Clomifene
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Clomid, Serophene, others[1] |
| Other names | Clomiphene; Chloramifene; Chloramiphene; MRL-41; MRL/41; NSC-35770 |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Selective estrogen receptor modulator; Progonadotropin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | High (>90%) |
| Metabolism | Liver CYP2D6 (with enterohepatic circulation)[2] |
| Metabolites | 4-Hydroxyclomiphene (4-OH-CLO), 4-Hydroxy-N-desethylclomiphene (4-OH-DE-CLO) |
| Elimination half-life | 4 – 7 days [2][3][4] active metabolites: |
| Excretion | Mainly feces, some in urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.826 |
| Chemical and physical data | |
| Formula | C26H28ClNO |
| Molar mass | 405.97 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome.[5] It is taken by mouth.[5]
Common side effects include pelvic pain and hot flashes.[5] Other side effects can include changes in vision, vomiting, trouble sleeping, ovarian cancer, and seizures.[5][6] It is not recommended in people with liver disease or abnormal vaginal bleeding of unknown cause or who are pregnant.[6][7] Clomifene is in the selective estrogen receptor modulator (SERM) family of medication and is a nonsteroidal medication.[7][8] It works by causing the release of GnRH by the hypothalamus, and subsequently gonadotropin from the anterior pituitary.[6]
Clomifene was approved for medical use in the United States in 1967.[5] It is on the World Health Organization's List of Essential Medicines, under the category "Ovulation inducers" (Complementary List).[9] Its introduction began the era of assisted reproductive technology.[10]
Clomifene (particularly the purified enclomiphene isomer) has also been found to have a powerful ability to boost or restore testosterone levels in hypogonadal men.[11]
Medical uses[edit]
Ovulation induction[edit]
Clomifene is one of several alternatives for ovulation induction in those who are infertile due to anovulation or oligoovulation.[12] Evidence is lacking for the use of clomifene in those who are infertile without a known reason.[13] In such cases, studies have observed a clinical pregnancy rate 5.6% per cycle with clomifene treatment vs. 1.3%–4.2% per cycle without treatment.[12]
Other uses[edit]
Clomifene has also been used with other assisted reproductive technology to increase success rates of these other modalities.[14]
Testosterone replacement therapy[edit]
Clomifene is sometimes used in the treatment of male hypogonadism as an alternative to testosterone replacement therapy.[15][non-primary source needed] It has been found to increase testosterone levels by 2- to 2.5-times in hypogonadal men at such dosages.[15][16] Despite the use of questionnaires in testosterone replacement comparator trials being called into question, clomifene's lower cost, therapeutic benefits, and greater value towards hypogonadism improvement have been noted.[17][non-primary source needed]
Clomifene consists of two stereoisomers in equal proportion: enclomifene and zuclomifene. Zuclomifene has pro-estrogenic properties, whereas enclomifene is pro-androgenic, i.e. it promotes testosterone production through stimulation of the HPG axis. For this reason, purified enclomifene isomer has been found to be twice as effective in boosting testosterone compared to the standard mix of both isomers.[11] Additionally, enclomifene has a half-life of just 10 hours,[4] but zuclomifene has a half-life on the order of several days to a week, so if the goal is to boost testosterone, taking regular clomifene may produce far longer-lasting pro-estrogenic effects than pro-androgenic effects.[18]
Gynecomastia[edit]
Clomifene has been used in the treatment of gynecomastia.[19] It has been found to be useful in the treatment of some cases of gynecomastia but it is not as effective as tamoxifen or raloxifene for this indication.[20] It has shown variable results for gynecomastia (probably because the zuclomifene isomer is estrogenic), and hence is not recommended for treatment of the condition.[21] Pure enclomifene isomer is likely to be more effective than clomifene at treating gynecomastia, because of the lack of the zuclomifene isomer (as noted above).[medical citation needed]
Due to its long half-life, zuclomifene can be detected in urine for at least 261 days after discontinuation[22] (261 days after discontinuation with a half-life of 30 days, there is still 0.24% of the peak level of zuclomifene being excreted, whereas with a half-life of 10 hours, enclomifene reaches the same 0.24% level in less than 4 days[medical citation needed]).
Because of its potential for boosting testosterone, clomifene is listed as banned for use by competitive sportsmen, both in and out of competition, by the World Anti-Doping Agency, absent an organic etiology of primary hypogonadism.[citation needed]
Contraindications[edit]
Contraindications include an allergy to the medication, pregnancy, prior liver problems, abnormal vaginal bleeding of unclear cause, ovarian cysts other than those due to polycystic ovarian syndrome, unmanaged adrenal or thyroid problems, and pituitary tumors.[7]
Side effects[edit]
The most common adverse drug reaction associated with the use of clomifene (>10% of people) is reversible ovarian enlargement.[7]
Less common effects (1–10% of people) include visual symptoms (blurred vision, double vision, floaters, eye sensitivity to light, scotomata), headaches, vasomotor flushes (or hot flashes), light sensitivity and pupil constriction, abnormal uterine bleeding and/or abdominal discomfort.[7]
Rare adverse events (<1% of people) include: high blood level of triglycerides, liver inflammation, reversible baldness and/or ovarian hyperstimulation syndrome.[7]
Clomifene can lead to multiple ovulation, hence increasing the chance of twins (10% of births instead of ~1% in the general population) and triplets.[medical citation needed]
Rates of birth defects and miscarriages do not appear to change with the use of clomifene for fertility.[7] Clomifene has been associated with liver abnormalities and a couple of cases of hepatotoxicity.[23]
Cancer risk[edit]
Some studies have suggested that clomifene if used for more than a year may increase the risk of ovarian cancer.[13] This may only be the case in those who have never been and do not become pregnant.[24] Subsequent studies have failed to support those findings.[12][25]
Clomifene has been shown to be associated with an increased risk of malignant melanomas and thyroid cancer.[3] Thyroid cancer risk was not associated with the number of pregnancies carried to viability.[26]
Pharmacology[edit]
Pharmacodynamics[edit]
Selective estrogen receptor modulator activity[edit]
Clomifene is a nonsteroidal triphenylethylene derivative that acts as a selective estrogen receptor modulator (SERM).[14] It consists of a non-racemic mixture of zuclomifene (~38%) and enclomifene (~62%), each of which has unique pharmacologic properties.[27] It is a mixed agonist and antagonist of the estrogen receptor (ER). Clomifene activates the ERα in the setting of low baseline estrogen levels and partially blocks the receptor in the context of high baseline estrogen levels.[16] Conversely, it is an antagonist of the ERβ.[16] Clomifene has antiestrogenic effects in the uterus.[28] There is little clinical research on the influence of clomifene in many target tissues, such as lipids, the cardiovascular system, and the breasts.[28][29] Positive effects of clomifene on bone have been observed.[16][28][29] Clomifene has been found to decrease insulin-like growth factor 1 (IGF-1) levels in women.[30]
Clomifene is a long-acting ER ligand, with a nuclear retention of greater than 48 hours.[31] Clomifene is a prodrug being activated via similar metabolic pathways as the related triphenylethylene SERMs tamoxifen and toremifene.[32][33] The affinity of clomifene for the ER relative to estradiol ranges from 0.1 to 12% in different studies, which is similar to the range for tamoxifen (0.06–16%).[34][35][36] 4-Hydroxyclomifene, a major active metabolite of clomifene, and afimoxifene (4-hydroxytamoxifen), a major active metabolite of tamoxifen, show 89–251% and 41–246% of the affinity of estradiol for the ER in human MCF-7 breast cancer cells, respectively.[37][38] The ER affinities of the isomers of 4-hydroxyclomifene were 285% for (E)-4-hydroxyclomifene and 16% for (Z)-4-hydroxyclomifene relative to estradiol.[37] 4-Hydroxy-N-desmethylclomifene has similar affinity to 4-hydroxyclomifene for the ER.[33] In one study, the affinities of clomifene and its metabolites for the ERα were ~100 nM for clomifene, ~2.4 nM for 4-hydroxyclomifene, ~125 nM for N-desmethylclomifene, and ~1.4 nM for 4-hydroxy-N-desmethylclomifene.[33]
Even though clomifene has some estrogenic effect, the antiestrogenic property is believed to be the primary source for stimulating ovulation.[5] Clomifene appears to act mostly in the hypothalamus where it depletes hypothalamic ERs and blocks the negative feedback effect of circulating endogenous estradiol, which in turn results in an increase in hypothalamic gonadotropin-releasing hormone (GnRH) pulse frequency and circulating concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH).[medical citation needed]
In normal physiologic female hormonal cycling, at seven days past ovulation, high levels of estrogen and progesterone produced from the corpus luteum inhibit GnRH, FSH, and LH at the hypothalamus and anterior pituitary.[medical citation needed] If fertilization does not occur in the post-ovulation period the corpus luteum disintegrates due to a lack of human chorionic gonadotropin (hCG).[medical citation needed] This would normally be produced by the embryo in the effort of maintaining progesterone and estrogen levels during pregnancy.[medical citation needed]
Therapeutically, clomifene is given early in the menstrual cycle to produce follicles.[medical citation needed] Follicles, in turn, produce the estrogen, which circulates in serum.[medical citation needed] In the presence of clomifene, the body perceives a low level of estrogen, similar to day 22 in the previous cycle.[medical citation needed] Since estrogen can no longer effectively exert negative feedback on the hypothalamus, GnRH secretion becomes more rapidly pulsatile, which results in increased pituitary gonadotropin release.[medical citation needed] (More rapid, lower amplitude pulses of GnRH lead to increased LH and FSH secretion, while more irregular, larger amplitude pulses of GnRH leads to a decrease in the ratio of LH to FSH.[medical citation needed]) Increased FSH levels cause the growth of more ovarian follicles, and subsequently rupture of follicles resulting in ovulation. Ovulation occurs most often 6 to 7 days after a course of clomifene.[medical citation needed]
In normal men, 50 mg/day clomifene for 8 months has been found to increase testosterone levels by around 870 ng/dL in younger men and by around 490 ng/dL in elderly men.[16] Estradiol levels increased by 62 pg/mL in younger men and by 40 pg/mL in elderly men.[16] These findings suggest that the progonadotropic effects of clomifene are stronger in younger men than in older men.[16] In men with hypogonadism, clomifene has been found to increase testosterone levels by 293 to 362 ng/dL and estradiol levels by 5.5 to 13 pg/mL.[16] In a large clinical study of men with low testosterone levels (<400 ng/dL), 25 mg/day clomifene increased testosterone levels from 309 ng/dL to 642 ng/dL after 3 months of therapy.[39] No significant changes in HDL cholesterol, triglycerides, fasting glucose, or prolactin levels were observed, although total cholesterol levels decreased significantly.[16][39]
| Medication | Breast | Bone | Liver | Uterus | Vagina | Brain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipids | Coagulation | SHBG | IGF-1 | Hot flashes | Gonadotropins | |||||||||
| Estradiol | + | + | + | + | + | + | + | + | + | + | ||||
| "Ideal SERM" | – | + | + | ± | ± | ± | – | + | + | ± | ||||
| Bazedoxifene | – | + | + | + | + | ? | – | ± | – | ? | ||||
| Clomifene | – | + | + | ? | + | + | – | ? | – | ± | ||||
| Lasofoxifene | – | + | + | + | ? | ? | ± | ± | – | ? | ||||
| Ospemifene | – | + | + | + | + | + | ± | ± | – | ± | ||||
| Raloxifene | – | + | + | + | + | + | ± | – | – | ± | ||||
| Tamoxifen | – | + | + | + | + | + | + | – | – | ± | ||||
| Toremifene | – | + | + | + | + | + | + | – | – | ± | ||||
| Effect: + = Estrogenic / agonistic. ± = Mixed or neutral. – = Antiestrogenic / antagonistic. Note: SERMs generally increase gonadotropin levels in hypogonadal and eugonadal men as well as premenopausal women (antiestrogenic) but decrease gonadotropin levels in postmenopausal women (estrogenic). Sources: See template. | ||||||||||||||
Other activities[edit]
Clomifene is an inhibitor of the conversion of desmosterol into cholesterol by the enzyme 24-dehydrocholesterol reductase.[40][41] Concerns about possible induction of desmosterolosis and associated symptoms such as cataracts and ichthyosis with extended exposure precluded the use of clomifene in the treatment of breast cancer.[40][41] Continuous use of clomifene has been found to increase desmosterol levels by 10% and continuous high doses of clomifene (200 mg/day) have been reported to produce visual disturbances.[42][43]
Pharmacokinetics[edit]
Clomifene produces N-desmethylclomifene, clomifenoxide (clomifene N-oxide), 4-hydroxyclomifene, and 4-hydroxy-N-desmethylclomifene as metabolites.[2][44] Clomifene is a prodrug most importantly of 4-hydroxyclomifene and 4-hydroxy-N-desmethylclomifene, which are the most active of its metabolites.[32][33] In one study, the peak levels after a single 50 mg dose of clomifene were 20.37 nmol/L for clomifene, 0.95 nmol/L for 4-hydroxyclomifene, and 1.15 nmol/L for 4-hydroxy-N-desmethylclomifene.[2]
Clomifene has an onset of action of 5 to 10 days following course of treatment and an elimination half-life about 4 - 7days.[2][4] In one study, after a single 50 mg dose of clomifene, the half-life of clomifene was 128 hours (5.3 days), of 4-hydroxyclomifene was 13 hours, and of 4-hydroxy-N-desmethylclomifene was 15 hours.[2] Individuals with the CYP2D6*10 allele showed longer half-lives for 4-hydroxyclomifene and 4-hydroxy-N-desmethylclomifene.[2] Primarily due to differences in CYP2D6 genetics, steady state concentrations and individual response to clomifene are highly variable.[45]
Most clomifene metabolism occurs in the liver, where it undergoes enterohepatic recirculation. Clomifene and its metabolites are excreted primarily through feces (42%), and excretion can occur up to 6 weeks after discontinuation.[27]
Chemistry[edit]
Clomifene is a triphenylethylene derivative. It is a mixture of two geometric isomers, the cis enclomifene ((E)-clomifene) form and trans zuclomifene ((Z)-clomifene) form. These two isomers contribute to the mixed estrogenic and antiestrogenic properties of clomifene.[10] The typical ratio of these isomers after synthesis is 38% zuclomiphene and 62% enclomiphene.[4] The United States Pharmacopeia specifies that clomifene preparations must contain between 30% and 50% zuclomiphene.[4]
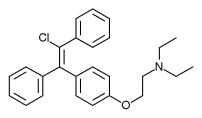 |
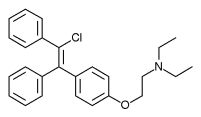 |
History[edit]
A team at William S. Merrell Chemical Company led by Frank Palopoli synthesized clomifene in 1956; after its biological activity was confirmed a patent was filed and issued in November 1959.[10][46] Scientists at Merrell had previously synthesized chlorotrianisene and ethamoxytriphetol.[10] Clomifene was studied in the treatment of advanced breast cancer during the period of 1964 to 1974 and was found to be effective but was abandoned due to concerns about desmosterolosis with extended use.[40][47][48] Short-term use (e.g. days to months) did not raise the same concerns and clomifene continued to be studied for other indications.[41][42]
| Antiestrogen | Dosage | Year(s) | Response rate | Adverse effects |
|---|---|---|---|---|
| Ethamoxytriphetol | 500–4,500 mg/day | 1960 | 25% | Acute psychotic episodes |
| Clomifene | 100–300 mg/day | 1964–1974 | 34% | Risks of cataracts |
| Nafoxidine | 180–240 mg/day | 1976 | 31% | Cataracts, ichthyosis, photophobia |
| Tamoxifen | 20–40 mg/day | 1971–1973 | 31% | Transient thrombocytopeniaa |
| Footnotes: a = "The particular advantage of this drug is the low incidence of troublesome side effects (25)." "Side effects were usually trivial (26)." Sources: [47][49] | ||||
Clinical studies were conducted under an Investigational New Drug Application; clomifene was third drug for which an IND had been filed under the 1962 Kefauver Harris Amendment to the Federal Food, Drug, and Cosmetic Act that had been passed in response to the thalidomide tragedy.[10] It was approved for marketing in 1967 under the brand name Clomid.[10][50] It was first used to treat cases of oligomenorrhea but was expanded to include treatment of anovulation when women undergoing treatment had higher than expected rates of pregnancy.[51]
The drug is widely considered to have been a revolution in the treatment of female infertility, the beginning of the modern era of assisted reproductive technology, and the beginning of what in the words of Eli Y. Adashi, was "the onset of the US multiple births epidemic".[10][52]
The company was acquired by Dow Chemical in 1980,[53][54] and in 1989 Dow Chemical acquired 67 percent interest of Marion Laboratories, which was renamed Marion Merrell Dow.[53] In 1995 Hoechst AG acquired the pharmaceutical business of Marion Merrell Dow.[55] Hoechst in turn became part of Aventis in 1999,[56]: 9–11 and subsequently a part of Sanofi.[57] It became the most widely prescribed drug for ovulation induction to reverse anovulation or oligoovulation.[58]
Society and culture[edit]
Brand names[edit]
Clomifene is marketed under many brand names worldwide, including Beclom, Bemot, Biogen, Blesifen, Chloramiphene, Clofert, Clomene, ClomHEXAL, Clomi, Clomid, Clomidac, Clomifen, Clomifencitrat, Clomifene, Clomifène, Clomifene citrate, Clomifeni citras, Clomifeno, Clomifert, Clomihexal, Clomiphen, Clomiphene, Clomiphene Citrate, Cloninn, Clostil, Clostilbegyt, Clovertil, Clovul, Dipthen, Dufine, Duinum, Fensipros, Fertab, Fertec, Fertex, Ferticlo, Fertil, Fertilan, Fertilphen, Fertin, Fertomid, Ferton, Fertotab, Fertyl, Fetrop, Folistim, Genoclom, Genozym, Hete, I-Clom, Ikaclomin, Klofit, Klomen, Klomifen, Lomifen, MER 41, Milophene, Ofertil, Omifin, Ova-mit, Ovamit, Ovinum, Ovipreg, Ovofar, Ovuclon, Ovulet, Pergotime, Pinfetil, Profertil, Prolifen, Provula, Reomen, Serofene, Serophene, Serpafar, Serpafar, Surole, Tocofeno, and Zimaquin.[1]
Regulation[edit]
Clomifene is included on the World Anti-Doping Agency list of illegal doping agents in sport.[59] It is listed because it is an "anti-estrogenic substance".[citation needed]
Research[edit]
Clomifene has been used almost exclusively for ovulation induction in premenopausal women, and has been studied very limitedly in postmenopausal women.[60]
Clomifene was studied for treatment and prevention of breast cancer, but issues with toxicity led to abandonment of this indication, as did the discovery of tamoxifen.[61] Like the structurally related drug triparanol, clomifene is known to inhibit the enzyme 24-dehydrocholesterol reductase and increase circulating desmosterol levels, making it unfavorable for extended use in breast cancer due to risk of side effects like irreversible cataracts.[62][63]
References[edit]
- ^ a b "International brands of clomifene -". Drugs.com. Archived from the original on 20 September 2016. Retrieved 11 September 2016.
- ^ a b c d e f g h i Kim MJ, Byeon JY, Kim YH, Kim SH, Lee CM, Jung EH, et al. (March 2018). "Effect of the CYP2D6*10 allele on the pharmacokinetics of clomiphene and its active metabolites". Archives of Pharmacal Research. 41 (3): 347–353. doi:10.1007/s12272-018-1005-7. PMID 29516347. S2CID 4034257.
- ^ a b Yilmaz S, Yilmaz Sezer N, Gönenç İM, İlhan SE, Yilmaz E (April 2018). "Safety of clomiphene citrate: a literature review". Cytotechnology. 70 (2): 489–495. doi:10.1007/s10616-017-0169-1. PMC 5851961. PMID 29159661.
- ^ a b c d e Mikkelson TJ, Kroboth PD, Cameron WJ, Dittert LW, Chungi V, Manberg PJ (September 1986). "Single-dose pharmacokinetics of clomiphene citrate in normal volunteers". Fertility and Sterility. 46 (3): 392–396. doi:10.1016/S0015-0282(16)49574-9. PMID 3091405.
- ^ a b c d e f "Clomiphene Citrate". The American Society of Health-System Pharmacists. Archived from the original on 14 September 2017. Retrieved 8 December 2016.
- ^ a b c World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 385–386. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c d e f g "Clomiphene citrate tablets label" (PDF). FDA. Archived (PDF) from the original on 27 September 2016. Retrieved 11 September 2016.
- ^ Ghumman S (2015). Principles and Practice of Controlled Ovarian Stimulation in ART. Springer. p. 65. ISBN 9788132216865. Archived from the original on 27 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b c d e f g Dickey RP, Holtkamp DE (1996). "Development, pharmacology and clinical experience with clomiphene citrate". Human Reproduction Update. 2 (6): 483–506. doi:10.1093/humupd/2.6.483. PMID 9111183.
- ^ a b Rodriguez KM, Pastuszak AW, Lipshultz LI (August 2016). "Enclomiphene citrate for the treatment of secondary male hypogonadism". Expert Opinion on Pharmacotherapy. 17 (11): 1561–7. doi:10.1080/14656566.2016.1204294. PMC 5009465. PMID 27337642.
- ^ a b c Practice Committee of the American Society for Reproductive Medicine (August 2013). "Use of clomiphene citrate in infertile women: a committee opinion". Fertility and Sterility. 100 (2): 341–8. doi:10.1016/j.fertnstert.2013.05.033. PMID 23809505.
- ^ a b Hughes E, Brown J, Collins JJ, Vanderkerchove P (January 2010). "Clomiphene citrate for unexplained subfertility in women". The Cochrane Database of Systematic Reviews. 2010 (1): CD000057. doi:10.1002/14651858.CD000057.pub2. PMC 7052733. PMID 20091498.
- ^ a b Seli E, Arici A. "Ovulation induction with clomiphene citrate". UpToDate. Retrieved 30 July 2019.
- ^ a b Bach PV, Najari BB, Kashanian JA (2016). "Adjunct Management of Male Hypogonadism". Current Sexual Health Reports. 8 (4): 231–239. doi:10.1007/s11930-016-0089-7. ISSN 1548-3584. S2CID 79220716.
- ^ a b c d e f g h i Trost LW, Khera M (July 2014). "Alternative treatment modalities for the hypogonadal patient". Current Urology Reports. 15 (7): 417. doi:10.1007/s11934-014-0417-2. PMID 24817260. S2CID 20304701.
- ^ DiGiorgio L, Sadeghi-Nejad H (December 2016). "Off label therapies for testosterone replacement". Translational Andrology and Urology. 5 (6): 844–849. doi:10.21037/tau.2016.08.15. PMC 5182219. PMID 28078215.
- ^ Helo S, Mahon J, Ellen J, Wiehle R, Fontenot G, Hsu K, et al. (January 2017). "Serum levels of enclomiphene and zuclomiphene in men with hypogonadism on long-term clomiphene citrate treatment". BJU International. 119 (1): 171–176. doi:10.1111/bju.13625. PMID 27511863. S2CID 5538782.
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1206–. ISBN 978-0-7817-1750-2.
- ^ Agrawal S, Ganie MA, Nisar S (2017). "Gynaecomastia". Basics of Human Andrology. Springer. pp. 451–458. doi:10.1007/978-981-10-3695-8_26. ISBN 978-981-10-3694-1.
- ^ Nordt CA, DiVasta AD (August 2008). "Gynecomastia in adolescents". Current Opinion in Pediatrics. 20 (4): 375–82. doi:10.1097/MOP.0b013e328306a07c. PMID 18622190. S2CID 205834072.
- ^ Miller GD, Moore C, Nair V, Hill B, Willick SE, Rogol AD, et al. (March 2019). "Hypothalamic-Pituitary-Testicular Axis Effects and Urinary Detection Following Clomiphene Administration in Males". The Journal of Clinical Endocrinology and Metabolism. 104 (3): 906–914. doi:10.1210/jc.2018-01159. PMID 30295816.
- ^ Cameron R, Feuer G, de la Iglesia F (6 December 2012). Drug-Induced Hepatotoxicity. Springer Science & Business Media. pp. 565–. ISBN 978-3-642-61013-4.
- ^ Trabert B, Lamb EJ, Scoccia B, Moghissi KS, Westhoff CL, Niwa S, et al. (December 2013). "Ovulation-inducing drugs and ovarian cancer risk: results from an extended follow-up of a large United States infertility cohort". Fertility and Sterility. 100 (6): 1660–6. doi:10.1016/j.fertnstert.2013.08.008. PMC 3873340. PMID 24011610.
- ^ Gadducci A, Guerrieri ME, Genazzani AR (January 2013). "Fertility drug use and risk of ovarian tumors: a debated clinical challenge". Gynecological Endocrinology. 29 (1): 30–5. doi:10.3109/09513590.2012.705382. PMID 22946709. S2CID 1240526.
- ^ Yu Q, Lv X, Liu K, Ma D, Wu Y, Dai W, et al. (2018). "Fertility Drugs Associated with Thyroid Cancer Risk: A Systematic Review and Meta-Analysis". BioMed Research International. 2018: 7191704. doi:10.1155/2018/7191704. PMC 5971354. PMID 29862285.
- ^ a b "ClomiPHENE (Professional Patient Advice)". Drugs.com. Retrieved 30 July 2019.
- ^ a b c Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L (2000). "A pharmacological review of selective oestrogen receptor modulators". Human Reproduction Update. 6 (3): 212–24. doi:10.1093/humupd/6.3.212. PMID 10874566.
- ^ a b Haskell SG (May 2003). "Selective estrogen receptor modulators". Southern Medical Journal. 96 (5): 469–76. doi:10.1097/01.SMJ.0000051146.93190.4A. PMID 12911186. S2CID 40607634.
- ^ Duarte FH, Jallad RS, Bronstein MD (November 2016). "Estrogens and selective estrogen receptor modulators in acromegaly". Endocrine. 54 (2): 306–314. doi:10.1007/s12020-016-1118-z. PMID 27704479. S2CID 10136018.
- ^ Runnebaum B, Rabe T (17 April 2013). Gynäkologische Endokrinologie und Fortpflanzungsmedizin: Band 1: Gynäkologische Endokrinologie. Springer-Verlag. pp. 88–. ISBN 978-3-662-07635-4.
- ^ a b Roche V, Zito WS, Lemke T, Williams DA (29 July 2019). Foye's Principles of Medicinal Chemistry. Wolters Kluwer Health. pp. 3010–. ISBN 978-1-4963-8587-1.
- ^ a b c d Obach RS (April 2013). "Pharmacologically active drug metabolites: impact on drug discovery and pharmacotherapy". Pharmacological Reviews. 65 (2): 578–640. doi:10.1124/pr.111.005439. PMID 23406671. S2CID 720243.
- ^ Wittliff JL, Kerr II DA, Andres SA (2005). "Estrogens IV: Estrogen-Like Pharmaceuticals". In Wexler P (ed.). Encyclopedia of Toxicology, 2nd Edition. Vol. Dib–L. Elsevier. pp. 254–258. ISBN 9780080548005.
- ^ Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, et al. (March 2000). "The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands". Toxicological Sciences. 54 (1): 138–53. doi:10.1093/toxsci/54.1.138. PMID 10746941.
- ^ Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, et al. (March 2001). "Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens". Chemical Research in Toxicology. 14 (3): 280–94. doi:10.1021/tx000208y. PMID 11258977.
- ^ a b Baumann RJ, Bush TL, Cross-Doersen DE, Cashman EA, Wright PS, Zwolshen JH, et al. (March 1998). "Clomiphene analogs with activity in vitro and in vivo against human breast cancer cells". Biochemical Pharmacology. 55 (6): 841–51. doi:10.1016/s0006-2952(97)00574-1. PMID 9586957.
- ^ Sutherland RL, Watts CK, Ruenitz PC (October 1986). "Definition of two distinct mechanisms of action of antiestrogens on human breast cancer cell proliferation using hydroxytriphenylethylenes with high affinity for the estrogen receptor". Biochemical and Biophysical Research Communications. 140 (2): 523–9. doi:10.1016/0006-291x(86)90763-1. PMID 3778464.
- ^ a b Rambhatla A, Mills JN, Rajfer J (2016). "The Role of Estrogen Modulators in Male Hypogonadism and Infertility". Reviews in Urology. 18 (2): 66–72. doi:10.3909/riu0711 (inactive 31 January 2024). PMC 5010627. PMID 27601965.
{{cite journal}}: CS1 maint: DOI inactive as of January 2024 (link) - ^ a b c Zhang X (16 October 2018). Estrogen Receptor and Breast Cancer: Celebrating the 60th Anniversary of the Discovery of ER. Springer. pp. 153–. ISBN 978-3-319-99350-8.
- ^ a b c Maximov PY, McDaniel RD, Jordan VC (23 July 2013). Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Science & Business Media. pp. 34–. ISBN 978-3-0348-0664-0.
- ^ a b Harper MJ (21 December 2013). "Pharmacological Control of Reproduction in Women". In Jucker E (ed.). Progress in Drug Research / Fortschritte der Arzneimittelforschung / Progrès des recherches pharmaceutiques. Birkhäuser. pp. 69–. ISBN 978-3-0348-7065-8.
- ^ Hormones and Breast Cancer. Elsevier. 25 June 2013. pp. 13–. ISBN 978-0-12-416676-9.
- ^ Analytical Profiles of Drug Substances and Excipients. Academic Press. 20 March 1998. pp. 113–. ISBN 978-0-08-086120-3. Archived from the original on 5 November 2017.
- ^ Rostami-Hodjegan A, Lennard MS, Tucker GT, Ledger WL (May 2004). "Monitoring plasma concentrations to individualize treatment with clomiphene citrate". Fertility and Sterility. 81 (5): 1187–1193. doi:10.1016/j.fertnstert.2003.07.044. PMID 15136073.
- ^ US 2,914,563, Allen RE, Palopoli FP, Schumann EL, Van Campen Jr MG, "Therapeutic composition", issued 24 November 1959, assigned to William S Merrill Company
- ^ a b Jensen EV, Jordan VC (June 2003). "The estrogen receptor: a model for molecular medicine". Clin. Cancer Res. 9 (6): 1980–9. PMID 12796359.
- ^ Howell A, Jordan VC (2013). "Adjuvant Antihormone Therapy". In Craig JV (ed.). Estrogen Action, Selective Estrogen Receptor Modulators And Women's Health: Progress And Promise. World Scientific. pp. 229–254. doi:10.1142/9781848169586_0010. ISBN 978-1-84816-959-3.
- ^ Howell A, Jordan VC (2013). "Adjuvant Antihormone Therapy". In Craig JV (ed.). Estrogen Action, Selective Estrogen Receptor Modulators And Women's Health: Progress And Promise. World Scientific. pp. 229–254. doi:10.1142/9781848169586_0010. ISBN 978-1-84816-959-3.
- ^ Holtkamp DE, Greslin JG, Root CA, Lerner LJ (October 1960). "Gonadotrophin inhibiting and anti-fecundity effects of chloramiphene". Proceedings of the Society for Experimental Biology and Medicine. 105: 197–201. doi:10.3181/00379727-105-26054. PMID 13715563. S2CID 1448466.
- ^ Hughes E, Collins J, Vandekerckhove P (2000). "Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea". The Cochrane Database of Systematic Reviews (2): CD000056. doi:10.1002/14651858.CD000056. PMID 10796477. (Retracted, see doi:10.1002/14651858.cd000056.pub2)
- ^ Adashi EY (Fall 2014). "Iatrogenic Birth Plurality: The Challenge and Its Possible Solution" (PDF). Harvard Health Policy Review. 14 (1): 9–10. Archived from the original (PDF) on 6 October 2016. Retrieved 12 September 2016.
- ^ a b Lee P (18 July 1989). "Dow Chemical to Get Control of Marion Labs : $5-Billion-Plus Deal Is an Effort to Diversify". Los Angeles Times. Archived from the original on 29 June 2016.
- ^ Williams W (11 February 1981). "Dow Broadens Product Lines". The New York Times. ISSN 0362-4331. Archived from the original on 6 October 2016.
- ^ "Hoechst AG to Buy Marion Merrell Dow / Acquisition worth over $7 billion". San Francisco Chronicle. Reuters. 5 May 1995. Archived from the original on 6 October 2016.
- ^ Arturo Bris and Christos Cabolis, Corporate Governance Convergence Through Cross-Border Mergers The Case of Aventis Archived 21 April 2014 at the Wayback Machine, Chapter 4 in Corporate Governance and Regulatory Impact on Mergers and Acquisitions: Research and Analysis on Activity Worldwide Since 1990. Eds Greg N. Gregoriou, Luc Renneboog. Academic Press, 26 July 2007
- ^ Timmons H, Bennhold K (27 April 2004). "France Helped Broker the Aventis-Sanofi Deal". The New York Times. Archived from the original on 5 November 2017.
- ^ Strauss JF, Barbieri RL (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 518–. ISBN 978-1-4557-2758-2. Archived from the original on 5 November 2017.
- ^ The WADA Prohibited List 2016 (listed as clomiphene) Archived 6 March 2016 at the Wayback Machine
- ^ Palacios S (March 2007). "The future of the new selective estrogen receptor modulators". Menopause International. 13 (1): 27–34. doi:10.1258/175404507780456791. PMID 17448265. S2CID 29053109.
- ^ Maximov PY, Lee TM, Jordan VC (May 2013). "The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice". Current Clinical Pharmacology. 8 (2): 135–55. doi:10.2174/1574884711308020006. PMC 3624793. PMID 23062036.
- ^ Hormones and Breast Cancer. Elsevier. 25 June 2013. pp. 13–. ISBN 978-0-12-416676-9. Archived from the original on 5 November 2017.
- ^ Maximov PY, McDaniel RE, Jordan VC (2013). "Tamoxifen Goes Forward Alone". Tamoxifen. Milestones in Drug Therapy. Springer. pp. 31–46. doi:10.1007/978-3-0348-0664-0_2. ISBN 978-3-0348-0663-3. ISSN 2296-6064.
