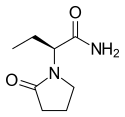
Levetiracetam
| |||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /lɛvɪtɪˈræsɪtæm/ | ||
| Trade names | Keppra, Elepsia, Spritam, others | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a699059 | ||
| License data | |||
| Pregnancy category |
| ||
| Routes of administration | By mouth, intravenous | ||
| Drug class | Racetam anticonvulsant | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | ≈100% | ||
| Protein binding | <10% | ||
| Metabolism | Enzymatic hydrolysis of acetamide group | ||
| Elimination half-life | 6–8 hrs | ||
| Excretion | Kidney | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| ECHA InfoCard | 100.121.571 | ||
| Chemical and physical data | |||
| Formula | C8H14N2O2 | ||
| Molar mass | 170.212 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Levetiracetam, sold under the brand name Keppra among others, is a medication used to treat epilepsy.[7] It is used for partial-onset, myoclonic, or tonic–clonic seizures and is taken either by mouth as an immediate or extended release formulation or by injection into a vein.[7]
Common side effects of levetiracetam include sleepiness, dizziness, feeling tired, and aggression.[7] Severe side effects may include psychosis, suicide, and allergic reactions such as Stevens–Johnson syndrome or anaphylaxis.[7] Levetiracetam is the S-enantiomer of etiracetam.[8] Its mechanism of action is not yet clear.[7]
Levetiracetam was approved for medical use in the United States in 1999[7] and is available as a generic medication.[9] In 2021, it was the 101st most commonly prescribed medication in the United States, with more than 6 million prescriptions.[10][11] It is on the World Health Organization's List of Essential Medicines.[12]
Medical uses[edit]
Focal epilepsy[edit]
Levetiracetam is effective as single-drug treatment for newly diagnosed focal epilepsy in adults.[13][14] It reduces focal seizures by 50% or more as an add-on medication.[15]
Partial-complex epilepsy[edit]
Levetiracetam is effective as add-on treatment for partial (focal) epilepsy.[16]
Generalized epilepsy[edit]
Levetiracetam is effective for treatment of generalized tonic-clonic epilepsy.[14] It has been approved in the United States as add-on treatment for myoclonic, and tonic-clonic seizures.[4] Levetiracetam has been approved in the European Union as a monotherapy treatment for epilepsy in the case of partial seizures or as an adjunctive therapy for partial, myoclonic, and tonic-clonic seizures.[17]
Levetiracetam is sometimes used off label to treat status epilepticus.[18][19]
Prevention of seizures[edit]
Based on low-quality evidence, levetiracetam is about as effective as phenytoin for prevention of early seizures after traumatic brain injury.[20] It may be effective for prevention of seizures associated with subarachnoid hemorrhages.[21]
Other[edit]
Levetiracetam has not been found to be useful for treatment of neuropathic pain,[22] nor for treatment of essential tremors.[23] Levetiracetam has not been found to be useful for treating all developmental disorders within the autism spectrum; [24][25] studies have only proven to be an effective treatment for partial, myoclonic, or tonic-clonic seizures associated with autism spectrum disorder.[26]
Special groups[edit]
Studies in female pregnant rats have shown minor fetal skeletal abnormalities when given maximum recommended human doses of levetiracetam orally throughout pregnancy and lactation.[medical citation needed]
Studies were conducted to look for increased adverse effects in the elderly population as compared to younger patients. One such study published in Epilepsy Research showed no significant increase in incidence of adverse symptoms experienced by young or elderly patients with central nervous system (CNS) disorders.[medical citation needed]
Adverse effects[edit]
The most common adverse effects of levetiracetam treatment include CNS effects such as somnolence, decreased energy, headache, dizziness, mood swings and coordination difficulties. These adverse effects are most pronounced in the first month of therapy. About 4% of patients dropped out of pre-approval clinical trials due to these side effects.[4]
About 13% of people taking levetiracetam experience adverse neuropsychiatric symptoms, which are usually mild. These include agitation, hostility, apathy, anxiety, emotional lability, and depression. Serious psychiatric adverse side effects that are reversed by drug discontinuation occur in about 1%. These include hallucinations, suicidal thoughts, or psychosis. These occurred mostly within the first month of therapy, but they could develop at any time during treatment.[27]
Although rare, Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), which appears as a painful spreading rash with redness and blistering and/or peeling skin, have been reported in patients treated with levetiracetam.[28] The incidence of SJS following exposure to anti-epileptics such as levetiracetam is about 1 in 3,000.[29]
Levetiracetam should not be used in people who have previously shown hypersensitivity to levetiracetam or any of the inactive ingredients in the tablet or oral solution. Such hypersensitivity reactions include, but are not limited to, unexplained rash with redness or blistered skin, difficulty breathing, and tightness in the chest or airways.[4]
In a study, the incidence of decreased bone mineral density of patients on levetiracetam was significantly higher than those for other epileptic medications.[30]
Suicide[edit]
Levetiracetam, along with other anti-epileptic drugs, can increase the risk of suicidal behavior or thoughts. People taking levetiracetam should be monitored closely for signs of worsening depression, suicidal thoughts or tendencies, or any altered emotional or behavioral states.[4]
Kidney and liver[edit]
Kidney impairment decreases the rate of elimination of levetiracetam from the body. Individuals with reduced kidney function may require dose adjustments. Kidney function can be estimated from the rate of creatinine clearance.[4]
Dose adjustment of levetiracetam is not necessary in liver impairment.[4]
Drug interactions[edit]
No significant pharmacokinetic interactions were observed between levetiracetam or its major metabolite and concomitant medications.[31] The pharmacokinetic profile of levetiracetam is not influenced by phenytoin, phenobarbital, primidone, carbamazepine, valproic acid, lamotrigine, gabapentin, digoxin, ethinylestradiol, or warfarin.[32]
Mechanism of action[edit]
The exact mechanism by which levetiracetam acts to treat epilepsy is unknown. Levetiracetam does not exhibit pharmacologic actions similar to that of classical anticonvulsants. It does not inhibit voltage-dependent Na+ channels, does not affect GABAergic transmission, and does not bind to GABAergic or glutamatergic receptors.[33] However, the drug binds to SV2A,[34] a synaptic vesicle glycoprotein, and inhibits presynaptic calcium channels,[35] reducing neurotransmitter release and acting as a neuromodulator. This is believed to impede impulse conduction across synapses.[36]
Pharmacokinetics[edit]
Absorption[edit]
The absorption of levetiracetam tablets and oral solution is rapid and essentially complete. The bioavailability of levetiracetam is close to 100 percent, and the effect of food on absorption is minor.[4]
Distribution[edit]
The volume of distribution of levetiracetam is similar to total body water. Levetiracetam modestly binds to plasma proteins (less than 10%).[4]
Metabolism[edit]
Levetiracetam does not undergo extensive metabolism, and the metabolites formed are not active and do not exert pharmacological activity. Metabolism of levetiracetam is not by liver cytochrome P450 enzymes, but through other metabolic pathways such as hydrolysis and hydroxylation.[4]
Excretion[edit]
In persons with normal kidney function, levetiracetam is eliminated from the body primarily by the kidneys with about 66 percent of the original drug passed unchanged into urine. The plasma half-life of levetiracetam in adults is about 6 to 8 hours[4], although the mean CSF half life of approx. 24 hours better reflects levels at site of action.[37]
Analogues[edit]
Brivaracetam, a chemical analogue to levetiracetam, is a racetam derivative with similar properties.
Society and culture[edit]
Levetiracetam is available as regular and extended release oral formulations and as intravenous formulations.[38]
The immediate release tablet has been available as a generic in the United States since 2008, and in the UK since 2011.[39][15] The patent for the extended release tablet will expire in 2028.[40]
The branded version Keppra is manufactured by UCB Pharmaceuticals S.A.[3][4][5][6]
In 2015, Aprecia's 3D-printed orally disintegrating tablet form of the drug was approved by the FDA, under the trade name Spritam.[41] Some have said that the drug has been improved by 3D printing, as the formula used now has improved disintegration properties.[42]
Legal status[edit]
Australia[edit]
Levetiracetam is a Schedule 4 substance in Australia under the Poisons Standard (February 2020).[43] A Schedule 4 substance is classified as "Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by State or Territory legislation to prescribe and should be available from a pharmacist on prescription."[43]
Japan[edit]
Under Japanese law, levetiracetam and other racetams cannot be brought into the country except for personal use by a traveler for whom it has been prescribed.[44] Travelers who plan to bring more than a month's worth must apply for an import certificate, known as a Yakkan Shoumei (薬監証明, yakkan shōmei).[45]
Research[edit]
Levetiracetam has been studied in the past for treating symptoms of neurobiological conditions such as Tourette syndrome,[46] and anxiety disorder.[47] However, its most serious adverse effects are behavioral, and its benefit-risk ratio in these conditions is not well understood.[47]
Levetiracetam is being tested as a drug to reduce hyperactivity in the hippocampus in Alzheimer's disease.[48]
Additionally, Levetiracetam has been experimentally shown to reduce Levodopa-induced dyskinesia,[49] a type of movement disorder, or dyskinesia associated with the use of Levodopa, a medication used to treat Parkinson's disease.
Of the ten medications evaluated in a 2023 systematic review of the literature, levetiracetam was found to be the only medication with sufficient evidence showing that it may cause seizure freedom in some infants.[50] Further, adverse effects from levetiracetam were rarely severe enough for the medication to be discontinued in this age group. Because available research included only 2 published studies reporting seizure freedom rates, however, the strength of the evidence was judged to be low.[50]
References[edit]
- ^ "Levetiracetam Use During Pregnancy". Drugs.com. Archived from the original on 6 March 2019. Retrieved 5 March 2019.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b "Keppra 100 mg/ml concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 24 October 2021. Retrieved 9 September 2020.
- ^ a b c d e f g h i j k l "Keppra- levetiracetam tablet, film coated Keppra- levetiracetam solution". DailyMed. 5 November 2019. Archived from the original on 7 August 2020. Retrieved 9 September 2020.
- ^ a b "Keppra XR- levetiracetam tablet, film coated, extended release". DailyMed. 4 November 2019. Archived from the original on 29 July 2021. Retrieved 9 September 2020.
- ^ a b "Keppra- levetiracetam injection, solution, concentrate". DailyMed. 4 November 2019. Archived from the original on 21 January 2016. Retrieved 9 September 2020.
- ^ a b c d e f "Levetiracetam Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 24 March 2019. Retrieved 14 January 2019.
- ^ Cavanna AE (2018). Behavioural Neurology of Anti-Epileptic Drugs: A Practical Guide. Oxford University Press. p. 17. ISBN 9780198791577.
- ^ British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 319. ISBN 9780857113382.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Levetiracetam - Drug Usage Statistics". ClinCalc. Archived from the original on 4 January 2024. Retrieved 14 January 2024.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Lattanzi S, Zaccara G, Giovannelli F, Grillo E, Nardone R, Silvestrini M, et al. (January 2019). "Antiepileptic monotherapy in newly diagnosed focal epilepsy. A network meta-analysis". Acta Neurologica Scandinavica. 139 (1): 33–41. doi:10.1111/ane.13025. PMID 30194755. S2CID 52174058.
- ^ a b Nevitt SJ, Sudell M, Cividini S, Marson AG, Tudur Smith C (April 2022). "Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data". The Cochrane Database of Systematic Reviews. 2022 (4): CD011412. doi:10.1002/14651858.CD011412.pub4. PMC 8974892. PMID 35363878.
- ^ a b Mbizvo GK, Dixon P, Hutton JL, Marson AG (September 2012). "Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review". The Cochrane Database of Systematic Reviews. 2012 (9): CD001901. doi:10.1002/14651858.cd001901.pub2. PMC 7061650. PMID 22972056.
- ^ Slater J, Chung S, Huynh L, Duh MS, Gorin B, McMicken C, et al. (July 2018). "Efficacy of antiepileptic drugs in the adjunctive treatment of refractory partial-onset seizures: Meta-analysis of pivotal trials". Epilepsy Research. 143: 120–129. doi:10.1016/j.eplepsyres.2017.10.004. PMID 29784458.
- ^ BNF 59. BMA & RPSGB. 2010.
- ^ Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. (Neurocritical Care Society Status Epilepticus Guideline Writing Committee) (August 2012). "Guidelines for the evaluation and management of status epilepticus". Neurocritical Care. 17 (1): 3–23. doi:10.1007/s12028-012-9695-z. PMID 22528274. S2CID 4675838.
- ^ Meierkord H, Boon P, Engelsen B, Göcke K, Shorvon S, Tinuper P, Holtkamp M (March 2010). "EFNS guideline on the management of status epilepticus in adults". European Journal of Neurology. 17 (3): 348–355. doi:10.1111/j.1468-1331.2009.02917.x. PMID 20050893. S2CID 1032139.
- ^ Khan NR, VanLandingham MA, Fierst TM, Hymel C, Hoes K, Evans LT, et al. (December 2016). "Should Levetiracetam or Phenytoin Be Used for Posttraumatic Seizure Prophylaxis? A Systematic Review of the Literature and Meta-analysis". Neurosurgery. 79 (6): 775–782. doi:10.1227/NEU.0000000000001445. PMID 27749510.
- ^ Shah D, Husain AM (December 2009). "Utility of levetiracetam in patients with subarachnoid hemorrhage". Seizure. 18 (10): 676–679. doi:10.1016/j.seizure.2009.09.003. PMID 19864168. S2CID 7555384.
- ^ Crawford-Faucher A, Huijon RM (July 2015). "The Role of Levetiracetam in Treating Chronic Neuropathic Pain Symptoms". American Family Physician. 92 (1): 23–24. PMID 26132123. Archived from the original on 29 August 2021. Retrieved 1 September 2017.
- ^ Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB, et al. (November 2011). "Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology". Neurology. 77 (19): 1752–1755. doi:10.1212/WNL.0b013e318236f0fd. PMC 3208950. PMID 22013182.
- ^ Volkmar F, Siegel M, Woodbury-Smith M, King B, McCracken J, State M (February 2014). "Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder". Journal of the American Academy of Child and Adolescent Psychiatry. 53 (2): 237–257. doi:10.1016/j.jaac.2013.10.013. PMID 24472258.
- ^ Hirota T, Veenstra-Vanderweele J, Hollander E, Kishi T (April 2014). "Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis". Journal of Autism and Developmental Disorders. 44 (4): 948–957. doi:10.1007/s10803-013-1952-2. PMID 24077782. S2CID 36509998.
- ^ Frye RE, Rossignol D, Casanova MF, Brown GL, Martin V, Edelson S, et al. (September 2013). "A review of traditional and novel treatments for seizures in autism spectrum disorder: findings from a systematic review and expert panel". Frontiers in Public Health. 1: 31. doi:10.3389/fpubh.2013.00031. PMC 3859980. PMID 24350200.
- ^ Gambardella A, Labate A, Colosimo E, Ambrosio R, Quattrone A (February 2008). "Monotherapy for partial epilepsy: focus on levetiracetam". Neuropsychiatric Disease and Treatment. 4 (1): 33–38. doi:10.2147/NDT.S1655. PMC 2515905. PMID 18728811.
- ^ Zou LP, Ding CH, Song ZJ, Li XF (December 2012). "Stevens-Johnson syndrome induced by levetiracetam". Seizure. 21 (10): 823–825. doi:10.1016/j.seizure.2012.09.005. PMID 23036769.
- ^ Griebel ML (1998). "Acute management of hypersensitivity reactions and seizures". Epilepsia. 39 (Suppl 7): S17–S21. doi:10.1111/j.1528-1157.1998.tb01680.x. PMID 9798757. S2CID 10305861.
- ^ Beniczky SA, Viken J, Jensen LT, Andersen NB (July 2012). "Bone mineral density in adult patients treated with various antiepileptic drugs". Seizure. 21 (6): 471–472. doi:10.1016/j.seizure.2012.04.002. PMID 22541979.
- ^ Browne TR, Szabo GK, Leppik IE, Josephs E, Paz J, Baltes E, Jensen CM (June 2000). "Absence of pharmacokinetic drug interaction of levetiracetam with phenytoin in patients with epilepsy determined by new technique". Journal of Clinical Pharmacology. 40 (6): 590–595. doi:10.1002/j.1552-4604.2000.tb05984.x. PMID 10868309. S2CID 35576679.
- ^ Gidal BE, Baltès E, Otoul C, Perucca E (2005). "Effect of levetiracetam on the pharmacokinetics of adjunctive antiepileptic drugs: a pooled analysis of data from randomized clinical trials". Epilepsy Research. 64 (1–2): 1–11. doi:10.1016/j.eplepsyres.2005.01.005. PMID 15823510. S2CID 13555324.
- ^ Deshpande LS, Delorenzo RJ (2014). "Mechanisms of levetiracetam in the control of status epilepticus and epilepsy". Frontiers in Neurology. 5: 11. doi:10.3389/fneur.2014.00011. PMC 3907711. PMID 24550884.
- ^ Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B (June 2004). "The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam". Proceedings of the National Academy of Sciences of the United States of America. 101 (26): 9861–9866. Bibcode:2004PNAS..101.9861L. doi:10.1073/pnas.0308208101. PMC 470764. PMID 15210974.
- ^ Vogl C, Mochida S, Wolff C, Whalley BJ, Stephens GJ (August 2012). "The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway". Molecular Pharmacology. 82 (2): 199–208. doi:10.1124/mol.111.076687. PMID 22554805. S2CID 8333373.
- ^ Rogawski MA (June 2006). "Diverse mechanisms of antiepileptic drugs in the development pipeline". Epilepsy Research. 69 (3): 273–294. doi:10.1016/j.eplepsyres.2006.02.004. PMC 1562526. PMID 16621450.
- ^ Steinhoff BJ, Staack AM (January 2019). "Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience". Therapeutic Advances in Neurological Disorders. 12: 1756286419873518. doi:10.1177/1756286419873518. PMC 6734620. PMID 31523280.
- ^ "Levetiracetam Injection Prescribing Information" (PDF). Archived (PDF) from the original on 1 November 2018. Retrieved 4 November 2015.
- ^ Branch Website Management. "Patent Terms Extended Under 35 USC §156". www.uspto.gov. Archived from the original on 15 November 2015. Retrieved 5 November 2015.
- ^ Webber K (12 September 2011). "FDA Access Data" (PDF). ANDA 091291. Department of Health and Human Services. Archived (PDF) from the original on 4 March 2016. Retrieved 4 November 2015.
- ^ "FDA approves the first 3D-printed drug product". KurzweilAI. 13 October 2015. Archived from the original on 16 October 2015. Retrieved 14 October 2015.
- ^ Mohammed AA, Algahtani MS, Ahmad MZ, Ahmad J, Kotta S (1 December 2021). "3D Printing in medicine: Technology overview and drug delivery applications". Annals of 3D Printed Medicine. 3D-Printed Medicine: From today's accomplishments to tomorrow's promises. 4: 100037. doi:10.1016/j.stlm.2021.100037. ISSN 2666-9641. S2CID 244421616.
- ^ a b Poisons Standard February 2020 Archived 2 March 2020 at the Wayback Machine. comlaw.gov.au
- ^ "Information for those who are bringing medicines for personal use into Japan". Japanese Ministry of Health, Labour and Welfare. Archived from the original on 12 November 2020. Retrieved 10 November 2020.
- ^ "Q&A for those who are importing medicines into Japan" (PDF). Archived from the original (PDF) on 11 November 2020. Retrieved 10 November 2020.
- ^ Martínez-Granero MA, García-Pérez A, Montañes F (June 2010). "Levetiracetam as an alternative therapy for Tourette syndrome". Neuropsychiatric Disease and Treatment. 6: 309–316. doi:10.2147/ndt.s6371. PMC 2898169. PMID 20628631.
- ^ a b Farooq MU, Bhatt A, Majid A, Gupta R, Khasnis A, Kassab MY (March 2009). "Levetiracetam for managing neurologic and psychiatric disorders". American Journal of Health-System Pharmacy. 66 (6): 541–561. doi:10.2146/ajhp070607. PMID 19265183.
- ^ Haberman RP, Branch A, Gallagher M (July 2017). "Targeting Neural Hyperactivity as a Treatment to Stem Progression of Late-Onset Alzheimer's Disease". Neurotherapeutics. 14 (3): 662–676. doi:10.1007/s13311-017-0541-z. PMC 5509635. PMID 28560709.
- ^ Du H, Nie S, Chen G, Ma K, Xu Y, Zhang Z, et al. (27 January 2015). Reichmann H, Alves da Costa C (eds.). "Levetiracetam Ameliorates L-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats Inducing Critical Molecular Changes in the Striatum". Parkinson's Disease. 2015 (1). London, England, United Kingdom: Hindawi Publishing Corporation: 253878. doi:10.1155/2015/253878. LCCN 2010247839. PMC 4322303. PMID 25692070.
- ^ a b Treadwell JR, Wu M, Tsou AY (October 2022). Management of Infantile Epilepsies (Report). Rockville (MD): Agency for Healthcare Research and Quality (AHRQ) (US). doi:10.23970/ahrqepccer252. PMID 36383706. 22(23)-EHC004 Report No.: 2021-SR-01. Archived from the original on 5 July 2023. Retrieved 12 July 2023.