |
|||
| authorlink = | work = |publisher = American Brachytherapy Society |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
|display-authors=etal}}</ref><ref name="Hsu-ABS"> |
|||
{{cite web |
|||
| url = http://www.americanbrachytherapy.org/guidelines/HDRTaskGroup.pdf |
|||
| title = American Brachytherapy Society prostate high-dose rate task group |
|||
| accessdate = 25 September 2009 |
|||
| author = Hsu I-C |
|||
| authorlink = | work = |publisher = American Brachytherapy Society |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
|display-authors=etal}}</ref> |
|||
Permanent seed implantation is suitable for patients with a localised tumour and good prognosis |
Permanent seed implantation is suitable for patients with a localised tumour and good prognosis |
||
<ref name="Batterman-2004" /><ref name="Merrick-ABS" /><ref name="Ash 2005"> |
<ref name="Batterman-2004" /><ref name="Merrick-ABS" /><ref name="Ash 2005"> |
||
{{Cite book | last = Ash D | authorlink = | editor = Hoskin P, Coyle C | year = 2005 | title = Radiotherpay in practice: brachytherapy | chapter = Prostate Cancer | publisher = Oxford University Press | location = New York | id =| author2 = |
|||
{{Cite book |
|||
| last = Ash D |
|||
| authorlink = |
|||
| editor = Hoskin P, Coyle C |
|||
| year = 2005 |
|||
| title = Radiotherpay in practice: brachytherapy |
|||
| chapter = Prostate Cancer |
|||
| publisher = Oxford University Press |
|||
| location = New York |
|||
| id = |
|||
| author2 = |
|||
and others| displayauthors = |
and others| displayauthors = |
||
1}}</ref><ref name="Morris-2009"> |
1}}</ref><ref name="Morris-2009"> |
||
{{Cite |
{{Cite journal| last1 = Morris | first1 = W. J.| first2 = M.| first3 = D.| first4 = M.| first5 = I.| first6 = A.| first7 = T.| first8 = M.| first9 = W.| last10 = Wu | first10 = J.| last11 = Lapointe | first11 = V.| last12 = Berthelet | first12 = E.| last13 = Pai | first13 = H.| last14 = Harrison | first14 = R.| last15 = Kwa | first15 = W.| last16 = Bucci | first16 = J.| last17 = Racz | first17 = V.| last18 = Woods | first18 = R.| title = Evaluation of Dosimetric Parameters and Disease Response After 125Iodine Transperineal Brachytherapy for Low- and Intermediate-Risk Prostate Cancer| journal = International Journal of Radiation OncologyBiologyPhysics| volume = 73| pages = 1432–1438| year = 2009 | doi = 10.1016/j.ijrobp.2008.07.042| last2 = Keyes| last3 = Palma| last4 = McKenzie| last5 = Spadinger| last6 = Agranovich| last7 = Pickles| last8 = Liu| last9 = Kwan| pmid = 19036530| issue = 5}}</ref> and has been shown to be a highly effective treatment to prevent the cancer from returning.<ref name="Pickles-2009" /><ref name="Batterman-2004" /> The survival rate is similar to that found with EBRT or surgery ([[radical prostatectomy]]), but with fewer side effects such as [[impotence]] and [[Urinary incontinence|incontinence]].<ref name="Frank-2007" /> The procedure can be completed quickly and patients are usually able to go home on the same day of treatment and return to normal activities after 1 to 2 days.<ref name="BMJGroup-2009"> |
||
{{cite web | url = http://www.guardian.co.uk/lifeandstyle/besttreatments/prostate-cancer-treatments-internal-radiotherapy-brachytherapy | title = Prostate cancer: internal radiotherapy (brachytherapy) | accessdate = 25 September 2009 | author = BMJ Group | authorlink = |date= June 2009 | work = |publisher = Guardian.co.uk | pages = |language = |quote = | archiveurl = |archivedate =}}</ref> |
|||
{{cite web |
|||
| url = http://www.guardian.co.uk/lifeandstyle/besttreatments/prostate-cancer-treatments-internal-radiotherapy-brachytherapy |
|||
| title = Prostate cancer: internal radiotherapy (brachytherapy) |
|||
| accessdate = 25 September 2009 |
|||
| author = BMJ Group |
|||
| authorlink = |date= June 2009 |
|||
| work = |publisher = Guardian.co.uk |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
}}</ref> |
|||
Permanent seed implantation is often a less invasive treatment option compared to the surgical removal of the prostate.<ref name="BMJGroup-2009" /> |
Permanent seed implantation is often a less invasive treatment option compared to the surgical removal of the prostate.<ref name="BMJGroup-2009" /> |
||
| Line 363: | Line 159: | ||
===Breast cancer=== |
===Breast cancer=== |
||
Radiation therapy is standard of care for women who have undergone [[lumpectomy]] or [[mastectomy]] surgery, and is an integral component of breast-conserving therapy.<ref name="GEC-ESTRO" />{{rp|Ch. 18}}<ref name="Keisch-ABS"> |
Radiation therapy is standard of care for women who have undergone [[lumpectomy]] or [[mastectomy]] surgery, and is an integral component of breast-conserving therapy.<ref name="GEC-ESTRO" />{{rp|Ch. 18}}<ref name="Keisch-ABS"> |
||
{{cite web | url = http://www.americanbrachytherapy.org/guidelines/HDRTaskGroup.pdf | title = American Brachytherapy Society breast brachytherapy task group | accessdate = 25 September 2009 | author = Keisch | authorlink = |date= February 2007 | work = |publisher = American Brachytherapy Society | pages = |language = |quote = | archiveurl = |archivedate =|display-authors=etal}}</ref> |
|||
{{cite web |
|||
| url = http://www.americanbrachytherapy.org/guidelines/HDRTaskGroup.pdf |
|||
| title = American Brachytherapy Society breast brachytherapy task group |
|||
| accessdate = 25 September 2009 |
|||
| author = Keisch |
|||
| authorlink = |date= February 2007 |
|||
| work = |publisher = American Brachytherapy Society |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
|display-authors=etal}}</ref> |
|||
Brachytherapy can be used after surgery, before chemotherapy or palliatively in the case of advanced disease.<ref name="Hoskin 2005"> |
Brachytherapy can be used after surgery, before chemotherapy or palliatively in the case of advanced disease.<ref name="Hoskin 2005"> |
||
{{Cite book | last = Hoskin P | authorlink = | editor = Hoskin P, Coyle C | year = 2005 | title = Radiotherapy in practice: brachytherapy | chapter = Breast Brachytherapy | publisher = Oxford University Press | location = New York | id = | isbn = 0-19-852940-6| author2 = |
|||
{{Cite book |
|||
| last = Hoskin P |
|||
| authorlink = |
|||
| editor = Hoskin P, Coyle C |
|||
| year = 2005 |
|||
| title = Radiotherapy in practice: brachytherapy |
|||
| chapter = Breast Brachytherapy |
|||
| publisher = Oxford University Press |
|||
| location = New York |
|||
| id = |
|||
| isbn = 0-19-852940-6 |
|||
| author2 = |
|||
and others| displayauthors = |
and others| displayauthors = |
||
1}}</ref> Brachytherapy to treat [[breast cancer]] is usually performed with HDR temporary brachytherapy. Post surgery, breast brachytherapy can be used as a “boost” following irradiation of the whole breast using EBRT.<ref name="Keisch-ABS" /><ref name="Polgár-2009"> |
1}}</ref> Brachytherapy to treat [[breast cancer]] is usually performed with HDR temporary brachytherapy. Post surgery, breast brachytherapy can be used as a “boost” following irradiation of the whole breast using EBRT.<ref name="Keisch-ABS" /><ref name="Polgár-2009"> |
||
{{Cite journal| first1 = C.| last1 = Polgár | first2 = T.| title = Current status and perspectives of brachytherapy for breast cancer| journal = International Journal of Clinical Oncology| volume = 14| issue = 1| pages = 7–0| year = 2009| pmid = 19225919 | doi = 10.1007/s10147-008-0867-y| last2 = Major}}</ref> |
|||
{{Cite pmid|19225919}}</ref> |
|||
More recently, brachytherapy alone is applied in a technique called APBI (accelerated partial breast irradiation), involving delivery of radiation to only the immediate region surrounding the original tumour.<ref name="Nelson-2009" /><ref name="Keisch-ABS" /><ref name="Polgár-2009" /> |
More recently, brachytherapy alone is applied in a technique called APBI (accelerated partial breast irradiation), involving delivery of radiation to only the immediate region surrounding the original tumour.<ref name="Nelson-2009" /><ref name="Keisch-ABS" /><ref name="Polgár-2009" /> |
||
The main benefit of breast brachytherapy compared to EBRT is that a high dose of radiation can be precisely applied to the tumour while sparing radiation to healthy breast tissues and underlying structures such as the ribs and lungs.<ref name="Hoskin 2005" /> APBI can typically be completed over the course of a week.<ref name="Nelson-2009" /> The option of brachytherapy may be particularly important in ensuring that working women, the elderly or women without easy access to a treatment centre, are able to benefit from breast-conserving therapy due to the short treatment course compared with EBRT (which often requires more visits over the course of 1–2 months).<ref name="Kelley 2007" /> Brachytherapy has demonstrated excellent local control of breast cancer at follow-up of up to 6 years post treatment.<ref name="Nelson-2009" /><ref name="King-2000"> |
The main benefit of breast brachytherapy compared to EBRT is that a high dose of radiation can be precisely applied to the tumour while sparing radiation to healthy breast tissues and underlying structures such as the ribs and lungs.<ref name="Hoskin 2005" /> APBI can typically be completed over the course of a week.<ref name="Nelson-2009" /> The option of brachytherapy may be particularly important in ensuring that working women, the elderly or women without easy access to a treatment centre, are able to benefit from breast-conserving therapy due to the short treatment course compared with EBRT (which often requires more visits over the course of 1–2 months).<ref name="Kelley 2007" /> Brachytherapy has demonstrated excellent local control of breast cancer at follow-up of up to 6 years post treatment.<ref name="Nelson-2009" /><ref name="King-2000"> |
||
{{Cite journal | pmid = 11113440 | year = 2000 | author1 = King | first2 = J. S. | first3 = R. R. | first4 = G. M. | first5 = T. G. | first6 = X. Z. | title = Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer | volume = 180 | issue = 4 | pages = 299–304 | journal = American journal of surgery | doi = 10.1016/S0002-9610(00)00454-2 | last2 = Bolton | last3 = Kuske | last4 = Fuhrman | last5 = Scroggins | last6 = Jiang}}</ref><ref name="Go´mez-Iturriaga-2008"> |
|||
{{Cite pmid|11113440}}</ref><ref name="Go´mez-Iturriaga-2008"> |
|||
{{Cite |
{{Cite journal| first1 = A.| last1 = Gomeziturriaga | first2 = L.| first3 = M.| first4 = F.| first5 = J.| first6 = O.| first7 = R.| title = Early breast cancer treated with conservative surgery, adjuvant chemotherapy, and delayed accelerated partial breast irradiation with high-dose-rate brachytherapy| journal = Brachytherapy| volume = 7| pages = 310–315| year = 2008 | doi = 10.1016/j.brachy.2008.04.006| pmid = 18778971| last2 = Pina| last3 = Cambeiro| last4 = Martínez-Regueira| last5 = Aramendía| last6 = Fernández-Hidalgo| last7 = Martínez-Monge| issue = 4}}</ref> A study is underway to compare patient outcomes of APBI in comparison to EBRT at up to 10 years after treatment.<ref name="Breast-cancer-trial"> |
||
{{cite web | url = http://clinicaltrials.gov/ct2/show/NCT00402519 | title = APBI Versus EBRT Therapy After Breast Conserving Surgery for Low-risk Breast Cancer | accessdate = 17 April 2010 | author = Clinicaltrials.gov | authorlink = |date= February 2010 | work = |publisher = | pages = |language = |quote = | archiveurl = |archivedate =}}</ref> |
|||
{{cite web |
|||
| url = http://clinicaltrials.gov/ct2/show/NCT00402519 |
|||
| title = APBI Versus EBRT Therapy After Breast Conserving Surgery for Low-risk Breast Cancer |
|||
| accessdate = 17 April 2010 |
|||
| author = Clinicaltrials.gov |
|||
| authorlink = |date= February 2010 |
|||
| work = |publisher = |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
}}</ref> |
|||
There are two methods that can be used to deliver breast brachytherapy: |
There are two methods that can be used to deliver breast brachytherapy: |
||
| Line 411: | Line 178: | ||
Interstitial breast brachytherapy involves the temporary placement of several flexible plastic catheters in the breast tissue. These are carefully positioned to allow optimal targeting of radiation to the treatment area while sparing the surrounding breast tissue.<ref name="Kelley 2007" /> The catheters are connected to an [[#Procedure|afterloader]], which delivers the planned radiation dose to the treatment area. Interstitial breast brachytherapy can be used as “boost” after EBRT, or as APBI.<ref name="Polgár-2009" /> |
Interstitial breast brachytherapy involves the temporary placement of several flexible plastic catheters in the breast tissue. These are carefully positioned to allow optimal targeting of radiation to the treatment area while sparing the surrounding breast tissue.<ref name="Kelley 2007" /> The catheters are connected to an [[#Procedure|afterloader]], which delivers the planned radiation dose to the treatment area. Interstitial breast brachytherapy can be used as “boost” after EBRT, or as APBI.<ref name="Polgár-2009" /> |
||
Intracavitary breast brachytherapy (also known as “balloon brachytherapy”) involves the placement of a single catheter into the breast cavity left after the removal of the tumour (lumpectomy).<ref name="Kelley 2007" /> The catheter can be placed at the time of the lumpectomy or postoperatively.<ref name="Kelley 2007" /> Via the catheter, a balloon is then inflated in the cavity. The catheter is then connected to an [[#Procedure|afterloader]], which delivers the radiation dose through the catheter and into the balloon. Currently, intracavitary breast brachytherapy is only routinely used for APBI.<ref name="Shah-2010">{{Cite |
Intracavitary breast brachytherapy (also known as “balloon brachytherapy”) involves the placement of a single catheter into the breast cavity left after the removal of the tumour (lumpectomy).<ref name="Kelley 2007" /> The catheter can be placed at the time of the lumpectomy or postoperatively.<ref name="Kelley 2007" /> Via the catheter, a balloon is then inflated in the cavity. The catheter is then connected to an [[#Procedure|afterloader]], which delivers the radiation dose through the catheter and into the balloon. Currently, intracavitary breast brachytherapy is only routinely used for APBI.<ref name="Shah-2010">{{Cite journal| last1 = Shah | first1 = A. P.| last2 = Strauss | first2 = J. B.| last3 = Kirk | first3 = M. C.| last4 = Chen | first4 = S. S.| last5 = Dickler | first5 = A.| title = A dosimetric analysis comparing electron beam with the MammoSite brachytherapy applicator for intact breast boost| journal = Physica Medica| volume = 26| issue = 2| pages = 80–87| year = 2010| pmid = 19836283| doi = 10.1016/j.ejmp.2009.08.004}}</ref> |
||
There are also devices that combine the features of interstitial and intracavitary breast brachytherapy (e.g. SAVI). These devices use multiple catheters but are inserted through a single-entry point in the breast. Studies suggest the use of multiple catheters enables physicians to target the radiation more precisely.<ref>Scanderbeg, D; Yashar, C; White, G; Rice, R; Pawlicki, T (2010). “Evaluation of Three APBI Techniques under NSABP B-39 Guidelines.” ''Journal of Applied Clinical Medical Physics'' 11 (1): 274-280.</ref><ref>Yashar, C; Blair, S; Wallace, A; Scanderbeg, D (2009). “Initial Clinical Experience with the Strut-Adjusted Volume Implant Brachytherapy Applicator for Accelerated Partial Breast Irradiation.” ''Brachytherapy'' 8: 367-372.</ref> |
There are also devices that combine the features of interstitial and intracavitary breast brachytherapy (e.g. SAVI). These devices use multiple catheters but are inserted through a single-entry point in the breast. Studies suggest the use of multiple catheters enables physicians to target the radiation more precisely.<ref>Scanderbeg, D; Yashar, C; White, G; Rice, R; Pawlicki, T (2010). “Evaluation of Three APBI Techniques under NSABP B-39 Guidelines.” ''Journal of Applied Clinical Medical Physics'' 11 (1): 274-280.</ref><ref>Yashar, C; Blair, S; Wallace, A; Scanderbeg, D (2009). “Initial Clinical Experience with the Strut-Adjusted Volume Implant Brachytherapy Applicator for Accelerated Partial Breast Irradiation.” ''Brachytherapy'' 8: 367-372.</ref> |
||
| Line 419: | Line 186: | ||
Brachytherapy for skin cancer provides good cosmetic results and clinical efficacy; studies with up to 5 years follow-up have shown that brachytherapy is highly effective in terms local control, and is comparable to EBRT.<ref name="Guix-2000"> |
Brachytherapy for skin cancer provides good cosmetic results and clinical efficacy; studies with up to 5 years follow-up have shown that brachytherapy is highly effective in terms local control, and is comparable to EBRT.<ref name="Guix-2000"> |
||
{{Cite journal | doi = 10.1016/S0360-3016(99)00547-7 | pmid = 10758310 | year = 2000 | author1 = Guix | first2 = F. | first3 = J. | first4 = C. | first5 = A. | first6 = J. | first7 = R. | title = Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds | volume = 47 | issue = 1 | pages = 95–102 | journal = International journal of radiation oncology, biology, physics | last2 = Finestres | last3 = Tello | last4 = Palma | last5 = Martinez | last6 = Guix | last7 = Guix}}</ref><ref name="Sedda-2008"> |
|||
{{Cite pmid|10758310}}</ref><ref name="Sedda-2008"> |
|||
{{Cite journal| first1 = A. F.| last1 = Sedda | first2 = G.| first3 = C.| first4 = A. M.| first5 = P.| title = Dermatological high-dose-rate brachytherapy for the treatment of basal and squamous cell carcinoma| journal = Clinical and Experimental Dermatology| volume = 33| pages = 745–749| year = 2008 | doi = 10.1111/j.1365-2230.2008.02852.x| pmid = 18681873| last2 = Rossi| last3 = Cipriani| last4 = Carrozzo| last5 = Donati| issue = 6}}</ref><ref name="Rio-2005"> |
|||
{{Cite pmid|18681873}}</ref><ref name="Rio-2005"> |
|||
{{Cite journal| first1 = E.| last1 = Rio | first2 = E.| first3 = C.| first4 = P.| first5 = S.| first6 = L.| first7 = C.| first8 = M.| first9 = B.| title = Interstitial brachytherapy of periorificial skin carcinomas of the face: A retrospective study of 97 cases| journal = International Journal of Radiation OncologyBiologyPhysics| volume = 63| issue = 3| pages = 753–757| year = 2005| pmid = 15927410 | doi = 10.1016/j.ijrobp.2005.03.027| last2 = Bardet| last3 = Ferron| last4 = Peuvrel| last5 = Supiot| last6 = Campion| last7 = Beauvillain De Montreuil| last8 = Mahe| last9 = Dreno}}</ref> Treatment times are typically short, providing convenience for patients.<ref name="Musmacher-2006"> |
|||
{{Cite pmid|15927410}}</ref> Treatment times are typically short, providing convenience for patients.<ref name="Musmacher-2006"> |
|||
{{Cite journal | author = Musmacher J | year = 2006 | title = High dose rate brachytherapy with surface applicators: Treatment for nonmelanomatous skin cancer | journal = Journal of Clinical Oncology | volume = 24 | issue = | page = 15543 | pmid = | url = | author2 = and others | displayauthors = 1 }}</ref> |
|||
{{Cite journal |
|||
| author = Musmacher J |
|||
| year = 2006 |
|||
| title = High dose rate brachytherapy with surface applicators: Treatment for nonmelanomatous skin cancer |
|||
| journal = Journal of Clinical Oncology |
|||
| volume = 24 |
|||
| issue = |
|||
| page = 15543 |
|||
| pmid = |
|||
| url = |
|||
| author2 = and others |
|||
| displayauthors = 1 |
|||
}}</ref> |
|||
It has been suggested that brachytherapy may become a standard of treatment for skin cancer in the near future.<ref name="Musmacher-2006" /> |
It has been suggested that brachytherapy may become a standard of treatment for skin cancer in the near future.<ref name="Musmacher-2006" /> |
||
===Coronary/vascular=== |
===Coronary/vascular=== |
||
Brachytherapy can be used in the treatment of [[Coronary stent#Restenosis|coronary in-stent restenosis]], in which a catheter is placed inside blood vessels, through which sources are inserted and removed.<ref name="ESC-2005"> |
Brachytherapy can be used in the treatment of [[Coronary stent#Restenosis|coronary in-stent restenosis]], in which a catheter is placed inside blood vessels, through which sources are inserted and removed.<ref name="ESC-2005"> |
||
{{Cite journal| first1 = A. /T. F. | first2 = S.| last5 = Colombo| last4 = Camici| last7 = Jørgensen | first3 = P. | first4 = F. F.| last3 = Avilés| last1 = Members | first5 = P. G.| last2 = Albertsson | first6 = A.| last8 = Marco | first7 = C. | first8 = E.| last6 = Hamm| last9 = Nordrehaug | first9 = J.| title = Guidelines for Percutaneous Coronary Interventions: the Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology| author14 = Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology | first13 = W.| last13 = Wijns | first12 = G. W.| last12 = Stone| journal = European Heart Journal | first11 = P.| volume = 26| issue = 8| last10 = Ruzyllo| last11 = Urban| pages = 804–847| year = 2005| pmid = 15769784 | first10 = W. | doi = 10.1093/eurheartj/ehi138}}</ref> |
|||
{{Cite pmid|15769784}}</ref> |
|||
In treating In-stent restenosis (ISR) Drug Eluting stents (DES) have been found to be superior to Intracoronary Brachytherapy (ICBT).<!--<ref name="Clinical Cardiology 5/10/11>--> |
In treating In-stent restenosis (ISR) Drug Eluting stents (DES) have been found to be superior to Intracoronary Brachytherapy (ICBT).<!--<ref name="Clinical Cardiology 5/10/11>--> |
||
However, there is continued interest in vascular brachytherapy for persistent restenosis in failed stents and vein grafts. |
However, there is continued interest in vascular brachytherapy for persistent restenosis in failed stents and vein grafts. |
||
The therapy has also been investigated for use in the treatment of [[peripheral vascular disease|peripheral vasculature stenosis]]<ref name="Sidawy-2002"> |
The therapy has also been investigated for use in the treatment of [[peripheral vascular disease|peripheral vasculature stenosis]]<ref name="Sidawy-2002"> |
||
{{Cite |
{{Cite journal | doi = 10.1067/mva.2002.123751 | pmid = 12021726 | year = 2002 | author1 = Sidawy | first2 = J. | first3 = R. | title = Peripheral vascular brachytherapy | volume = 35 | issue = 5 | pages = 1041–1047 | journal = Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter | last2 = Weiswasser | last3 = Waksman}}</ref> and considered for the treatment of [[atrial fibrillation]].<ref name="Pérez-Castellano-2006"> |
||
{{Cite journal| last1 = Perez-castellano | first1 = N.| first2 = J.| first3 = P.| first4 = P.| first5 = M.| first6 = M. J.| first7 = C.| first8 = J. M.| first9 = J.| last10 = Fernández-Ortiz | first10 = A.| last11 = Vano | first11 = E.| last12 = MacAya | first12 = C.| title = Pathological Effects of Pulmonary Vein beta-Radiation in a Swine Model| journal = Journal of Cardiovascular Electrophysiology| volume = 17| issue = 6| pages = 662–669| year = 2006| pmid = 16836719 | doi = 10.1111/j.1540-8167.2006.00462.x| last2 = Villacastín| last3 = Aragoncillo| last4 = Fantidis| last5 = Sabaté| last6 = García-Torrent| last7 = Prieto| last8 = Corral| last9 = Moreno}}</ref> |
|||
{{Cite pmid|16836719}}</ref> |
|||
==Side effects== |
==Side effects== |
||
| Line 451: | Line 206: | ||
===Acute=== |
===Acute=== |
||
Acute side effects associated with brachytherapy include localised bruising, swelling, bleeding, discharge or discomfort within the implanted region. These usually resolve within a few days following completion of treatment.<ref name="Macmillan-brachytherapy"> |
Acute side effects associated with brachytherapy include localised bruising, swelling, bleeding, discharge or discomfort within the implanted region. These usually resolve within a few days following completion of treatment.<ref name="Macmillan-brachytherapy"> |
||
{{cite web | url = http://www.macmillan.org.uk/Cancerinformation/Cancertreatment/Treatmenttypes/Radiotherapy/Beingtreated/Brachytherapy.aspx | title = Brachytherapy | accessdate = 25 September 2009 | author = Macmillan Cancer Support | authorlink = | work = |publisher = | pages = |language = |quote = | archiveurl = |archivedate =}}</ref> |
|||
{{cite web |
|||
| url = http://www.macmillan.org.uk/Cancerinformation/Cancertreatment/Treatmenttypes/Radiotherapy/Beingtreated/Brachytherapy.aspx |
|||
| title = Brachytherapy |
|||
| accessdate = 25 September 2009 |
|||
| author = Macmillan Cancer Support |
|||
| authorlink = | work = |publisher = |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
}}</ref> |
|||
Patients may also feel fatigued for a short period following treatment.<ref name="Macmillan-brachytherapy" /><ref name="Fieler-1997"> |
Patients may also feel fatigued for a short period following treatment.<ref name="Macmillan-brachytherapy" /><ref name="Fieler-1997"> |
||
{{Cite journal | pmid = 9127366 | year = 1997 | author1 = Fieler | title = Side effects and quality of life in patients receiving high-dose rate brachytherapy | volume = 24 | issue = 3 | pages = 545–553 | journal = Oncology nursing forum}}</ref> |
|||
{{Cite pmid|9127366}}</ref> |
|||
Brachytherapy treatment for cervical or prostate cancer can cause acute and transient urinary symptoms such as urinary retention, urinary incontinence or painful urination (dysuria).<ref name="Frank-2007" /><ref name="Doust-2004"> |
Brachytherapy treatment for cervical or prostate cancer can cause acute and transient urinary symptoms such as urinary retention, urinary incontinence or painful urination (dysuria).<ref name="Frank-2007" /><ref name="Doust-2004"> |
||
{{Cite journal | pmid = 15301172 | year = 2004 | author1 = Doust | first2 = E. | first3 = G. | first4 = M. | first5 = D. | title = A systematic review of brachytherapy. Is it an effective and safe treatment for localised prostate cancer? | volume = 33 | issue = 7 | pages = 525–529 | journal = Australian family physician | last2 = Miller | last3 = Duchesne | last4 = Kitchener | last5 = Weller}}</ref><ref name="Magné-2009"> |
|||
{{Cite pmid|15301172}}</ref><ref name="Magné-2009"> |
|||
{{Cite journal| last1 = Magné | first1 = N.| first2 = N. C.| first3 = E.| first4 = P.| first5 = P.| first6 = D.| first7 = C.| first8 = P.| first9 = C.| title = Patterns of care and outcome in elderly cervical cancer patients: A special focus on brachytherapy| journal = Radiotherapy and Oncology| volume = 91| issue = 2| pages = 197–201| year = 2009| pmid = 18954913 | doi = 10.1016/j.radonc.2008.08.011| last2 = Mancy| last3 = Chajon| last4 = Duvillard| last5 = Pautier| last6 = Castaigne| last7 = Lhommé| last8 = Morice| last9 = Haie-Meder}}</ref> |
|||
{{Cite pmid|18954913}}</ref> |
|||
Transient increased bowel frequency, diarrhoea, constipation or minor rectal bleeding, may also occur.<ref name="Frank-2007" /><ref name="Doust-2004" /><ref name="Magné-2009" /> Acute and subacute side effects usually resolve over a matter of days or a few weeks. In the case of permanent (seed) brachytherapy for prostate cancer, there is a small chance that some seeds may migrate out of the treatment region into the bladder or urethra and be passed in the urine. |
Transient increased bowel frequency, diarrhoea, constipation or minor rectal bleeding, may also occur.<ref name="Frank-2007" /><ref name="Doust-2004" /><ref name="Magné-2009" /> Acute and subacute side effects usually resolve over a matter of days or a few weeks. In the case of permanent (seed) brachytherapy for prostate cancer, there is a small chance that some seeds may migrate out of the treatment region into the bladder or urethra and be passed in the urine. |
||
Brachytherapy for skin cancer may result in a shedding of the outer layers of skin (desquamation) around the area of treatment in the weeks following therapy, which typically heals in 5–8 weeks.<ref name="GEC-ESTRO" />{{rp|Ch. 28}} If the cancer is located on the lip, ulceration may occur as a result of brachytherapy, but usually resolves after 4–6 weeks.<ref name="Casino-2006"> |
Brachytherapy for skin cancer may result in a shedding of the outer layers of skin (desquamation) around the area of treatment in the weeks following therapy, which typically heals in 5–8 weeks.<ref name="GEC-ESTRO" />{{rp|Ch. 28}} If the cancer is located on the lip, ulceration may occur as a result of brachytherapy, but usually resolves after 4–6 weeks.<ref name="Casino-2006"> |
||
{{Cite journal | author = Casino AR | year = 2006 | title = Brachytherapy in lip cancer | journal = Medicina Oral | volume = 11 | issue = | pages = E223–9 | pmid = | url = | author2 = and others | displayauthors = 1 }}</ref> |
|||
{{Cite journal |
|||
| author = Casino AR |
|||
| year = 2006 |
|||
| title = Brachytherapy in lip cancer |
|||
| journal = Medicina Oral |
|||
| volume = 11 |
|||
| issue = |
|||
| pages = E223–9 |
|||
| pmid = |
|||
| url = |
|||
| author2 = and others |
|||
| displayauthors = 1 |
|||
}}</ref> |
|||
Most of the acute side effects associated with brachytherapy can be treated with medication or through dietary changes, and usually disappear over time (typically a matter of weeks), once the treatment is completed. The acute side effects of HDR brachytherapy are broadly similar to EBRT.<ref name="Fieler-1997" /> |
Most of the acute side effects associated with brachytherapy can be treated with medication or through dietary changes, and usually disappear over time (typically a matter of weeks), once the treatment is completed. The acute side effects of HDR brachytherapy are broadly similar to EBRT.<ref name="Fieler-1997" /> |
||
| Line 491: | Line 226: | ||
Brachytherapy for breast or skin cancer may cause scar tissue to form around the treatment area. In the case of breast brachytherapy, fat necrosis may occur as a result of fatty acids entering the breast tissues. This can cause the breast tissue to become swollen and tender. Fat necrosis is a benign condition and typically occurs 4–12 months after treatment and affects about 2% of patients.<ref name="Vicini-2009"> |
Brachytherapy for breast or skin cancer may cause scar tissue to form around the treatment area. In the case of breast brachytherapy, fat necrosis may occur as a result of fatty acids entering the breast tissues. This can cause the breast tissue to become swollen and tender. Fat necrosis is a benign condition and typically occurs 4–12 months after treatment and affects about 2% of patients.<ref name="Vicini-2009"> |
||
{{Cite journal| last1 = Vicini | first1 = F.| first2 = P. D.| first3 = C. A.| first4 = A. J.| first5 = D.| first6 = H. C.| first7 = M. A.| first8 = V. J.| first9 = H. M.| last10 = Lyden | first10 = M.| title = Three-year analysis of treatment efficacy, cosmesis, and toxicity by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation (APBI)| journal = Cancer| volume = 112| issue = 4| pages = 758–766| year = 2008| pmid = 18181095 | doi = 10.1002/cncr.23227| last2 = Beitsch| last3 = Quiet| last4 = Keleher| last5 = Garcia| last6 = Snider Jr| last7 = Gittleman| last8 = Zannis| last9 = Kuerer}}</ref><ref name="Humonc-breast"> |
|||
{{Cite pmid|18181095}}</ref><ref name="Humonc-breast"> |
|||
{{cite web | url = http://www.humonc.wisc.edu/modules/mediawiki/index.php?title=Breast_brachytherapy | title = Breast brachytherapy | accessdate = 25 September 2009 | author = Department of Human Oncology, University of Wisconsin School of Medicine and Public Health | authorlink = | work = |publisher = | pages = |language = |quote = | archiveurl = |archivedate =}} {{Dead link|date=April 2012|bot=H3llBot}}</ref> |
|||
{{cite web |
|||
| url = http://www.humonc.wisc.edu/modules/mediawiki/index.php?title=Breast_brachytherapy |
|||
| title = Breast brachytherapy |
|||
| accessdate = 25 September 2009 |
|||
| author = Department of Human Oncology, University of Wisconsin School of Medicine and Public Health |
|||
| authorlink = | work = |publisher = |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
}} {{Dead link|date=April 2012|bot=H3llBot}}</ref> |
|||
==Safety around others== |
==Safety around others== |
||
Patients often ask if they need to have special safety precautions around family and friends after receiving brachytherapy. If temporary brachytherapy is used, no radioactive sources remain in the body after treatment. Therefore, there is no radiation risk to friends or family from being in close proximity with them.<ref name=rtanswers.com>{{Cite web |
Patients often ask if they need to have special safety precautions around family and friends after receiving brachytherapy. If temporary brachytherapy is used, no radioactive sources remain in the body after treatment. Therefore, there is no radiation risk to friends or family from being in close proximity with them.<ref name=rtanswers.com>{{Cite web |url= http://www.rtanswers.com/treatmentinformation/treatmenttypes/brachytherapy.aspx |title=Treatment Types: Brachytherapy |work=RT Answers|publisher=American Society for Radiation Oncology |accessdate=April 18, 2010}}</ref> |
||
|url= http://www.rtanswers.com/treatmentinformation/treatmenttypes/brachytherapy.aspx |
|||
|title=Treatment Types: Brachytherapy |
|||
|work=RT Answers|publisher=American Society for Radiation Oncology |
|||
|accessdate=April 18, 2010 |
|||
}}</ref> |
|||
If permanent brachytherapy is used, low dose radioactive sources (seeds) are left in the body after treatment - the radiation levels are very low and decrease over time. In addition, the irradiation only affects tissues within a few millimeters of the radioactive sources (i.e. the tumour being treated). As a precaution, some people receiving permanent brachytherapy may be advised to not hold any small children or be too close to pregnant women for a short time after treatment. Radiation oncologists or nurses can provide specific instructions to patients and advise for how long they need to be careful.<ref name=rtanswers.com /> |
If permanent brachytherapy is used, low dose radioactive sources (seeds) are left in the body after treatment - the radiation levels are very low and decrease over time. In addition, the irradiation only affects tissues within a few millimeters of the radioactive sources (i.e. the tumour being treated). As a precaution, some people receiving permanent brachytherapy may be advised to not hold any small children or be too close to pregnant women for a short time after treatment. Radiation oncologists or nurses can provide specific instructions to patients and advise for how long they need to be careful.<ref name=rtanswers.com /> |
||
| Line 536: | Line 258: | ||
[[File:Brachytherapy treatment planning.jpg|thumb|right|250px|Refinement of the treatment plan during a brachytherapy procedure.]] |
[[File:Brachytherapy treatment planning.jpg|thumb|right|250px|Refinement of the treatment plan during a brachytherapy procedure.]] |
||
To identify the optimal spatial and temporal distribution of radiation sources within the applicators of the implanted tissue or cavity, the treatment planning software allows virtual radiation sources to be placed within the virtual patient. The software shows a graphical representation of the distribution of the irradiation. This serves as a guide for the brachytherapy team to refine the distribution of the sources and provide a treatment plan that is optimally tailored to the anatomy of each patient before actual delivery of the irradiation begins.<ref name="GYNOpt-2009"> |
To identify the optimal spatial and temporal distribution of radiation sources within the applicators of the implanted tissue or cavity, the treatment planning software allows virtual radiation sources to be placed within the virtual patient. The software shows a graphical representation of the distribution of the irradiation. This serves as a guide for the brachytherapy team to refine the distribution of the sources and provide a treatment plan that is optimally tailored to the anatomy of each patient before actual delivery of the irradiation begins.<ref name="GYNOpt-2009"> |
||
{{Cite journal | author = Trnková P. |author2=Pötter R. |author3=Baltas D. |author4=Karabis A. |author5=Fidarova E. |author6=Dimopoulos J. |author7=Georg D. |author8=Kirisits C. | year = 2009 | title = New inverse planning technology for image-guided cervical cancer brachytherapy: Description and evaluation within a clinical frame | journal = Radiotherapy and Oncology | volume = 93 | issue = 2 | pages = 331–340 | pmid = 19846230 | url = http://www.pi-medical.gr/sites/default/files/New%20inverse%20planning%20technology%20for%20image-guided%20cervical%20cancer%20brachytherapy_1.pdf | doi = 10.1016/j.radonc.2009.10.004 }}</ref> This approach is sometimes called ‘dose-painting’. |
|||
{{Cite journal |
|||
| author = Trnková P. |
|||
|author2=Pötter R. |author3=Baltas D. |author4=Karabis A. |author5=Fidarova E. |author6=Dimopoulos J. |author7=Georg D. |author8=Kirisits C. |
|||
| year = 2009 |
|||
| title = New inverse planning technology for image-guided cervical cancer brachytherapy: Description and evaluation within a clinical frame |
|||
| journal = Radiotherapy and Oncology |
|||
| volume = 93 |
|||
| issue = 2 |
|||
| pages = 331–340 |
|||
| pmid = 19846230 |
|||
| url = http://www.pi-medical.gr/sites/default/files/New%20inverse%20planning%20technology%20for%20image-guided%20cervical%20cancer%20brachytherapy_1.pdf |
|||
| doi = 10.1016/j.radonc.2009.10.004 |
|||
}}</ref> This approach is sometimes called ‘dose-painting’. |
|||
===Treatment delivery=== |
===Treatment delivery=== |
||
| Line 611: | Line 321: | ||
==Electronic brachytherapy== |
==Electronic brachytherapy== |
||
Electronic brachytherapy involves placement of miniature low energy x-ray tube sources into a pre-positioned applicator within body/tumour cavities to rapidly deliver high doses to target tissues while maintaining low doses to distant non-target tissues.<ref name="AAPM-report"> |
Electronic brachytherapy involves placement of miniature low energy x-ray tube sources into a pre-positioned applicator within body/tumour cavities to rapidly deliver high doses to target tissues while maintaining low doses to distant non-target tissues.<ref name="AAPM-report"> |
||
{{cite web | url = http://www.aapm.org/pubs/reports/RPT_152.pdf | title = The 2007 AAPM response to the CRCPD request for recommendations for the CRCPD's model regulations for electronic brachytherapy | accessdate = 17 April 2010 | author = American Association of Physicists in Medicine | authorlink = |date= February 2009 | work = |publisher = American Association of Physicists in Medicine | pages = |language = |quote = | archiveurl = |archivedate =}}</ref> |
|||
{{cite web |
|||
| url = http://www.aapm.org/pubs/reports/RPT_152.pdf |
|||
| title = The 2007 AAPM response to the CRCPD request for recommendations for the CRCPD's model regulations for electronic brachytherapy |
|||
| accessdate = 17 April 2010 |
|||
| author = American Association of Physicists in Medicine |
|||
| authorlink = |date= February 2009 |
|||
| work = |publisher = American Association of Physicists in Medicine |
|||
| pages = |language = |quote = |
|||
| archiveurl = |archivedate = |
|||
}}</ref> |
|||
==Environmental hazard== |
==Environmental hazard== |
||
Revision as of 14:16, 16 August 2015
| Brachytherapy | |
|---|---|
| ICD-10-PCS | D?1 |
| ICD-9-CM | 92.27 |
| MeSH | D001918 |
Brachytherapy (from the Greek word βραχύς brachys, meaning "short-distance"), also known as internal radiotherapy, sealed source radiotherapy, curietherapy or endocurietherapy, is a form of radiotherapy where a sealed radiation source is placed inside or next to the area requiring treatment. Brachytherapy is commonly used as an effective treatment for cervical, prostate, breast, and skin cancer and can also be used to treat tumours in many other body sites.[1]
Brachytherapy can be used alone or in combination with other therapies such as surgery, external beam radiotherapy (EBRT) and chemotherapy.
Brachytherapy contrasts with unsealed source radiotherapy in which a therapeutic radionuclide (radioisotope) is injected into the body to chemically localize to the tissue requiring destruction. It also contrasts to EBRT, in which high-energy x-rays (or occasionally gamma-rays from a radioisotope like cobalt-60) are directed at the tumour from outside the body. Brachytherapy instead involves the precise placement of short-range radiation-sources (radioisotopes) directly at the site of the cancerous tumour. These are enclosed in a protective capsule or wire, which allows the ionizing radiation to escape to treat and kill surrounding tissue but prevents the charge of radioisotope from moving or dissolving in body fluids. The capsule may be removed later, or (with some radioisotopes) it may be allowed to remain in place.[1]: Ch. 1 [2] A key feature of brachytherapy is that the irradiation affects only a very localized area around the radiation sources. Exposure to radiation of healthy tissues farther away from the sources is therefore reduced. In addition, if the patient moves or if there is any movement of the tumour within the body during treatment, the radiation sources retain their correct position in relation to the tumour. These characteristics of brachytherapy provide advantages over EBRT - the tumour can be treated with very high doses of localised radiation whilst reducing the probability of unnecessary damage to surrounding healthy tissues.[1]: Ch. 1 [2]
A course of brachytherapy can be completed in less time than other radiotherapy techniques. This can help reduce the chance for surviving cancer cells to divide and grow in the intervals between each radiotherapy dose.[2] Patients typically have to make fewer visits to the radiotherapy clinic compared with EBRT, and the treatment is often performed on an outpatient basis. This makes treatment accessible and convenient for many patients.[3][4] These features of brachytherapy mean that most patients are able to tolerate the brachytherapy procedure very well.
Brachytherapy represents an effective treatment option for many types of cancer. Treatment results have demonstrated that the cancer cure rates of brachytherapy are either comparable to surgery and EBRT or are improved when used in combination with these techniques.[5][6][7][8][9][10][11][12] In addition, brachytherapy is associated with a low risk of serious adverse side effects.[13][14]
The global market for brachytherapy reached US$680 million in 2013, of which the High-Dose Rate (HDR) and LDR segments accounted for 70%. Microspheres and electronic brachytherapy commanded the remaining 30%. The brachytherapy market is expected to reach over US$2.4 billion in 2030, growing by 8% annually, mainly driven by the microspheres market as well as electronic brachytherapy, which is gaining significant interest worldwide as a user-friendly technology.[15]
History
Brachytherapy dates back to 1901 (shortly after the discovery of radioactivity by Henri Becquerel in 1896) when Pierre Curie suggested to Henri-Alexandre Danlos that a radioactive source could be inserted into a tumour.[16][17] It was found that the radiation caused the tumour to shrink.[17] Independently, Alexander Graham Bell also suggested the use of radiation in this way.[17] In the early twentieth century, techniques for the application of brachytherapy were pioneered at the Curie institute in Paris by Danlos and at St Luke's and Memorial Hospital in New York by Robert Abbe.[1]: Ch. 1 [17]
Interstitial radium therapy was common in the 1930s.[1]: Ch. 1 Gold seeds filled with radon were used as early as 1942[18] until at least 1958.[19] Gold shells were selected by Gino Failla around 1920 to shield beta rays while passing gamma rays.[20] Cobalt needles were also used briefly after world war II.[1]: Ch. 1 Radon and cobalt were replaced by radioactive tantalum and gold, before iridium rose in prominence.[1]: Ch. 1 First used in 1958, iridium is the most commonly used artificial source for brachytherapy today.[1]: Ch. 1
Following initial interest in brachytherapy in Europe and the US, its use declined in the middle of the twentieth century due to the problem of radiation exposure to operators from the manual application of the radioactive sources.[17][21] However, the development of remote afterloading systems, which allow the radiation to be delivered from a shielded safe, and the use of new radioactive sources in the 1950s and 1960s, reduced the risk of unnecessary radiation exposure to the operator and patients.[16] This, together with more recent advancements in three-dimensional imaging modalities, computerised treatment planning systems and delivery equipment has made brachytherapy a safe and effective treatment for many types of cancer today.[1]: Ch. 1
Types
Different types of brachytherapy can be defined according to (1) the placement of the radiation sources in the target treatment area, (2) the rate or ‘intensity’ of the irradiation dose delivered to the tumour, and (3) the duration of dose delivery.
Source placement
The two main types of brachytherapy treatment in terms of the placement of the radioactive source are interstitial and contact.
- In the case of interstitial brachytherapy, the sources are placed directly in the target tissue of the affected site, such as the prostate or breast.[1]: Ch. 1
- Contact brachytherapy involves placement of the radiation source in a space next to the target tissue.[1]: Ch. 1 This space may be a body cavity (intracavitary brachytherapy) such as the cervix, uterus or vagina; a body lumen (intraluminal brachytherapy) such as the trachea or oesophagus; or externally (surface brachytherapy) such as the skin.[1]: Ch. 1 A radiation source can also be placed in blood vessels (intravascular brachytherapy) for the treatment of coronary in-stent restenosis.[22]
Dose rate
- Main article: Dose from radioactive seeds
The dose rate of brachytherapy refers to the level or ‘intensity’ with which the radiation is delivered to the surrounding medium and is expressed in Grays per hour (Gy/h).
- Low-dose rate(LDR) brachytherapy involves implanting radiation sources that emit radiation at a rate of up to 2 Gy·h−1.[23] LDR brachytherapy is commonly used for cancers of the oral cavity,[24] oropharynx,[24] sarcomas[1]: Ch. 27 and prostate cancer[1]: Ch. 20 [25]
- Medium-dose rate (MDR) brachytherapy is characterized by a medium rate of dose delivery, ranging between 2 Gy·h−1 to 12 Gy·h−1.[23]
- High-dose rate (HDR) brachytherapy is when the rate of dose delivery exceeds 12 Gy·h−1.[23] The most common applications of HDR brachytherapy are in tumours of the cervix, esophagus, lungs, breasts and prostate.[1] Most HDR treatments are performed on an outpatient basis, but this is dependent on the treatment site.[26]
- Pulsed-dose rate (PDR) brachytherapy involves short pulses of radiation, typically once an hour, to simulate the overall rate and effectiveness of LDR treatment. Typical tumour sites treated by PDR brachytherapy are gynaecological[1]: Ch. 14 and head and neck cancers.[24]
Duration of dose delivery
The placement of radiation sources in the target area can be temporary or permanent.
- Temporary brachytherapy involves placement of radiation sources for a set duration (usually a number of minutes or hours) before being withdrawn.[1]: Ch. 1 The specific treatment duration will depend on many different factors, including the required rate of dose delivery and the type, size and location of the cancer. In LDR and PDR brachytherapy, the source typically stays in place up to 24 hours before being removed, while in HDR brachytherapy this time is typically a few minutes.[27]
- Permanent brachytherapy, also known as seed implantation, involves placing small LDR radioactive seeds or pellets (about the size of a grain of rice) in the tumour or treatment site and leaving them there permanently to gradually decay. Over a period of weeks or months, the level of radiation emitted by the sources will decline to almost zero. The inactive seeds then remain in the treatment site with no lasting effect.[28] Permanent brachytherapy is most commonly used in the treatment of prostate cancer.[25]
Clinical applications
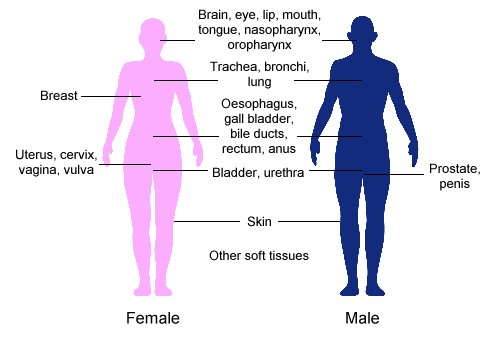
Brachytherapy is commonly used to treat cancers of the cervix, prostate, breast, and skin.[1]
Brachytherapy can also be used in the treatment of tumours of the brain, eye, head and neck region (lip, floor of mouth, tongue, nasopharynx and oropharynx),[24] respiratory tract (trachea and bronchi), digestive tract (oesophagus, gall bladder, bile-ducts, rectum, anus),[29] urinary tract (bladder, urethra, penis), female reproductive tract (uterus, vagina, vulva), and soft tissues.[1]
As the radiation sources can be precisely positioned at the tumour treatment site, brachytherapy enables a high dose of radiation to be applied to a small area. Furthermore, because the radiation sources are placed in or next to the target tumour, the sources maintain their position in relation to the tumour when the patient moves or if there is any movement of the tumour within the body. Therefore, the radiation sources remain accurately targeted. This enables clinicians to achieve a high level of dose conformity – i.e. ensuring the whole of the tumour receives an optimal level of radiation. It also reduces the risk of damage to healthy tissue, organs or structures around the tumour,[26] thus enhancing the chance of cure and preservation of organ function.
The use of HDR brachytherapy enables overall treatment times to be reduced compared with EBRT.[30][31] Patients receiving brachytherapy generally have to make fewer visits for radiotherapy compared with EBRT, and overall radiotherapy treatment plans can be completed in less time.[32] Many brachytherapy procedures are performed on an outpatient basis. This convenience may be particularly relevant for patients who have to work, older patients, or patients who live some distance from treatment centres, to ensure that they have access to radiotherapy treatment and adhere to treatment plans. Shorter treatment times and outpatient procedures can also help improve the efficiency of radiotherapy clinics.[33][34]
Brachytherapy can be used with the aim of curing the cancer in cases of small or locally advanced tumours, provided the cancer has not metastasized (spread to other parts of the body). In appropriately selected cases, brachytherapy for primary tumours often represents a comparable approach to surgery, achieving the same probability of cure and with similar side effects.[35][36] However, in locally advanced tumours, surgery may not routinely provide the best chance of cure and is often not technically feasible to perform. In these cases radiotherapy, including brachytherapy, offers the only chance of cure.[37][38] In more advanced disease stages, brachytherapy can be used as palliative treatment for symptom relief from pain and bleeding.
In cases where the tumour is not easily accessible or is too large to ensure an optimal distribution of irradiation to the treatment area, brachytherapy can be combined with other treatments, such as EBRT and/or surgery.[1]: Ch. 1 Combination therapy of brachytherapy exclusively with chemotherapy is rare.[39]
Cervical cancer
Brachytherapy is commonly used in the treatment of early or locally confined cervical cancer and is a standard of care in many countries.[1]: Ch. 14 [40][41][42][43] Cervical cancer can be treated with either LDR, PDR or HDR brachytherapy.[7][42][44] Used in combination with EBRT, brachytherapy can provide better outcomes than EBRT alone.[5] The precision of brachytherapy enables a high dose of targeted radiation to be delivered to the cervix, while minimising radiation exposure to adjacent tissues and organs.[41][42][45][46]
The chances of staying free of disease (disease-free survival) and of staying alive (overall survival) are similar for LDR, PDR and HDR treatments.[38][47] However, a key advantage of HDR treatment is that each dose can be delivered on an outpatient basis with a short administration time[5] providing greater convenience for many patients.
Prostate cancer
Brachytherapy to treat prostate cancer can be given either as permanent LDR seed implantation or as temporary HDR brachytherapy.[1]: Ch. 20 [48][49]
Permanent seed implantation is suitable for patients with a localised tumour and good prognosis [8][48][50][51] and has been shown to be a highly effective treatment to prevent the cancer from returning.[6][8] The survival rate is similar to that found with EBRT or surgery (radical prostatectomy), but with fewer side effects such as impotence and incontinence.[14] The procedure can be completed quickly and patients are usually able to go home on the same day of treatment and return to normal activities after 1 to 2 days.[3] Permanent seed implantation is often a less invasive treatment option compared to the surgical removal of the prostate.[3]
Temporary HDR brachytherapy is a newer approach to treating prostate cancer, but is currently less common than seed implantation. It is predominately used as to provide an extra dose in addition to EBRT (known as ‘”boost” therapy) as it offers an alternative method to deliver a high dose of radiation therapy that conforms to the shape of the tumour within the prostate, while sparing radiation exposure to surrounding tissues.[9][10][11][49][50][52] HDR brachytherapy as a boost for prostate cancer also means that the EBRT course can be shorter than when EBRT is used alone.[9][10][37][52]
Breast cancer
Radiation therapy is standard of care for women who have undergone lumpectomy or mastectomy surgery, and is an integral component of breast-conserving therapy.[1]: Ch. 18 [53] Brachytherapy can be used after surgery, before chemotherapy or palliatively in the case of advanced disease.[54] Brachytherapy to treat breast cancer is usually performed with HDR temporary brachytherapy. Post surgery, breast brachytherapy can be used as a “boost” following irradiation of the whole breast using EBRT.[53][55] More recently, brachytherapy alone is applied in a technique called APBI (accelerated partial breast irradiation), involving delivery of radiation to only the immediate region surrounding the original tumour.[12][53][55]
The main benefit of breast brachytherapy compared to EBRT is that a high dose of radiation can be precisely applied to the tumour while sparing radiation to healthy breast tissues and underlying structures such as the ribs and lungs.[54] APBI can typically be completed over the course of a week.[12] The option of brachytherapy may be particularly important in ensuring that working women, the elderly or women without easy access to a treatment centre, are able to benefit from breast-conserving therapy due to the short treatment course compared with EBRT (which often requires more visits over the course of 1–2 months).[4] Brachytherapy has demonstrated excellent local control of breast cancer at follow-up of up to 6 years post treatment.[12][56][57] A study is underway to compare patient outcomes of APBI in comparison to EBRT at up to 10 years after treatment.[58]
There are two methods that can be used to deliver breast brachytherapy:
- Interstitial breast brachytherapy using multiple catheters
- Intracavitary breast brachytherapy using a balloon catheter
Interstitial breast brachytherapy involves the temporary placement of several flexible plastic catheters in the breast tissue. These are carefully positioned to allow optimal targeting of radiation to the treatment area while sparing the surrounding breast tissue.[4] The catheters are connected to an afterloader, which delivers the planned radiation dose to the treatment area. Interstitial breast brachytherapy can be used as “boost” after EBRT, or as APBI.[55]
Intracavitary breast brachytherapy (also known as “balloon brachytherapy”) involves the placement of a single catheter into the breast cavity left after the removal of the tumour (lumpectomy).[4] The catheter can be placed at the time of the lumpectomy or postoperatively.[4] Via the catheter, a balloon is then inflated in the cavity. The catheter is then connected to an afterloader, which delivers the radiation dose through the catheter and into the balloon. Currently, intracavitary breast brachytherapy is only routinely used for APBI.[59]
There are also devices that combine the features of interstitial and intracavitary breast brachytherapy (e.g. SAVI). These devices use multiple catheters but are inserted through a single-entry point in the breast. Studies suggest the use of multiple catheters enables physicians to target the radiation more precisely.[60][61]
Skin cancer
HDR brachytherapy for nonmelanomatous skin cancer, such as basal cell carcinoma and squamous cell carcinoma, provides an alternative treatment option to surgery. This is especially relevant for cancers on the nose, ears, eyelids or lips, where surgery may cause disfigurement or require extensive reconstruction.[1]: Ch. 28 Various applicators can be used to ensure close contact between the radiation source(s) and the skin, which conform to the curvature of the skin and help ensure precision delivery of the optimal irradiation dose.[1]: Ch. 28
Brachytherapy for skin cancer provides good cosmetic results and clinical efficacy; studies with up to 5 years follow-up have shown that brachytherapy is highly effective in terms local control, and is comparable to EBRT.[62][63][64] Treatment times are typically short, providing convenience for patients.[65] It has been suggested that brachytherapy may become a standard of treatment for skin cancer in the near future.[65]
Coronary/vascular
Brachytherapy can be used in the treatment of coronary in-stent restenosis, in which a catheter is placed inside blood vessels, through which sources are inserted and removed.[66] In treating In-stent restenosis (ISR) Drug Eluting stents (DES) have been found to be superior to Intracoronary Brachytherapy (ICBT). However, there is continued interest in vascular brachytherapy for persistent restenosis in failed stents and vein grafts. The therapy has also been investigated for use in the treatment of peripheral vasculature stenosis[67] and considered for the treatment of atrial fibrillation.[68]
Side effects
The likelihood and nature of potential acute, sub-acute or long-term side-effects associated with brachytherapy depends on the location of the tumour being treated and the type of brachytherapy being used.
Acute
Acute side effects associated with brachytherapy include localised bruising, swelling, bleeding, discharge or discomfort within the implanted region. These usually resolve within a few days following completion of treatment.[69] Patients may also feel fatigued for a short period following treatment.[69][70]
Brachytherapy treatment for cervical or prostate cancer can cause acute and transient urinary symptoms such as urinary retention, urinary incontinence or painful urination (dysuria).[14][71][72] Transient increased bowel frequency, diarrhoea, constipation or minor rectal bleeding, may also occur.[14][71][72] Acute and subacute side effects usually resolve over a matter of days or a few weeks. In the case of permanent (seed) brachytherapy for prostate cancer, there is a small chance that some seeds may migrate out of the treatment region into the bladder or urethra and be passed in the urine.
Brachytherapy for skin cancer may result in a shedding of the outer layers of skin (desquamation) around the area of treatment in the weeks following therapy, which typically heals in 5–8 weeks.[1]: Ch. 28 If the cancer is located on the lip, ulceration may occur as a result of brachytherapy, but usually resolves after 4–6 weeks.[73]
Most of the acute side effects associated with brachytherapy can be treated with medication or through dietary changes, and usually disappear over time (typically a matter of weeks), once the treatment is completed. The acute side effects of HDR brachytherapy are broadly similar to EBRT.[70]
Long-term
In a small number of people, brachytherapy may cause long-term side effects due to damage or disruption of adjacent tissues or organs. Long-term side effects are usually mild or moderate in nature. For example, urinary and digestive problems may persist as a result of brachytherapy for cervical or prostate cancer, and may require ongoing management.[14][71][72]
Brachytherapy for prostate cancer may cause erectile dysfunction in approximately 15-30% of patients.[1]: Ch. 20 [28] However, the risk of erectile dysfunction is related to age (older men are at a greater risk than younger men) and also the level of erectile function prior to receiving brachytherapy. In patients who do experience erectile dysfunction, the majority of cases can successfully be treated with drugs such as Viagra.[1]: Ch. 20 Importantly, the risk of erectile dysfunction after brachytherapy is less than after radical prostatectomy.[35][71]
Brachytherapy for breast or skin cancer may cause scar tissue to form around the treatment area. In the case of breast brachytherapy, fat necrosis may occur as a result of fatty acids entering the breast tissues. This can cause the breast tissue to become swollen and tender. Fat necrosis is a benign condition and typically occurs 4–12 months after treatment and affects about 2% of patients.[74][75]
Safety around others
Patients often ask if they need to have special safety precautions around family and friends after receiving brachytherapy. If temporary brachytherapy is used, no radioactive sources remain in the body after treatment. Therefore, there is no radiation risk to friends or family from being in close proximity with them.[76]
If permanent brachytherapy is used, low dose radioactive sources (seeds) are left in the body after treatment - the radiation levels are very low and decrease over time. In addition, the irradiation only affects tissues within a few millimeters of the radioactive sources (i.e. the tumour being treated). As a precaution, some people receiving permanent brachytherapy may be advised to not hold any small children or be too close to pregnant women for a short time after treatment. Radiation oncologists or nurses can provide specific instructions to patients and advise for how long they need to be careful.[76]
Procedure

Initial planning
In order to accurately plan the brachytherapy procedure, a thorough clinical examination is performed to understand the characteristics of the tumour. In addition, a range of imaging modalities can be used to visualise the shape and size of the tumour and its relation to surrounding tissues and organs. These include x-ray radiography, ultrasound, computed axial tomography (CT or CAT) scans and magnetic resonance imaging (MRI).[1]: Ch. 5 The data from many of these sources can be used to create a 3D visualisation of the tumour and the surrounding tissues.[1]: Ch. 5
Using this information, a plan of the optimal distribution of the radiation sources can be developed. This includes consideration of how the source carriers (applicators), which are used to deliver the radiation to the treatment site, should be placed and positioned.[1]: Ch. 5 Applicators are non-radioactive and are typically needles or plastic catheters. The specific type of applicator used will depend on the type of cancer being treated and the characteristics of the target tumour.[1]: Ch. 5
This initial planning helps to ensure that ‘cold spots’ (too little irradiation) and ‘hot spots’ (too much irradiation) are avoided during treatment, as these can respectively result in treatment failure and side-effects.[45]
Insertion and imaging of the applicator(s)
Before radioactive sources can be delivered to the tumour site, the applicators have to be inserted and correctly positioned in line with the initial planning.
Imaging techniques, such as x-ray, fluoroscopy and ultrasound are typically used to help guide the placement of the applicators to their correct positions and to further refine the treatment plan.[1]: Ch. 5 CAT scans and MRI can also be used.[1]: Ch. 5 Once the applicators are inserted, they are held in place against the skin using sutures or adhesive tape to prevent them from moving. Once the applicators are confirmed as being in the correct position, further imaging can be performed to guide detailed treatment planning.[1]: Ch. 5

Creation of a virtual patient
The images of the patient with the applicators in situ are imported into treatment planning software and the patient is brought into a dedicated shielded room for treatment. The treatment planning software enables multiple 2D images of the treatment site to be translated into a 3D ‘virtual patient’, within which the position of the applicators can be defined.[1]: Ch. 5 The spatial relationships between the applicators, the treatment site and the surrounding healthy tissues within this ‘virtual patient’ are a copy of the relationships in the actual patient.
Optimizing the irradiation plan

To identify the optimal spatial and temporal distribution of radiation sources within the applicators of the implanted tissue or cavity, the treatment planning software allows virtual radiation sources to be placed within the virtual patient. The software shows a graphical representation of the distribution of the irradiation. This serves as a guide for the brachytherapy team to refine the distribution of the sources and provide a treatment plan that is optimally tailored to the anatomy of each patient before actual delivery of the irradiation begins.[77] This approach is sometimes called ‘dose-painting’.
Treatment delivery
The radiation sources used for brachytherapy are always enclosed within a non-radioactive capsule. The sources can be delivered manually, but are more commonly delivered through a technique known as ‘afterloading’.
Manual delivery of brachytherapy is limited to a few LDR applications, due to risk of radiation exposure to clinical staff.[27]
In contrast, afterloading involves the accurate positioning of non-radioactive applicators in the treatment site, which are subsequently loaded with the radiation sources. In manual afterloading, the source is delivered into the applicator by the operator.
Remote afterloading systems provide protection from radiation exposure to healthcare professionals by securing the radiation source in a shielded safe. Once the applicators are correctly positioned in the patient, they are connected to an ‘afterloader’ machine (containing the radioactive sources) through a series of connecting guide tubes. The treatment plan is sent to the afterloader, which then controls the delivery of the sources along the guide tubes into the pre-specified positions within the applicator. This process is only engaged once staff are removed from the treatment room. The sources remain in place for a pre-specified length of time, again following the treatment plan, following which they are returned along the tubes to the afterloader.
On completion of delivery of the radioactive sources, the applicators are carefully removed from the body. Patients typically recover quickly from the brachytherapy procedure, enabling it to often be performed on an outpatient basis.[26]
Between 2003 and 2012 in United States community hospitals, the rate of hospital stays with brachytherapy (internal radiation therapy) had a 24.4 percent average annual decrease among adults aged 45-64 years and a 27.3 percent average annual decrease among adults aged 65-84 years. Brachytherapy was the OR procedure with the greatest change in occurrence among hospital stays paid by Medicare and private insurance.[78]
Radiation sources
Commonly used radiation sources (radionuclides) for brachytherapy[79]
| Radionuclide | Type | Half-life | Energy |
|---|---|---|---|
| Cesium-137 (137Cs) | β−- particles | 30.17 years | 0.662 MeV |
| Cobalt-60 (60Co) | β−- particles | 5.26 years | 1.17, 1.33 MeV |
| Iridium-192 (192Ir) | γ-rays | 73.8 days | 0.38 MeV (mean) |
| Iodine-125 (125I) | Electron Capture, ε | 59.6 days | 27.4, 31.4 and 35.5 keV |
| Palladium-103 (103Pd) | Electron Capture, ε | 17.0 days | 21 keV (mean) |
| Ruthenium-106 (106Ru) | β−- particles | 1.02 years | 3.54 MeV |
| Radium-226 (226Ra) | β−- particles | 1599 years |
Electronic brachytherapy
Electronic brachytherapy involves placement of miniature low energy x-ray tube sources into a pre-positioned applicator within body/tumour cavities to rapidly deliver high doses to target tissues while maintaining low doses to distant non-target tissues.[80]
Environmental hazard
Due to the small size of brachytherapy sources and low control in early decades, there is a risk that some of these have escaped into the environment to become orphaned sources. A radium needle was found in a Prague playground in 2011, radiating 500 µSv/h from one metre away.[81][82][83]
See also
- External beam radiotherapy
- Prostate brachytherapy
- Targeted Intra-operative radiotherapy TARGIT
- Unsealed source radiotherapy
- Nuclear medicine
- Intraoperative radiation therapy
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj Gerbaulet, Alain; Pötter, Richard; Mazeron, Jean-Jacques; Meertens, Harm; Limbergen, Erik Van, eds. (2002). The GEC ESTRO handbook of brachytherapy. Leuven, Belgium: European Society for Therapeutic Radiology and Oncology. ISBN 978-90-804532-6-5.
- ^ a b c
Stewart AJ; et al. (2007). "Radiobiological concepts for brachytherapy". In Devlin P (ed.). Brachytherapy. Applications and Techniques. Philadelphia: LWW.
{{cite book}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b c BMJ Group (June 2009). "Prostate cancer: internal radiotherapy (brachytherapy)". Guardian.co.uk. Retrieved 25 September 2009.
- ^ a b c d e
Kelley JR; et al. (2007). "Breast brachytherapy". In Devlin P (ed.). Brachytherapy. Applications and Techniques. Philadelphia: LWW.
{{cite book}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b c
Viswanathan AN; et al. (2007). "Gynecologic brachytherapy". In Devlin P (ed.). Brachytherapy. Applications and Techniques. Philadelphia: LWW.
{{cite book}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Pickles, T.; Keyes, M.; Morris, W. J. (2009). "Brachytherapy or Conformal External Radiotherapy for Prostate Cancer: A Single-Institution Matched-Pair Analysis". International Journal of Radiation OncologyBiologyPhysics. 76 (1): 43–49. doi:10.1016/j.ijrobp.2009.01.081. PMID 19570619.
- ^ a b Haie-meder, C.; Chargari, C.; Rey, A.; Dumas, I.; Morice, P.; Magné, N. (2009). "DVH parameters and outcome for patients with early-stage cervical cancer treated with preoperative MRI-based low dose rate brachytherapy followed by surgery". Radiotherapy and Oncology. 93 (2): 316–321. doi:10.1016/j.radonc.2009.05.004. PMID 19586673.
- ^ a b c Battermann, J.; Boon, T.; Moerland, M. (2004). "Results of permanent prostate brachytherapy, 13 years of experience at a single institution". Radiotherapy and Oncology. 71 (1): 23–28. doi:10.1016/j.radonc.2004.01.020. PMID 15066292.
- ^ a b c Galalae, R.; Martinez, A.; Mate, T.; Mitchell, C.; Edmundson, G.; Nuernberg, N.; Eulau, S.; Gustafson, G.; Gribble, M.; Kovács, G. (2004). "Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer". International Journal of Radiation OncologyBiologyPhysics. 58 (4): 1048–1055. doi:10.1016/j.ijrobp.2003.08.003. PMID 15001244.
- ^ a b c Hoskin, P. J.; Motohashi, K.; Bownes, P.; Bryant, L.; Ostler, P. (2007). "High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomised phase three trial". Radiotherapy and Oncology. 84 (2): 114–120. doi:10.1016/j.radonc.2007.04.011. PMID 17531335.
- ^ a b Pieters, B. R.; De Back, D. Z.; Koning, C. C. E.; Zwinderman, A. H. (2009). "Comparison of three radiotherapy modalities on biochemical control and overall survival for the treatment of prostate cancer: A systematic review". Radiotherapy and Oncology. 93 (2): 168–173. doi:10.1016/j.radonc.2009.08.033. PMID 19748692.
- ^ a b c d Nelson, J. C.; Beitsch, P. D.; Vicini, F. A.; Quiet, C. A.; Garcia, D.; Snider, H. C.; Gittleman, M. A.; Zannis, V. J.; Whitworth, P. W.; Fine, R. E.; Keleher, A. J.; Kuerer, H. M. (2009). "Four-year clinical update from the American Society of Breast Surgeons MammoSite brachytherapy trial". The American Journal of Surgery. 198 (1): 83–91. doi:10.1016/j.amjsurg.2008.09.016. PMID 19268900.
- ^ Ferrer, M.; Suárez, J.; Guedea, F.; Fernández, P.; MacÍas, V.; Mariño, A.; Hervas, A.; Herruzo, I.; Ortiz, M.; Villavicencio, H.; Craven-Bratle, J.; Garin, O.; Aguiló, F.; Multicentric Spanish Group of Clinically Localized Prostate Cancer (2008). "Health-Related Quality of Life 2 Years After Treatment with Radical Prostatectomy, Prostate Brachytherapy, or External Beam Radiotherapy in Patients with Clinically Localized Prostate Cancer". International Journal of Radiation OncologyBiologyPhysics. 72 (2): 421–432. doi:10.1016/j.ijrobp.2007.12.024. PMID 18325680.
- ^ a b c d e Frank, S.; Pisters, L.; Davis, J.; Lee, A.; Bassett, R.; Kuban, D. (2007). "An Assessment of Quality of Life Following Radical Prostatectomy, High Dose External Beam Radiation Therapy and Brachytherapy Iodine Implantation as Monotherapies for Localized Prostate Cancer". The Journal of Urology. 177 (6): 2151–2156. doi:10.1016/j.juro.2007.01.134. PMID 17509305.
- ^ http://www.prlog.org/12390829-brachytherapy-market-recovery-to-reach-us-2-4-billion.html
- ^ a b Gupta VK. (1995). "Brachytherapy – past, present and future". Journal of Medical Physics. 20: 31–38.
- ^ a b c d e Nag S. "A brief history of brachytherapy". Retrieved 25 September 2009.
- ^ Goldstein, N. (1975). "Radon seed implants. Residual radioactivity after 33 years". Archives of dermatology. 111 (6): 757–759. doi:10.1001/archderm.1975.01630180085013. PMID 1137421.
- ^ Winston, P. (June 1958). "Carcinoma of the Trachea Treated by Radon Seed Implantation". The Journal of Laryngology & Otology. 72 (6): 496–499. doi:10.1017/S0022215100054232.
- ^ Oak Ridge Associated Universitie. "Seeds (ca. 1940s - 1960s)". Health Physics Historical Instrumentation Collection. Retrieved 12 November 2012.
- ^ Aronowitz, J. (2008). "The "Golden Age" of prostate brachytherapy: A cautionary tale". Brachytherapy. 7 (1): 55–59. doi:10.1016/j.brachy.2007.12.004. PMID 18299114.
- ^ Giap H and Tripuraneni P. (2007). "Vascular brachytherapy". In Devlin P (ed.). Brachytherapy. Applications and Techniques. Philadelphia: LWW.
- ^ a b c
Thomadsen BR; et al. (2005). Brachytherapy Physics. Medical Physics Publishing.
{{cite book}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b c d Mazeron, J. J.; Ardiet, J. M.; Haie-Méder, C.; Kovács, G. R.; Levendag, P.; Peiffert, D.; Polo, A.; Rovirosa, A.; Strnad, V. (2009). "GEC-ESTRO recommendations for brachytherapy for head and neck squamous cell carcinomas". Radiotherapy and Oncology. 91 (2): 150–156. doi:10.1016/j.radonc.2009.01.005. PMID 19329209.
- ^ a b
Koukourakis G; et al. (2009). "Brachytherapy for prostate cancer: A systematic review". Adv Urol. 26 (1): 63–8. PMID 2735748.
{{cite journal}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b c Nag S. (2004). "High dose rate brachytherapy: its clinical applications and treatment guidelines". Technology in Cancer Research and Treatment. 3 (3): 269–87. PMID 15161320.
- ^ a b Flynn A; et al. (2005). "Isotopes and delivery systems for brachytherapy". In Hoskin P, Coyle C (ed.). Radiotherapy in practice: brachytherapy. New York: Oxford University Press.
{{cite book}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Moule, R. N.; Hoskin, P. J. (2009). "Non-surgical treatment of localised prostate cancer". Surgical Oncology. 18 (3): 255–267. doi:10.1016/j.suronc.2009.03.006. PMID 19442516.
- ^ Dvorák; Jandík, P.; Melichar, B.; Jon, B.; Mergancová, J.; Zoul, Z.; Vacek, Z.; Petera, J. (2002). "Intraluminal high dose rate brachytherapy in the treatment of bile duct and gallbladder carcinomas". Hepato-gastroenterology. 49 (46): 916–917. PMID 12143240.
- ^
Joseph, K. J.; Alvi, R.; Skarsgard, D.; Tonita, J.; Pervez, N.; Small, C.; Tai, P. (2008). "Analysis of health related quality of life (HRQoL) of patients with clinically localized prostate cancer, one year after treatment with external beam radiotherapy (EBRT) alone versus EBRT and high dose rate brachytherapy (HDRBT)". Radiation Oncology. 3: 20. doi:10.1186/1748-717X-3-20. PMC 2494997. PMID 18627617.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Holmboe; Concato, J. (2000). "Treatment decisions for localized prostate cancer: asking men what's important". Journal of general internal medicine. 15 (10): 694–701. doi:10.1046/j.1525-1497.2000.90842.x. PMC 1495597. PMID 11089712.
- ^ Hoskin P, Coyle C, ed. (2005). Radiotherapy in practice: brachytherapy. New York: Oxford University Press. ISBN 0-19-852940-6.
- ^ Guedea, F.; Ventura, M.; Mazeron, J.; Torrecilla, J.; Bilbao, P.; Borràs, J. (2008). "Patterns of Care for Brachytherapy in Europe: Facilities and resources in brachytherapy in the European area". Brachytherapy. 7 (3): 223–230. doi:10.1016/j.brachy.2008.03.001. PMID 18579448.
- ^
Quang TS; et al. (2007). "Technological evolution in the treatment of prostate cancer". Oncology. 21.
{{cite journal}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Guedea, F.; Ferrer, M.; Pera, J.; Aguiló, F.; Boladeras, A.; Suárez, J. F.; Cunillera, O.; Ferrer, F.; Pardo, Y.; Martínez, E.; Ventura, M. (2009). "Quality of life two years after radical prostatectomy, prostate brachytherapy or external beam radiotherapy for clinically localised prostate cancer: The Catalan Institute of Oncology/Bellvitge Hospital experience". Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 11 (7): 470–478. doi:10.1007/s12094-009-0387-x. PMID 19574206.
- ^ Litwin, M. S.; Gore, J. L.; Kwan, L.; Brandeis, J. M.; Lee, S. P.; Withers, H. R.; Reiter, R. E. (2007). "Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer". Cancer. 109 (11): 2239–2247. doi:10.1002/cncr.22676. PMID 17455209.
- ^ a b Pistis, F.; Guedea, F.; Pera, J.; Gutierrez, C.; Ventura, M.; Polo, A.; Martinez, E.; Boladeras, A.; Ferrer, F.; Gabriele, P.; Linares, L. (2009). "External beam radiotherapy plus high-dose-rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology". Brachytherapy. 9 (1): 15–22. doi:10.1016/j.brachy.2009.05.001. PMID 19734106.
- ^ a b Lertsanguansinchai, P.; Lertbutsayanukul, C.; Shotelersuk, K.; Khorprasert, C.; Rojpornpradit, P.; Chottetanaprasith, T.; Srisuthep, A.; Suriyapee, S.; Jumpangern, C.; Tresukosol, D.; Charoonsantikul, C. (2004). "Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma". International Journal of Radiation OncologyBiologyPhysics. 59 (5): 1424–1431. doi:10.1016/j.ijrobp.2004.01.034. PMID 15275728.
- ^
Roddiger SJ; et al. (2006). "Neoadjuvant interstitial high-dose-rate (HDR) brachytherapy combined with systemic chemotherapy in patients with breast cancer". Strahlenther Onkol. 182 (1): 22–9. doi:10.1007/s00066-006-1454-7. PMID 16404517.
{{cite journal}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Gaffney, D.; Du Bois, A.; Narayan, K.; Reed, N.; Toita, T.; Pignata, S.; Blake, P.; Portelance, L.; Sadoyze, A.; Pötter, R.; Colombo, A.; Randall, M.; Mirza, M. R.; Trimble, E. L. (2007). "Practice Patterns of Radiotherapy in Cervical Cancer Among Member Groups of the Gynecologic Cancer Intergroup (GCIG)". International Journal of Radiation OncologyBiologyPhysics. 68 (2): 485–490. doi:10.1016/j.ijrobp.2006.12.013. PMID 17336465.
- ^ a b National Institute for Health and Clinical Excellence (March 2006). "High dose rate brachytherapy for carcinoma of the cervix". NICE. Retrieved 25 September 2009.
- ^ a b c Viswanathan AN; et al. "American Brachytherapy Society cervical cancer brachytherapy task group" (PDF). American Brachytherapy Society. Retrieved 25 September 2009.
- ^ Viswanathan, A. N.; Erickson, B. A. (2009). "Three-Dimensional Imaging in Gynecologic Brachytherapy: A Survey of the American Brachytherapy Society". International Journal of Radiation OncologyBiologyPhysics. 76 (1): 104–109. doi:10.1016/j.ijrobp.2009.01.043. PMID 19619956.
- ^ Kim, D. H.; Wang-Chesebro, A. .; Weinberg, V. .; Pouliot, J. .; Chen, L. M.; Speight, J. .; Littell, R. .; Hsu, I. C. (2009). "High–Dose Rate Brachytherapy Using Inverse Planning Simulated Annealing for Locoregionally Advanced Cervical Cancer: A Clinical Report with 2-Year Follow-Up". International Journal of Radiation OncologyBiologyPhysics. 75 (5): 1329–1334. doi:10.1016/j.ijrobp.2009.01.002. PMID 19409728.
- ^ a b Potter, R.; Kirisits, C.; Fidarova, E.; Dimopoulos, J.; Berger, D.; Tanderup, K.; Lindegaard, J. (2008). "Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma". Acta Oncologica. 47 (7): 1325–1336. doi:10.1080/02841860802282794. PMID 18661430.
- ^ Pötter, R.; Haie-Meder, C.; Van Limbergen, E. V.; Barillot, I.; De Brabandere, M. D.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; Petrow, P.; Rownd, J.; Kirisits, C.; Gec Estro Working, G. (2006). "Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology". Radiotherapy and Oncology. 78 (1): 67–77. doi:10.1016/j.radonc.2005.11.014. PMID 16403584.
- ^ Hareyama, M. .; Sakata, K. I.; Oouchi, A. .; Nagakura, H. .; Shido, M. .; Someya, M. .; Koito, K. . (2002). "High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix". Cancer. 94 (1): 117–124. doi:10.1002/cncr.10207. PMID 11815967.
- ^ a b Merrick GS; et al. "American Brachytherapy Society prostate low-dose rate task group" (PDF). American Brachytherapy Society. Retrieved 25 September 2009.
- ^ a b Hsu I-C; et al. "American Brachytherapy Society prostate high-dose rate task group" (PDF). American Brachytherapy Society. Retrieved 25 September 2009.
- ^ a b
Ash D; et al. (2005). "Prostate Cancer". In Hoskin P, Coyle C (ed.). Radiotherpay in practice: brachytherapy. New York: Oxford University Press.
{{cite book}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Morris, W. J.; Keyes, M.; Palma, D.; McKenzie, M.; Spadinger, I.; Agranovich, A.; Pickles, T.; Liu, M.; Kwan, W.; Wu, J.; Lapointe, V.; Berthelet, E.; Pai, H.; Harrison, R.; Kwa, W.; Bucci, J.; Racz, V.; Woods, R. (2009). "Evaluation of Dosimetric Parameters and Disease Response After 125Iodine Transperineal Brachytherapy for Low- and Intermediate-Risk Prostate Cancer". International Journal of Radiation OncologyBiologyPhysics. 73 (5): 1432–1438. doi:10.1016/j.ijrobp.2008.07.042. PMID 19036530.
- ^ a b
Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.4065/83.12.1364, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.4065/83.12.1364instead. - ^ a b c Keisch; et al. (February 2007). "American Brachytherapy Society breast brachytherapy task group" (PDF). American Brachytherapy Society. Retrieved 25 September 2009.
- ^ a b
Hoskin P; et al. (2005). "Breast Brachytherapy". In Hoskin P, Coyle C (ed.). Radiotherapy in practice: brachytherapy. New York: Oxford University Press. ISBN 0-19-852940-6.
{{cite book}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b c Polgár, C.; Major, T. (2009). "Current status and perspectives of brachytherapy for breast cancer". International Journal of Clinical Oncology. 14 (1): 7–0. doi:10.1007/s10147-008-0867-y. PMID 19225919.
- ^ King; Bolton, J. S.; Kuske, R. R.; Fuhrman, G. M.; Scroggins, T. G.; Jiang, X. Z. (2000). "Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer". American journal of surgery. 180 (4): 299–304. doi:10.1016/S0002-9610(00)00454-2. PMID 11113440.
- ^ Gomeziturriaga, A.; Pina, L.; Cambeiro, M.; Martínez-Regueira, F.; Aramendía, J.; Fernández-Hidalgo, O.; Martínez-Monge, R. (2008). "Early breast cancer treated with conservative surgery, adjuvant chemotherapy, and delayed accelerated partial breast irradiation with high-dose-rate brachytherapy". Brachytherapy. 7 (4): 310–315. doi:10.1016/j.brachy.2008.04.006. PMID 18778971.
- ^ Clinicaltrials.gov (February 2010). "APBI Versus EBRT Therapy After Breast Conserving Surgery for Low-risk Breast Cancer". Retrieved 17 April 2010.
- ^ Shah, A. P.; Strauss, J. B.; Kirk, M. C.; Chen, S. S.; Dickler, A. (2010). "A dosimetric analysis comparing electron beam with the MammoSite brachytherapy applicator for intact breast boost". Physica Medica. 26 (2): 80–87. doi:10.1016/j.ejmp.2009.08.004. PMID 19836283.
- ^ Scanderbeg, D; Yashar, C; White, G; Rice, R; Pawlicki, T (2010). “Evaluation of Three APBI Techniques under NSABP B-39 Guidelines.” Journal of Applied Clinical Medical Physics 11 (1): 274-280.
- ^ Yashar, C; Blair, S; Wallace, A; Scanderbeg, D (2009). “Initial Clinical Experience with the Strut-Adjusted Volume Implant Brachytherapy Applicator for Accelerated Partial Breast Irradiation.” Brachytherapy 8: 367-372.
- ^ Guix; Finestres, F.; Tello, J.; Palma, C.; Martinez, A.; Guix, J.; Guix, R. (2000). "Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds". International journal of radiation oncology, biology, physics. 47 (1): 95–102. doi:10.1016/S0360-3016(99)00547-7. PMID 10758310.
- ^ Sedda, A. F.; Rossi, G.; Cipriani, C.; Carrozzo, A. M.; Donati, P. (2008). "Dermatological high-dose-rate brachytherapy for the treatment of basal and squamous cell carcinoma". Clinical and Experimental Dermatology. 33 (6): 745–749. doi:10.1111/j.1365-2230.2008.02852.x. PMID 18681873.
- ^ Rio, E.; Bardet, E.; Ferron, C.; Peuvrel, P.; Supiot, S.; Campion, L.; Beauvillain De Montreuil, C.; Mahe, M.; Dreno, B. (2005). "Interstitial brachytherapy of periorificial skin carcinomas of the face: A retrospective study of 97 cases". International Journal of Radiation OncologyBiologyPhysics. 63 (3): 753–757. doi:10.1016/j.ijrobp.2005.03.027. PMID 15927410.
- ^ a b
Musmacher J; et al. (2006). "High dose rate brachytherapy with surface applicators: Treatment for nonmelanomatous skin cancer". Journal of Clinical Oncology. 24: 15543.
{{cite journal}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Members, A. /T. F.; Albertsson, S.; Avilés, P.; Camici, F. F.; Colombo, P. G.; Hamm, A.; Jørgensen, C.; Marco, E.; Nordrehaug, J.; Ruzyllo, W.; Urban, P.; Stone, G. W.; Wijns, W.; Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology (2005). "Guidelines for Percutaneous Coronary Interventions: the Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology". European Heart Journal. 26 (8): 804–847. doi:10.1093/eurheartj/ehi138. PMID 15769784.
- ^ Sidawy; Weiswasser, J.; Waksman, R. (2002). "Peripheral vascular brachytherapy". Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 35 (5): 1041–1047. doi:10.1067/mva.2002.123751. PMID 12021726.
- ^ Perez-castellano, N.; Villacastín, J.; Aragoncillo, P.; Fantidis, P.; Sabaté, M.; García-Torrent, M. J.; Prieto, C.; Corral, J. M.; Moreno, J.; Fernández-Ortiz, A.; Vano, E.; MacAya, C. (2006). "Pathological Effects of Pulmonary Vein beta-Radiation in a Swine Model". Journal of Cardiovascular Electrophysiology. 17 (6): 662–669. doi:10.1111/j.1540-8167.2006.00462.x. PMID 16836719.
- ^ a b Macmillan Cancer Support. "Brachytherapy". Retrieved 25 September 2009.
- ^ a b Fieler (1997). "Side effects and quality of life in patients receiving high-dose rate brachytherapy". Oncology nursing forum. 24 (3): 545–553. PMID 9127366.
- ^ a b c d Doust; Miller, E.; Duchesne, G.; Kitchener, M.; Weller, D. (2004). "A systematic review of brachytherapy. Is it an effective and safe treatment for localised prostate cancer?". Australian family physician. 33 (7): 525–529. PMID 15301172.
- ^ a b c Magné, N.; Mancy, N. C.; Chajon, E.; Duvillard, P.; Pautier, P.; Castaigne, D.; Lhommé, C.; Morice, P.; Haie-Meder, C. (2009). "Patterns of care and outcome in elderly cervical cancer patients: A special focus on brachytherapy". Radiotherapy and Oncology. 91 (2): 197–201. doi:10.1016/j.radonc.2008.08.011. PMID 18954913.
- ^
Casino AR; et al. (2006). "Brachytherapy in lip cancer". Medicina Oral. 11: E223–9.
{{cite journal}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Vicini, F.; Beitsch, P. D.; Quiet, C. A.; Keleher, A. J.; Garcia, D.; Snider Jr, H. C.; Gittleman, M. A.; Zannis, V. J.; Kuerer, H. M.; Lyden, M. (2008). "Three-year analysis of treatment efficacy, cosmesis, and toxicity by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation (APBI)". Cancer. 112 (4): 758–766. doi:10.1002/cncr.23227. PMID 18181095.
- ^ Department of Human Oncology, University of Wisconsin School of Medicine and Public Health. "Breast brachytherapy". Retrieved 25 September 2009. [dead link]
- ^ a b "Treatment Types: Brachytherapy". RT Answers. American Society for Radiation Oncology. Retrieved April 18, 2010.
- ^ Trnková P.; Pötter R.; Baltas D.; Karabis A.; Fidarova E.; Dimopoulos J.; Georg D.; Kirisits C. (2009). "New inverse planning technology for image-guided cervical cancer brachytherapy: Description and evaluation within a clinical frame" (PDF). Radiotherapy and Oncology. 93 (2): 331–340. doi:10.1016/j.radonc.2009.10.004. PMID 19846230.
- ^ Fingar KR, Stocks C, Weiss AJ, Steiner CA (December 2014). "Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003-2012". HCUP Statistical Brief #186. Rockville, MD: Agency for Healthcare Research and Quality.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ http://www.nndc.bnl.gov/wallet/wc7.html
- ^ American Association of Physicists in Medicine (February 2009). "The 2007 AAPM response to the CRCPD request for recommendations for the CRCPD's model regulations for electronic brachytherapy" (PDF). American Association of Physicists in Medicine. Retrieved 17 April 2010.
- ^ ""Radioactive" little cylinder found underground in a park in Podolí". iDNES.cz. 29 September 2011. Retrieved 12 November 2012.
- ^ Motl, Luboš. "Why a small cylinder buried in Prague radiates 500 μSv/h?". Retrieved 12 November 2012.
- ^ Falvey, Christian (29 September 2011). "Passerby stumbles upon radioactive playground thanks to wristwatch". Radio Prague. Retrieved 21 November 2012.
External links
- European Society for Therapeutic Radiology and Oncology (ESTRO)
- American Society for Therapeutic Radiology and Oncology (ASTRO)
- American Brachytherapy Society (ABS)
- BrachyAcademy - The peer-to-peer education platform for brachy professionals
- About Brachytherapy - a resource for patients and healthcare professionals
- Brachytherapy – an international disciplinary journal
- Brachytherapy at Cancer Treatment Centers of America
- Journal of Contemporary Brachytherapy
- Cancerbackup – Brachytherapy
- About breast brachytherapy
- Brachytherapy for breast cancer
- Prostate brachytherapy advisory group
- Understanding Brachytherapy: resources, FAQs, glossary
- Elekta information on Brachytherapy service provision
- A support and discussion group for Prostate Brachytherapy patients.
- Brachytherapy until 1903: questions of priority (research note)
