Methylphenylpiracetam: Difference between revisions
Removed (4R,5S) from IUPACName. |
Rewrite in progress. |
||
| Line 26: | Line 26: | ||
}} |
}} |
||
''' |
'''Methylphenylpiracetam''' is a [[derivative (chemistry)|derivative]] of [[piracetam]] and a positive [[allosteric modulator]] of the [[sigma-1 receptor]].<ref name="">{{cite journal | year = 2015 | title = Novel positive allosteric modulators of sigma-1 receptor | vauthors = Vavers E, Zvejniece L, Veinberg G, Svalbe B, Domracheva I, Vilskersts R, Dambrova M | doi = 10.1186/2193-1801-4-S1-P51 | accessdate = 2016-01-01 | quote = <small>The R-configuration enantiomers of methylphenylpiracetam are more active positive allosteric modulators of Sigma-1 receptor than S-configuration enantiomers.</small>}}</ref><ref name="pmid24490863">{{Cite journal |
||
| pmid = 24490863 |
| pmid = 24490863 |
||
| pmc = 3969087 |
| pmc = 3969087 |
||
Revision as of 05:19, 1 January 2016
| |
| Names | |
|---|---|
| IUPAC name
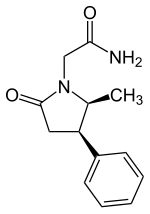
2-(5-Methyl-2-oxo-4-phenyl-pyrrolidin-1-yl)-acetamide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C13H16N2O2 | |
| Molar mass | 232.283 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methylphenylpiracetam is a derivative of piracetam and a positive allosteric modulator of the sigma-1 receptor.[1][2][3] It is a racetam and is the (4R,5S) stereoisomer of 2-(5-methyl-2-oxo-4-phenyl-pyrrolidin-1-yl)-acetamide.[2] It differs from phenylpiracetam by having a methyl group.[2]
E1R enhances cognition and has efficacy against cholinergic dysfunction in mice without affecting locomotor activity.[2]
Effects
Pretreatment with E1R enhanced the σ1R agonist PRE-084's stimulating effect and facilitated passive avoidance retention.[2] It alleviated scopolamine-induced cognitive impairment.[2]
Because E1R had no effect on locomotor activity, it was found to be free of potential motor side effects.[2]
Enantiomers
The (4R,5R) enantiomer is also a σ1R PAM.[3] In contrast, the optical antipodes (4S,5R) and (4S,5S) are less effective as a σ1R PAM.[3]
| Enantiomer | σ1R PAM effect %[3] |
|---|---|
| erythro-(4R,5S) | 222 ± 37 |
| threo-(4R,5R) | 191 ± 23 |
| erythro-(4S,5R) | 141 ± 40 |
| threo-(4S,5S) | 147 ± 31 |
See also
References
- ^ Vavers E, Zvejniece L, Veinberg G, Svalbe B, Domracheva I, Vilskersts R, Dambrova M (2015). "Novel positive allosteric modulators of sigma-1 receptor". doi:10.1186/2193-1801-4-S1-P51.
The R-configuration enantiomers of methylphenylpiracetam are more active positive allosteric modulators of Sigma-1 receptor than S-configuration enantiomers.
{{cite journal}}:|access-date=requires|url=(help); Cite journal requires|journal=(help)CS1 maint: unflagged free DOI (link) - ^ a b c d e f g Zvejniece, L; Vavers, E; Svalbe, B; Vilskersts, R; Domracheva, I; Vorona, M; Veinberg, G; Misane, I; Stonans, I; Kalvinsh, I; Dambrova, M (2014). "The cognition-enhancing activity of E1R, a novel positive allosteric modulator of sigma-1 receptors". British Journal of Pharmacology. 171 (3): 761–71. doi:10.1111/bph.12506. PMC 3969087. PMID 24490863.
- ^ a b c d Veinberg, G; Vorona, M; Zvejniece, L; Vilskersts, R; Vavers, E; Liepinsh, E; Kazoka, H; Belyakov, S; Mishnev, A; Kuznecovs, J; Vikainis, S; Orlova, N; Lebedev, A; Ponomaryov, Y; Dambrova, M (2013). "Synthesis and biological evaluation of 2-(5-methyl-4-phenyl-2-oxopyrrolidin-1-yl)-acetamide stereoisomers as novel positive allosteric modulators of sigma-1 receptor". Bioorganic & Medicinal Chemistry. 21 (10): 2764–71. doi:10.1016/j.bmc.2013.03.016. PMID 23582449.
External links
Further reading
- US patent 8791273, Kalvins, et al., "4R,5S-enantiomer of 2-(5-methyl-2-oxo-4-phenyl-pyrrolidin-1-yl)-acetamide with nootropic activity", issued 2014-07-29
- Veinberg G, Vavers E, Orlova N, Kuznecovs J, Domracheva I, Vorona M, Zvejniece L, Dambrova M (2015). "Stereochemistry of phenylpiracetam and its methyl derivative: improvement of the pharmacological profile". Chemistry of Heterocyclic Compounds. 51 (7): 601–606.
