Oxandrolone: Difference between revisions
Exercisephys (talk | contribs) m →top: specify oral in comment |
Exercisephys (talk | contribs) →top: add MEDRS ref for hereditary angioedema |
||
| Line 51: | Line 51: | ||
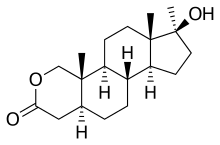
'''Oxandrolone''', also known as '''Oxandrin''', is an [[anabolic steroid]] first made by Raphael Pappo while at [[Searle Laboratories]], now [[Pfizer|Pfizer Inc.]], under the trademark '''Anavar''', and introduced into the United States in 1964. It is a synthetic derivative of [[dihydrotestosterone]] with an oxygen atom replacing the 2 carbon and methylation in the 17 position. |
'''Oxandrolone''', also known as '''Oxandrin''', is an [[anabolic steroid]] first made by Raphael Pappo while at [[Searle Laboratories]], now [[Pfizer|Pfizer Inc.]], under the trademark '''Anavar''', and introduced into the United States in 1964. It is a synthetic derivative of [[dihydrotestosterone]] with an oxygen atom replacing the 2 carbon and methylation in the 17 position. |
||
The drug was prescribed to promote muscle regrowth in disorders which cause involuntary weight loss, and is used as part of treatment for [[HIV/AIDS]]. It had also been shown to be partially successful in treating cases of [[osteoporosis]]. However, in part due to bad publicity from its abuses by [[bodybuilding|bodybuilders]], production of Anavar was discontinued by Searle Laboratories in 1989. It was picked up by Bio-Technology General Corporation, which changed its name to [[Savient Pharmaceuticals]] who, following successful clinical trials in 1995, released it under the tradename Oxandrin.<ref name="llewellyn"/> BTG subsequently won approvals for [[orphan drug]] status by the [[Food and Drug Administration]] (FDA) for treating [[alcoholic hepatitis]], [[Turner syndrome]], and HIV-induced [[wasting syndrome|weight loss]]. It is also indicated as an offset to [[protein catabolism]] caused by long-term administration of [[corticosteroids]]. In addition, the drug has shown positive results in treating [[anemia]] and [[hereditary angioedema]].<ref name="llewellyn"/> Oxandrolone is also well-established as a safe treatment for patients recovering from severe burns.<ref name="LiGuo2016">{{cite journal|last1=Li|first1=Hui|last2=Guo|first2=Yinan|last3=Yang|first3=Zhenyu|last4=Roy|first4=Mridul|last5=Guo|first5=Qulian|title=The efficacy and safety of oxandrolone treatment for patients with severe burns: A systematic review and meta-analysis|journal=Burns|volume=42|issue=4|year=2016|pages=717–727|issn=03054179|doi=10.1016/j.burns.2015.08.023}}</ref> |
The drug was prescribed to promote muscle regrowth in disorders which cause involuntary weight loss, and is used as part of treatment for [[HIV/AIDS]]. It had also been shown to be partially successful in treating cases of [[osteoporosis]]. However, in part due to bad publicity from its abuses by [[bodybuilding|bodybuilders]], production of Anavar was discontinued by Searle Laboratories in 1989. It was picked up by Bio-Technology General Corporation, which changed its name to [[Savient Pharmaceuticals]] who, following successful clinical trials in 1995, released it under the tradename Oxandrin.<ref name="llewellyn"/> BTG subsequently won approvals for [[orphan drug]] status by the [[Food and Drug Administration]] (FDA) for treating [[alcoholic hepatitis]], [[Turner syndrome]], and HIV-induced [[wasting syndrome|weight loss]]. It is also indicated as an offset to [[protein catabolism]] caused by long-term administration of [[corticosteroids]]. In addition, the drug has shown positive results in treating [[anemia]] and [[hereditary angioedema]].<ref name="llewellyn"/><ref name="Bork2012">{{cite journal|last1=Bork|first1=Konrad|title=Current Management Options for Hereditary Angioedema|journal=Current Allergy and Asthma Reports|volume=12|issue=4|year=2012|pages=273–280|issn=1529-7322|doi=10.1007/s11882-012-0273-4}}</ref> Oxandrolone is also well-established as a safe treatment for patients recovering from severe burns.<ref name="LiGuo2016">{{cite journal|last1=Li|first1=Hui|last2=Guo|first2=Yinan|last3=Yang|first3=Zhenyu|last4=Roy|first4=Mridul|last5=Guo|first5=Qulian|title=The efficacy and safety of oxandrolone treatment for patients with severe burns: A systematic review and meta-analysis|journal=Burns|volume=42|issue=4|year=2016|pages=717–727|issn=03054179|doi=10.1016/j.burns.2015.08.023}}</ref> |
||
<!-- Many people believe that oxandrolone is considerably less hepatotoxic than most oral anabolics, but I don't know of a reliable source for that in the general case. We'll make due with this for now. --> |
<!-- Many people believe that oxandrolone is considerably less hepatotoxic than most oral anabolics, but I don't know of a reliable source for that in the general case. We'll make due with this for now. --> |
||
Revision as of 22:49, 18 June 2016
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604024 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97% |
| Metabolism | Hepatic |
| Elimination half-life | 9 hours |
| Excretion | Urinary:90%; Fecal:7% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.158 |
| Chemical and physical data | |
| Formula | C19H30O3 |
| Molar mass | 306.44 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Oxandrolone, also known as Oxandrin, is an anabolic steroid first made by Raphael Pappo while at Searle Laboratories, now Pfizer Inc., under the trademark Anavar, and introduced into the United States in 1964. It is a synthetic derivative of dihydrotestosterone with an oxygen atom replacing the 2 carbon and methylation in the 17 position.
The drug was prescribed to promote muscle regrowth in disorders which cause involuntary weight loss, and is used as part of treatment for HIV/AIDS. It had also been shown to be partially successful in treating cases of osteoporosis. However, in part due to bad publicity from its abuses by bodybuilders, production of Anavar was discontinued by Searle Laboratories in 1989. It was picked up by Bio-Technology General Corporation, which changed its name to Savient Pharmaceuticals who, following successful clinical trials in 1995, released it under the tradename Oxandrin.[2] BTG subsequently won approvals for orphan drug status by the Food and Drug Administration (FDA) for treating alcoholic hepatitis, Turner syndrome, and HIV-induced weight loss. It is also indicated as an offset to protein catabolism caused by long-term administration of corticosteroids. In addition, the drug has shown positive results in treating anemia and hereditary angioedema.[2][3] Oxandrolone is also well-established as a safe treatment for patients recovering from severe burns.[4]
Although some orally-administered anabolic steroids are hepatotoxic, oxandrolone causes little or no detectable stress to burn victims' livers at clinical doses.[4]
Oxandrolone has long been used in small doses to treat idiopathic short stature in children. Although it accelerates growth, it is unlikely to increase adult height, and it is has therefore largely been replaced by growth hormone for this use.[5]
In the United States, oxandrolone is categorized as a Schedule III controlled substance under the Controlled Substances Act.[6]
Dosage
Children with idiopathic short stature are typically given low doses,[5] while people recovering from burns are typically given about medium doses.[4] Bodybuilders often take doses much higher than these.[citation needed]
Names
It has also been sold under the trade names Lonavar (Argentina, Australia), Lipidex (Brazil), Antitriol (Spain), Anatrophill (France), and Protivar.[2]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c Llewellyn, William (2011). William Llewellyn's Anabolics. Jupiter, FL: Molecular Nutrition, LLC. p. 610-616. ISBN 978-0-9828280-1-4.
- ^ Bork, Konrad (2012). "Current Management Options for Hereditary Angioedema". Current Allergy and Asthma Reports. 12 (4): 273–280. doi:10.1007/s11882-012-0273-4. ISSN 1529-7322.
- ^ a b c Li, Hui; Guo, Yinan; Yang, Zhenyu; Roy, Mridul; Guo, Qulian (2016). "The efficacy and safety of oxandrolone treatment for patients with severe burns: A systematic review and meta-analysis". Burns. 42 (4): 717–727. doi:10.1016/j.burns.2015.08.023. ISSN 0305-4179.
- ^ a b Wit, Jan M.; Oostdijk, Wilma (2015). "Novel approaches to short stature therapy". Best Practice & Research Clinical Endocrinology & Metabolism. 29 (3): 353–366. doi:10.1016/j.beem.2015.01.003. ISSN 1521-690X.
- ^ "Controlled Substances Act". United States Food and Drug Administration. 11 June 2009. Retrieved 17 June 2016.
External links
- Oxandrin Homepage, savientpharma.com (via archive.org)
- Oxandrin Label, fda.gov (retrieved 23 October 2009)
- "Oxandrolone Side Effects, Interactions and Information". drugs.com.
