Red algae: Difference between revisions
mNo edit summary |
m Journal cites:, added 18 DOIs, added 2 PMIDs, added 3 PMCs, templated 14 journal cites |
||
| Line 10: | Line 10: | ||
}} |
}} |
||
'''Red algae''', or '''Rhodophyta''' ({{IPAc-en|r|oʊ|ˈ|d|ɒ|f|ᵻ|t|ə}} {{respell|roh|DOF|it|ə}}, {{IPAc-en|ˌ|r|oʊ|d|ə|ˈ|f|aɪ|t|ə}} {{respell|ROH|də|FY|tə}}; {{ety|grc|''ῥόδον'' (rhodon)|rose||''φυτόν'' (phyton)|plant}}), are one of the oldest groups of [[eukaryotic]] [[algae]].<ref name=Lee2008>{{Cite book|author=Lee, R.E.|title=Phycology|edition=4th|year=2008|isbn= 978-0-521-63883-8|publisher= Cambridge University Press|postscript=<!--None-->}}</ref> The Rhodophyta also comprises one of the largest phyla of algae, containing over 7,000 currently recognized species with taxonomic revisions ongoing.<ref name=":0">{{Cite web|url=http://www.algaebase.org/browse/taxonomy/?id=97240|title=Algaebase|last=Guiry|first=M.D.|last2=Guiry|first2=G.M.|date=2016|website=www.algaebase.org|access-date=November 20, 2016}}</ref> The majority of species (6,793) are found in the [[Florideophyceae]] ([[Class (biology)|class]]), and mostly consist of [[multicellular]], [[ocean|marine]] algae, including many notable [[seaweed]]s.<ref name=":0" /><ref name="Thomas 02">{{cite book|title=Seaweeds|publisher=Life Series. [[Natural History Museum, London|Natural History Museum]], London|year=2002|isbn=978-0-565-09175-0|author=D. Thomas}}</ref> Approximately 5% of the red algae occur in freshwater environments with greater concentrations found in warmer areas.<ref name=":2">{{Cite journal|last=Sheath|first=Robert G.|year=1984|title=The biology of freshwater red algae|url=|journal=Progress Phycological Research|volume=3|pages=89–157|via=}}</ref> Except for two coastal cave dwelling species in the asexual class [[Cyanidiophyceae]], that diverged from other red algae about 1.3 billion years ago,<ref> |
'''Red algae''', or '''Rhodophyta''' ({{IPAc-en|r|oʊ|ˈ|d|ɒ|f|ᵻ|t|ə}} {{respell|roh|DOF|it|ə}}, {{IPAc-en|ˌ|r|oʊ|d|ə|ˈ|f|aɪ|t|ə}} {{respell|ROH|də|FY|tə}}; {{ety|grc|''ῥόδον'' (rhodon)|rose||''φυτόν'' (phyton)|plant}}), are one of the oldest groups of [[eukaryotic]] [[algae]].<ref name=Lee2008>{{Cite book|author=Lee, R.E.|title=Phycology|edition=4th|year=2008|isbn= 978-0-521-63883-8|publisher= Cambridge University Press|postscript=<!--None-->}}</ref> The Rhodophyta also comprises one of the largest phyla of algae, containing over 7,000 currently recognized species with taxonomic revisions ongoing.<ref name=":0">{{Cite web|url=http://www.algaebase.org/browse/taxonomy/?id=97240|title=Algaebase|last=Guiry|first=M.D.|last2=Guiry|first2=G.M.|date=2016|website=www.algaebase.org|access-date=November 20, 2016}}</ref> The majority of species (6,793) are found in the [[Florideophyceae]] ([[Class (biology)|class]]), and mostly consist of [[multicellular]], [[ocean|marine]] algae, including many notable [[seaweed]]s.<ref name=":0" /><ref name="Thomas 02">{{cite book|title=Seaweeds|publisher=Life Series. [[Natural History Museum, London|Natural History Museum]], London|year=2002|isbn=978-0-565-09175-0|author=D. Thomas}}</ref> Approximately 5% of the red algae occur in freshwater environments with greater concentrations found in warmer areas.<ref name=":2">{{Cite journal|last=Sheath|first=Robert G.|year=1984|title=The biology of freshwater red algae|url=|journal=Progress Phycological Research|volume=3|pages=89–157|via=}}</ref> Except for two coastal cave dwelling species in the asexual class [[Cyanidiophyceae]], that diverged from other red algae about 1.3 billion years ago,<ref>{{cite journal| pmc=3526103 | pmid=23267354 | doi=10.3389/fmicb.2012.00426 | volume=3 | title=Extreme environments as potential drivers of convergent evolution by exaptation: the Atacama Desert Coastal Range case | year=2012 | journal=Front Microbiol | page=426 | last1 = Azua-Bustos | first1 = A | last2 = González-Silva | first2 = C | last3 = Arenas-Fajardo | first3 = C | last4 = Vicuña | first4 = R}}</ref> there are no terrestrial species, which may be due to an evolutionary bottleneck where the last common ancestor lost about 25% of its core genes and much of its evolutionary plasticity.<ref>[http://deenr.rutgers.edu/Huan_Qiu_red_algae.html Why don't we live on a red planet?]</ref> |
||
The red algae form a distinct group characterized by having eukaryotic cells without [[flagella]] and [[centriole]]s, [[chloroplast]]s that lack external [[endoplasmic reticulum]] and contain unstacked (stroma) [[thylakoid]]s, and use [[phycobiliprotein]]s as [[accessory pigment]]s, which give them their red color.<ref name="Woelkerling 90">{{cite book|title=Biology of the Red Algae|publisher=[[Cambridge University Press]], Cambridge|year=1990|isbn=978-0-521-34301-5|pages=1–6|chapter=An introduction|author=W. J. Woelkerling|editor1=K. M. Cole|editor2=R. G. Sheath}}</ref> Red algae store sugars as [[floridean starch]], which is a type of starch that consists of highly branched [[amylopectin]] without [[amylose]],<ref name=Viola>{{cite journal|last1=Viola|first1=R.|last2=Nyvall|first2=P.|last3=Pedersén|first3=M.|year=2001|title=The unique features of starch metabolism in red algae|journal=Proceedings of the Royal Society of London B|volume=268|issue=1474|pages=1417–1422|doi=10.1098/rspb.2001.1644|pmid=11429143|pmc=1088757}}</ref> as food reserves outside their plastids. Most red algae are also [[multicellular]], macroscopic, marine, and [[sexual reproduction|reproduce sexually]]. The red algal life history is typically an [[alternation of generations]] that may have three generations rather than two.<ref>{{cite web|url=http://autocww.colorado.edu/~toldy2/E64ContentFiles/AlgaeAndFungi/Algae.html|title=Algae|publisher=autocww.colorado.edu}}</ref> |
The red algae form a distinct group characterized by having eukaryotic cells without [[flagella]] and [[centriole]]s, [[chloroplast]]s that lack external [[endoplasmic reticulum]] and contain unstacked (stroma) [[thylakoid]]s, and use [[phycobiliprotein]]s as [[accessory pigment]]s, which give them their red color.<ref name="Woelkerling 90">{{cite book|title=Biology of the Red Algae|publisher=[[Cambridge University Press]], Cambridge|year=1990|isbn=978-0-521-34301-5|pages=1–6|chapter=An introduction|author=W. J. Woelkerling|editor1=K. M. Cole|editor2=R. G. Sheath}}</ref> Red algae store sugars as [[floridean starch]], which is a type of starch that consists of highly branched [[amylopectin]] without [[amylose]],<ref name=Viola>{{cite journal|last1=Viola|first1=R.|last2=Nyvall|first2=P.|last3=Pedersén|first3=M.|year=2001|title=The unique features of starch metabolism in red algae|journal=Proceedings of the Royal Society of London B|volume=268|issue=1474|pages=1417–1422|doi=10.1098/rspb.2001.1644|pmid=11429143|pmc=1088757}}</ref> as food reserves outside their plastids. Most red algae are also [[multicellular]], macroscopic, marine, and [[sexual reproduction|reproduce sexually]]. The red algal life history is typically an [[alternation of generations]] that may have three generations rather than two.<ref>{{cite web|url=http://autocww.colorado.edu/~toldy2/E64ContentFiles/AlgaeAndFungi/Algae.html|title=Algae|publisher=autocww.colorado.edu}}</ref> |
||
| Line 18: | Line 18: | ||
Red algae are divided into the [[Cyanidiophyceae]], a class of unicellular and [[Thermoacidophile|thermoacidophilic]] [[extremophile]]s found in sulphuric hot springs and other acidic environments,<ref>{{Cite journal|last=Ciniglia|first=C.|last2=Yoon|first2=H.|last3=Pollio|first3=A.|last4=Bhattacharya|first4=D.|year=2004|title=Hidden biodiversity of the extremophilic Cyanidiales red algae|url=|journal=Molecular Ecology|volume=13|issue=7|pages=1827–1838|doi=10.1111/j.1365-294X.2004.02180.x|pmid=15189206}}</ref> an adaptation partly made possible by [[horizontal gene transfer]]s from prokaryotes,<ref>[https://www.sciencemag.org/news/2019/01/plants-and-animals-sometimes-take-genes-bacteria-study-algae-suggests Plants and animals sometimes take genes from bacteria, study of algae suggests - Sciencemag.org]</ref> with about 1% of their genome having this origin,<ref>[https://elifesciences.org/articles/45017 The genomes of polyextremophilic cyanidiales contain 1% horizontally transferred genes with diverse adaptive functions]</ref> and two sister clades called SCRP (Stylonematophyceae, Compsopogonophyceae, Rhodellophyceae and Porphyridiophyceae) and BF (Bangiophyceae and Florideophyceae), which are found in both marine and freshwater environments. The SCRP clade are microalgae, consisting of both unicellular forms and multicellular microscopic filaments and blades. The BF are macroalgae, seaweed that usually do not grow to more than about 50 cm in length, but a few species can reach lengths of 2 m.<ref> |
Red algae are divided into the [[Cyanidiophyceae]], a class of unicellular and [[Thermoacidophile|thermoacidophilic]] [[extremophile]]s found in sulphuric hot springs and other acidic environments,<ref>{{Cite journal|last=Ciniglia|first=C.|last2=Yoon|first2=H.|last3=Pollio|first3=A.|last4=Bhattacharya|first4=D.|year=2004|title=Hidden biodiversity of the extremophilic Cyanidiales red algae|url=|journal=Molecular Ecology|volume=13|issue=7|pages=1827–1838|doi=10.1111/j.1365-294X.2004.02180.x|pmid=15189206}}</ref> an adaptation partly made possible by [[horizontal gene transfer]]s from prokaryotes,<ref>[https://www.sciencemag.org/news/2019/01/plants-and-animals-sometimes-take-genes-bacteria-study-algae-suggests Plants and animals sometimes take genes from bacteria, study of algae suggests - Sciencemag.org]</ref> with about 1% of their genome having this origin,<ref>[https://elifesciences.org/articles/45017 The genomes of polyextremophilic cyanidiales contain 1% horizontally transferred genes with diverse adaptive functions]</ref> and two sister clades called SCRP (Stylonematophyceae, Compsopogonophyceae, Rhodellophyceae and Porphyridiophyceae) and BF (Bangiophyceae and Florideophyceae), which are found in both marine and freshwater environments. The SCRP clade are microalgae, consisting of both unicellular forms and multicellular microscopic filaments and blades. The BF are macroalgae, seaweed that usually do not grow to more than about 50 cm in length, but a few species can reach lengths of 2 m.<ref>{{cite journal| pmc=5547612 | pmid=28716924 | doi=10.1073/pnas.1703088114 | volume=114 | title=Insights into the red algae and eukaryotic evolution from the genome of <i>Porphyra umbilicalis</i> (Bangiophyceae, Rhodophyta) | year=2017 | journal=Proc Natl Acad Sci U S A | pages=E6361-E6370 | last1 = Brawley | first1 = SH }}</ref><ref>{{cite journal| pmc=5547612 | pmid=28716924 | doi=10.1073/pnas.1703088114 | volume=114 | title=Insights into the red algae and eukaryotic evolution from the genome of <i>Porphyra umbilicalis</i> (Bangiophyceae, Rhodophyta) | year=2017 | journal=Proc Natl Acad Sci U S A | pages=E6361-E6370 | last1 = Brawley | first1 = SH }}</ref> Most rhodophytes are marine with a worldwide distribution, and are often found at greater depths compared to other seaweeds. While this was formerly attributed to the presence of pigments (such as [[phycoerythrin]]) that would permit red algae to inhabit greater depths than other macroalgae by chromatic adaption, recent evidence calls this into question (e.g. the discovery of green algae at great depth in the Bahamas).<ref>{{Cite journal|last=Norris|first=J. N.|last2=Olsen|first2=J. L.|date=1991|title=Deep-water green algae from the Bahamas, including Cladophora vandenhoekii sp. nov. (Cladophorales)|journal=Phycologia|language=en|volume=30|issue=4|pages=315–328|doi=10.2216/i0031-8884-30-4-315.1|issn=0031-8884}}</ref> Some marine species are found on sandy shores, while most others can be found attached to rocky substrata.<ref name=":3">{{Cite book|title=Biology of the Red Algae|last=Kain|first=J.M.|last2=Norton|first2=T.A.|publisher=Cambridge University Press|year=1990|isbn=978-0521343015|editor-last=Cole|editor-first=J.M.|location=Cambridge, U.K.|pages=377–423|chapter=Marine Ecology|editor-last2=Sheath|editor-first2=R.G.|via=}}</ref> Freshwater species account for 5% of red algal diversity, but they also have a worldwide distribution in various habitats;<ref name=":2" /> they generally prefer clean, high-flow streams with clear waters and rocky bottoms, but with some exceptions.<ref name=Eloranta2004>{{Cite journal |
||
|url = http://www.oandhs.org/files/60.pdf |
|url = http://www.oandhs.org/files/60.pdf |
||
|journal = International Journal of Oceanography and Hydrobiology |
|journal = International Journal of Oceanography and Hydrobiology |
||
| Line 175: | Line 175: | ||
Some sources (such as Lee) place all red algae into the class "Rhodophyceae". (Lee's organization is not a comprehensive classification, but a selection of orders considered common or important.<ref name="Lee2008withpage">{{cite book|author=Robert Edward Lee|title=Phycology|url=https://books.google.com/books?id=gfoIAFHgusgC&pg=PA107|accessdate=31 January 2011|year=2008|publisher=Cambridge University Press|isbn=978-0-521-68277-0|pages=107}}</ref>) |
Some sources (such as Lee) place all red algae into the class "Rhodophyceae". (Lee's organization is not a comprehensive classification, but a selection of orders considered common or important.<ref name="Lee2008withpage">{{cite book|author=Robert Edward Lee|title=Phycology|url=https://books.google.com/books?id=gfoIAFHgusgC&pg=PA107|accessdate=31 January 2011|year=2008|publisher=Cambridge University Press|isbn=978-0-521-68277-0|pages=107}}</ref>) |
||
A subphylum - Proteorhodophytina - has been proposed to encompass the existing classes [[Compsopogonophyceae]], [[Porphyridiophyceae]], [[Rhodellophyceae]] and [[Stylonematophyceae]].<ref>Muñoz-Gómez SA |
A subphylum - Proteorhodophytina - has been proposed to encompass the existing classes [[Compsopogonophyceae]], [[Porphyridiophyceae]], [[Rhodellophyceae]] and [[Stylonematophyceae]].<ref>{{cite journal | last1 = Muñoz-Gómez | first1 = SA | last2 = Mejía-Franco | first2 = FG | last3 = Durnin | first3 = K | last4 = Colp | first4 = M | last5 = Grisdale | first5 = CJ | last6 = Archibald | first6 = JM | last7 = Ch | first7 = Slamovits | year = 2017 | title = The new red algal subphylum Proteorhodophytina comprises the largest and most divergent plastid genomes known | url = | journal = Curr Biol | volume = 27 | issue = 11| pages = 1677–1684 }}</ref> This proposal was made on the basis of the analysis of the plastid genomes. |
||
{{See also|Eukaryote#Phylogeny}} |
{{See also|Eukaryote#Phylogeny}} |
||
| Line 198: | Line 198: | ||
==Morphology== |
==Morphology== |
||
Red algal morphology is diverse ranging from [[unicellular]] forms to complex parenchymatous and non- parenchymatous thallus.<ref>Goff |
Red algal morphology is diverse ranging from [[unicellular]] forms to complex parenchymatous and non- parenchymatous thallus.<ref>{{cite journal | last1 = Goff | first1 = L. J. | last2 = Coleman | first2 = A. W. | year = 1986 | title = A Novel Pattern of Apical Cell Polyploidy, Sequential Polyploidy Reduction and Intercellular Nuclear Transfer in the Red Alga Polysiphonia | url = https://doi.org/10.1002/j.1537-2197.1986.tb08558.x | journal = American Journal of Botany | volume = 73 | issue = 8| pages = 1109–1130 | doi=10.1002/j.1537-2197.1986.tb08558.x}}</ref> Red algae have double [[cell wall]]s.<ref name=Fritsch1945/> The outer layers contain the polysaccharides [[agarose]] and agaropectin that can be extracted from the cell walls by boiling as [[agar]].<ref name=Fritsch1945/> The internal walls are mostly cellulose.<ref name=Fritsch1945/> They also have the most gene-rich plastid genomes known.<ref>[https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0059001 Evolution of Red Algal Plastid Genomes: Ancient Architectures, Introns, Horizontal Gene Transfer, and Taxonomic Utility of Plastid Markers - PLOS]</ref> |
||
===Cell Structure=== |
===Cell Structure=== |
||
Red algae do not have flagella and centrioles during their entire life cycle. Presence of normal spindle fibres, microtubules, un-stacked photosynthetic membranes, presence of phycobilin pigment granules.,<ref>W. J. Woelkerling (1990). "An introduction". In K. M. Cole; R. G. Sheath (eds.). Biology of the Red Algae. Cambridge University Press, Cambridge. pp. 1–6. {{ISBN|978-0-521-34301-5}}.</ref> presence of pit connection between cells filamentous genera, absence of chloroplast endoplasmic reticulum are the distinguishing characters of red algal cell structure.<ref>Scott |
Red algae do not have flagella and centrioles during their entire life cycle. Presence of normal spindle fibres, microtubules, un-stacked photosynthetic membranes, presence of phycobilin pigment granules.,<ref>W. J. Woelkerling (1990). "An introduction". In K. M. Cole; R. G. Sheath (eds.). Biology of the Red Algae. Cambridge University Press, Cambridge. pp. 1–6. {{ISBN|978-0-521-34301-5}}.</ref> presence of pit connection between cells filamentous genera, absence of chloroplast endoplasmic reticulum are the distinguishing characters of red algal cell structure.<ref>{{cite journal | last1 = Scott | first1 = J. | last2 = Cynthia | first2 = B. | last3 = Schornstein | first3 = K. | last4 = Thomas | first4 = J. | year = 1980 | title = Ultrastructure of Cell Division and Reproductive Differentiation of Male Plants in the Florideophyceae (Rhodophyta): Cell Division in Polysiphonia1 | url = https://doi.org/10.1111/j.1529-8817.1980.tb03068.x | journal = Journal of Phycology | volume = 16 | issue = 4| pages = 507–524 | doi=10.1111/j.1529-8817.1980.tb03068.x}}</ref> |
||
</ref> |
|||
====Chloroplasts==== |
====Chloroplasts==== |
||
Presence of the water-soluble pigments called phycobilins ([[phycocyanobilin]], [[phycoerythrobilin]], [[phycourobilin]] and [[phycobiliviolin]]), which are localized into [[phycobilisomes]], gives red algae their distinctive color.<ref>Gantt |
Presence of the water-soluble pigments called phycobilins ([[phycocyanobilin]], [[phycoerythrobilin]], [[phycourobilin]] and [[phycobiliviolin]]), which are localized into [[phycobilisomes]], gives red algae their distinctive color.<ref>{{cite journal | last1 = Gantt | first1 = E | year = 1969 | title = Properties and Ultrastructure of Phycoerythrin From Porphyridium cruentum12 | url = | journal = Plant Physiology | volume = 44 | issue = 11| pages = 1629–1638 }}</ref> Chloroplast contains evenly spaced and ungrouped thylakoids.<ref>The Fine Structure of Algal Cells—1st Edition. (n.d.). Retrieved October 16, 2019, from https://www.elsevier.com/books/the-fine-structure-of-algal-cells/dodge/978-0-12-219150-3 |
||
| ⚫ | </ref> Double membrane of chloroplast envelope surrounds the chloroplast. Absence of grana and attachment of phycobilisomes on the stromal surface of the thylakoid membrane are other distinguishing characters of red algal chloroplast.<ref>{{cite journal | last1 = Tsekos | first1 = I. | last2 = Reiss | first2 = H.-D. | last3 = Orfanidis | first3 = S. | last4 = Orologas | first4 = N. | year = 1996 | title = Ultrastructure and supramolecular organization of photosynthetic membranes of some marine red algae | url = https://doi.org/10.1111/j.1469-8137.1996.tb01923.x | journal = New Phytologist | volume = 133 | issue = 4| pages = 543–551 | doi=10.1111/j.1469-8137.1996.tb01923.x}}</ref> |
||
</ref> Chloroplast contains evenly spaced and ungrouped thylakoids.<ref>The Fine Structure of Algal Cells—1st Edition. (n.d.). Retrieved October 16, 2019, from https://www.elsevier.com/books/the-fine-structure-of-algal-cells/dodge/978-0-12-219150-3 |
|||
| ⚫ | </ref> Double membrane of chloroplast envelope surrounds the chloroplast. Absence of grana and attachment of phycobilisomes on the stromal surface of the thylakoid membrane are other distinguishing characters of red algal chloroplast.<ref>Tsekos |
||
</ref> |
|||
===Storage products=== |
===Storage products=== |
||
The major photosynthetic products include floridoside (major product), D‐isofloridoside, digeneaside, mannitol, sorbitol, dulcitol etc.<ref>Karsten |
The major photosynthetic products include floridoside (major product), D‐isofloridoside, digeneaside, mannitol, sorbitol, dulcitol etc.<ref>{{cite journal | last1 = Karsten | first1 = U. | last2 = West | first2 = J. A. | last3 = Zuccarello | first3 = G. C. | last4 = Engbrodt | first4 = R. | last5 = Yokoyama | first5 = A. | last6 = Hara | first6 = Y. | last7 = Brodie | first7 = J. | year = 2003 | title = Low Molecular Weight Carbohydrates of the Bangiophycidae (Rhodophyta)1 | url = https://doi.org/10.1046/j.1529-8817.2003.02192.x | journal = Journal of Phycology | volume = 39 | issue = 3| pages = 584–589 | doi=10.1046/j.1529-8817.2003.02192.x}}</ref> Floridean starch (similar to amylopectin in land plants), a long term storage product, is deposited freely (scattered) in the cytoplasm.<ref>Lee, R. E. (1974). Chloroplast structure and starch grain production as phylogenetic indicators in the lower Rhodophyceae. British Phycological Journal, 9(3), 291–295. https://doi.org/10.1080/00071617400650351 |
||
</ref> The concentration of photosynthetic products are altered by the environmental conditions like change in pH, the salinity of medium, change in light intensity, nutrient limitation etc.<ref>Joniyas, A., Surif, M., & Dehgahi, R. (2016). Effect of Nutrient and Light Intensity on Nutrient Uptakes of Gracilaria manilaensis. https://doi.org/10.12983/ijsres-2016-p0173-0185 |
</ref> The concentration of photosynthetic products are altered by the environmental conditions like change in pH, the salinity of medium, change in light intensity, nutrient limitation etc.<ref>Joniyas, A., Surif, M., & Dehgahi, R. (2016). Effect of Nutrient and Light Intensity on Nutrient Uptakes of Gracilaria manilaensis. https://doi.org/10.12983/ijsres-2016-p0173-0185 |
||
</ref><ref>Low Molecular Weight Carbohydrates in Red Algae – an Ecophysiological and Biochemical Perspective | SpringerLink. (n.d.). Retrieved October 16, 2019, from https://link.springer.com/chapter/10.1007/978-90-481-3795-4_24 |
</ref><ref>Low Molecular Weight Carbohydrates in Red Algae – an Ecophysiological and Biochemical Perspective | SpringerLink. (n.d.). Retrieved October 16, 2019, from https://link.springer.com/chapter/10.1007/978-90-481-3795-4_24 |
||
| Line 300: | Line 297: | ||
*''[[Porphyridium purpureum]]'', [[Porphyridiophyceae]]<ref>{{cite journal | last1 = Bhattacharya | display-authors = etal | year = 2013 | title = Genome of the red alga Porphyridium purpureum | url = | journal = Nature Communications | volume = 4 | issue = | page = 1941 | doi = 10.1038/ncomms2931 | pmid = 23770768 | pmc = 3709513 | bibcode = 2013NatCo...4.1941B }}</ref> |
*''[[Porphyridium purpureum]]'', [[Porphyridiophyceae]]<ref>{{cite journal | last1 = Bhattacharya | display-authors = etal | year = 2013 | title = Genome of the red alga Porphyridium purpureum | url = | journal = Nature Communications | volume = 4 | issue = | page = 1941 | doi = 10.1038/ncomms2931 | pmid = 23770768 | pmc = 3709513 | bibcode = 2013NatCo...4.1941B }}</ref> |
||
*''[[Porphyra umbilicalis]]'', [[Bangiophyceae]]<ref>{{cite journal|display-authors=4|last1=Brawley|first1=SH|last2=Blouin|first2=NA|last3=Ficko-Blean|first3=E|last4=Wheeler|first4=GL|last5=Lohr|first5=M|last6=Goodson|first6=HV|last7=Jenkins|first7=JW|last8=Blaby-Haas|first8=CE|last9=Helliwell|first9=KE|last10=Chan|first10=CX|last11=Marriage|first11=TN|last12=Bhattacharya|first12=D|last13=Klein|first13=AS|last14=Badis|first14=Y|last15=Brodie|first15=J|last16=Cao|first16=Y|last17=Collén|first17=J|last18=Dittami|first18=SM|last19=Gachon|first19=CMM|last20=Green|first20=BR|last21=Karpowicz|first21=SJ|last22=Kim|first22=JW|last23=Kudahl|first23=UJ|last24=Lin|first24=S|last25=Michel|first25=G|last26=Mittag|first26=M|last27=Olson|first27=BJSC|last28=Pangilinan|first28=JL|last29=Peng|first29=Y|last30=Qiu|first30=H|last31=Shu|first31=S|last32=Singer|first32=JT|last33=Smith|first33=AG|last34=Sprecher|first34=BN|last35=Wagner|first35=V|last36=Wang|first36=W|last37=Wang|first37=ZY|last38=Yan|first38=J|last39=Yarish|first39=C|last40=Zäuner-Riek|first40=S|last41=Zhuang|first41=Y|last42=Zou|first42=Y|last43=Lindquist|first43=EA|last44=Grimwood|first44=J|last45=Barry|first45=KW|last46=Rokhsar|first46=DS|last47=Schmutz|first47=J|last48=Stiller|first48=JW|last49=Grossman|first49=AR|last50=Prochnik|first50=SE| title=Insights into the red algae and eukaryotic evolution from the genome of ''Porphyra umbilicalis'' (Bangiophyceae, Rhodophyta)|journal=[[Proceedings of the National Academy of Sciences of the United States of America]]|date=1 August 2017|volume=114|issue=31|pages=E6361–E6370|doi=10.1073/pnas.1703088114|pmid=28716924|pmc=5547612}}</ref> |
*''[[Porphyra umbilicalis]]'', [[Bangiophyceae]]<ref>{{cite journal|display-authors=4|last1=Brawley|first1=SH|last2=Blouin|first2=NA|last3=Ficko-Blean|first3=E|last4=Wheeler|first4=GL|last5=Lohr|first5=M|last6=Goodson|first6=HV|last7=Jenkins|first7=JW|last8=Blaby-Haas|first8=CE|last9=Helliwell|first9=KE|last10=Chan|first10=CX|last11=Marriage|first11=TN|last12=Bhattacharya|first12=D|last13=Klein|first13=AS|last14=Badis|first14=Y|last15=Brodie|first15=J|last16=Cao|first16=Y|last17=Collén|first17=J|last18=Dittami|first18=SM|last19=Gachon|first19=CMM|last20=Green|first20=BR|last21=Karpowicz|first21=SJ|last22=Kim|first22=JW|last23=Kudahl|first23=UJ|last24=Lin|first24=S|last25=Michel|first25=G|last26=Mittag|first26=M|last27=Olson|first27=BJSC|last28=Pangilinan|first28=JL|last29=Peng|first29=Y|last30=Qiu|first30=H|last31=Shu|first31=S|last32=Singer|first32=JT|last33=Smith|first33=AG|last34=Sprecher|first34=BN|last35=Wagner|first35=V|last36=Wang|first36=W|last37=Wang|first37=ZY|last38=Yan|first38=J|last39=Yarish|first39=C|last40=Zäuner-Riek|first40=S|last41=Zhuang|first41=Y|last42=Zou|first42=Y|last43=Lindquist|first43=EA|last44=Grimwood|first44=J|last45=Barry|first45=KW|last46=Rokhsar|first46=DS|last47=Schmutz|first47=J|last48=Stiller|first48=JW|last49=Grossman|first49=AR|last50=Prochnik|first50=SE| title=Insights into the red algae and eukaryotic evolution from the genome of ''Porphyra umbilicalis'' (Bangiophyceae, Rhodophyta)|journal=[[Proceedings of the National Academy of Sciences of the United States of America]]|date=1 August 2017|volume=114|issue=31|pages=E6361–E6370|doi=10.1073/pnas.1703088114|pmid=28716924|pmc=5547612}}</ref> |
||
* ''[[Gracilaria changii]]'', [[Gracilariales]] <ref>Ho |
* ''[[Gracilaria changii]]'', [[Gracilariales]] <ref>{{cite journal | last1 = Ho | first1 = C.-L. | last2 = Lee | first2 = W.-K. | last3 = Lim | first3 = E.-L. | year = 2018 | title = Unraveling the nuclear and chloroplast genomes of an agar producing red macroalga, Gracilaria changii (Rhodophyta, Gracilariales) | url = https://doi.org/10.1016/j.ygeno.2017.09.003 | journal = Genomics | volume = 110 | issue = 2| pages = 124–133 | doi=10.1016/j.ygeno.2017.09.003}}</ref> |
||
* ''[[Galdieria phlegrea]]'', [[Cyanidiophytina]] <ref>Qiu |
* ''[[Galdieria phlegrea]]'', [[Cyanidiophytina]] <ref>{{cite journal | last1 = Qiu | first1 = H. | last2 = Price | first2 = D. C. | last3 = Weber | first3 = A. P. M. | last4 = Reeb | first4 = V. | last5 = Yang | first5 = E. C. | last6 = Lee | first6 = J. M. | last7 = Bhattacharya | first7 = D. | year = 2013 | title = Adaptation through horizontal gene transfer in the cryptoendolithic red alga Galdieria phlegrea | url = https://doi.org/10.1016/j.cub.2013.08.046 | journal = Current Biology | volume = 23 | issue = 19| pages = R865–R866 | doi=10.1016/j.cub.2013.08.046}}</ref> |
||
* ''[[Gracilariopsis lemaneiformis]]'', [[Gracilariales]] <ref>Zhou |
* ''[[Gracilariopsis lemaneiformis]]'', [[Gracilariales]] <ref>{{cite journal | last1 = Zhou | first1 = W. | last2 = Hu | first2 = Y. | last3 = Sui | first3 = Z. | last4 = Fu | first4 = F. | last5 = Wang | first5 = J. | last6 = Chang | first6 = L. | last7 = Li | first7 = B. | year = 2013 | title = Genome Survey Sequencing and Genetic Background Characterization of Gracilariopsis lemaneiformis (Rhodophyta) Based on Next-Generation Sequencing | url = https://doi.org/10.1371/journal.pone.0069909 | journal = PLOS ONE | volume = 8 | issue = 7| page = e69909 | doi=10.1371/journal.pone.0069909}}</ref> |
||
</ref> |
|||
* ''[[Gracilariopsis chorda]]'', [[Gracilariales]] <ref>JunMo Lee, Eun Chan Yang, Louis Graf, Ji Hyun Yang, Huan Qiu, Udi Zelzion, Cheong Xin Chan, Timothy G Stephens, Andreas P M Weber, Ga Hun Boo, Sung Min Boo, Kyeong Mi Kim, Younhee Shin, Myunghee Jung, Seung Jae Lee, Hyung-Soon Yim, Jung-Hyun Lee, Debashish Bhattacharya, Hwan Su Yoon, Analysis of the Draft Genome of the Red Seaweed Gracilariopsis chorda Provides Insights into Genome Size Evolution in Rhodophyta, Molecular Biology and Evolution, Volume 35, Issue 8, August 2018, Pages 1869–1886, https://doi.org/10.1093/molbev/msy081 |
* ''[[Gracilariopsis chorda]]'', [[Gracilariales]] <ref>JunMo Lee, Eun Chan Yang, Louis Graf, Ji Hyun Yang, Huan Qiu, Udi Zelzion, Cheong Xin Chan, Timothy G Stephens, Andreas P M Weber, Ga Hun Boo, Sung Min Boo, Kyeong Mi Kim, Younhee Shin, Myunghee Jung, Seung Jae Lee, Hyung-Soon Yim, Jung-Hyun Lee, Debashish Bhattacharya, Hwan Su Yoon, Analysis of the Draft Genome of the Red Seaweed Gracilariopsis chorda Provides Insights into Genome Size Evolution in Rhodophyta, Molecular Biology and Evolution, Volume 35, Issue 8, August 2018, Pages 1869–1886, https://doi.org/10.1093/molbev/msy081 |
||
</ref> |
</ref> |
||
| Line 339: | Line 335: | ||
==Human consumption== |
==Human consumption== |
||
Red algae has a long history of being utilized as an important source of nutritional, functional food ingredients and pharmaceutical substances.<ref name="doi.org">Wang, T., Jónsdóttir, R., Kristinsson, H. G., Hreggvidsson, G. O., Jónsson, J. Ó., Thorkelsson, G., & Ólafsdóttir, G. (2010). Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT - Food Science and Technology, 43(9), 1387–1393. https://doi.org/10.1016/j.lwt.2010.05.010</ref> Many of the edible red algae are a rich source of antioxidants, have high amount of protein content, minerals, trace elements, vitamins and essential fatty acids.<ref>MacArtain |
Red algae has a long history of being utilized as an important source of nutritional, functional food ingredients and pharmaceutical substances.<ref name="doi.org">Wang, T., Jónsdóttir, R., Kristinsson, H. G., Hreggvidsson, G. O., Jónsson, J. Ó., Thorkelsson, G., & Ólafsdóttir, G. (2010). Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT - Food Science and Technology, 43(9), 1387–1393. https://doi.org/10.1016/j.lwt.2010.05.010</ref> Many of the edible red algae are a rich source of antioxidants, have high amount of protein content, minerals, trace elements, vitamins and essential fatty acids.<ref>{{cite journal | last1 = MacArtain | first1 = P. | last2 = Gill | first2 = C. I. R. | last3 = Brooks | first3 = M. | last4 = Campbell | first4 = R. | last5 = Rowland | first5 = I. R. | year = 2007 | title = Nutritional Value of Edible Seaweeds | url = https://doi.org/10.1111/j.1753-4887.2007.tb00278.x | journal = Nutrition Reviews | volume = 65 | issue = 12| pages = 535–543 | doi=10.1111/j.1753-4887.2007.tb00278.x}}</ref> Traditionally red algae are eaten raw, in salads, soups, meal and condiments. Several species are important food crops, in particular members of the genus ''[[Porphyra]]'', variously known as [[nori]] (Japan), ''[[gim (food)|gim]]'' (Korea), 紫菜 (China), or [[laver (seaweed)|laver]] (Britain). [[Dulse]] (''[[Palmaria palmata]]'')<ref>{{cite web |url=http://www.seaveg.co.uk/dulse.html |publisher=Quality Sea Veg |title=Dulse: ''Palmaria palmata'' |accessdate=2007-06-28}}</ref> is another important British species.<ref>{{cite book |author1=T. F. Mumford |author2=A. Muira |lastauthoramp=yes |chapter=''Porphyra'' as food: cultivation and economics |title=Algae and Human Affairs |editor=C. A. Lembi |editor2=J. Waaland |year=1988 |publisher=[[Cambridge University Press]], Cambridge |isbn=978-0-521-32115-0}}</ref> These rhodophyte foods are high in vitamins and protein and are easily grown; for example, [[nori]] cultivation in Japan goes back more than three centuries. Some of the red algal species like ''[[Gracilaria]]'' and ''[[Laurencia]]'' are found to be rich in [[polyunsaturated fatty acids]] (eicopentaenoic acid, docohexaenoic acid, [[arachidonic acid]])<ref>Gressler, V., Yokoya, N. S., Fujii, M. T., Colepicolo, P., Filho, J. M., Torres, R. P., & Pinto, E. (2010). Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chemistry, 120(2), 585–590. https://doi.org/10.1016/j.foodchem.2009.10.028 |
||
</ref> and have protein content up to 47% of total biomass.<ref name="doi.org"/> Where a big portion of world population is not getting insufficient amount of daily iodine intake, a 150 ug/day requirement of iodine is obtained from a single gram of red algae.<ref>Hoek, C. van den, Mann, D.G. and Jahns, H.M. 1995. Algae An Introduction to Phycology. Cambridge University Press, Cambridge. {{ISBN|0 521 30419 9}} |
</ref> and have protein content up to 47% of total biomass.<ref name="doi.org"/> Where a big portion of world population is not getting insufficient amount of daily iodine intake, a 150 ug/day requirement of iodine is obtained from a single gram of red algae.<ref>Hoek, C. van den, Mann, D.G. and Jahns, H.M. 1995. Algae An Introduction to Phycology. Cambridge University Press, Cambridge. {{ISBN|0 521 30419 9}} |
||
</ref> Red algae, like ''[[Gracilaria]]'', ''[[Gelidium]]'', ''[[Euchema]]'', ''[[Porphyra]]'', ''[[Acanthophora]]'', and ''[[Palmaria (alga)|Palmaria]]'' |
</ref> Red algae, like ''[[Gracilaria]]'', ''[[Gelidium]]'', ''[[Euchema]]'', ''[[Porphyra]]'', ''[[Acanthophora]]'', and ''[[Palmaria (alga)|Palmaria]]'' |
||
are primarily known for their industrial use for phycocolloids (agar, align, furcellaran and carrageenan) as thickening agent, textiles, food, anticoagulants, water-binding agents etc.<ref>Dhargalkar VK, Verlecar XN. Southern Ocean Seaweeds: a resource for exploration in food and drugs. Aquaculture 2009; 287: 229-42.</ref> Dulse (''Palmaria palmata'') is one of the most consumed red algae and is a good source of iodine, protein, magnesium and calcium.<ref> |
are primarily known for their industrial use for phycocolloids (agar, align, furcellaran and carrageenan) as thickening agent, textiles, food, anticoagulants, water-binding agents etc.<ref>Dhargalkar VK, Verlecar XN. Southern Ocean Seaweeds: a resource for exploration in food and drugs. Aquaculture 2009; 287: 229-42.</ref> Dulse (''Palmaria palmata'') is one of the most consumed red algae and is a good source of iodine, protein, magnesium and calcium.<ref>{{cite journal | last1 = Musa Jibril | first1 = Sauban | last2 = Hassan Jakada | first2 = Bello | last3 = Yunusa Umar | first3 = Harisu | last4 = Abdul-qadir Ahmad | first4 = Tahir | year = 2016 | title = Importance of Some Algal Species as a Source of Food and Supplement. Int.J.Curr.Microbiol.App | url = | journal = Sci | volume = 5 | issue = 5| pages = 186–193 }}</ref> China, Japan, Republic of Korea are the top producers of seaweeds.<ref>Manivannan, K., Thirumaran, G., Karthikai, D.G., Anantharaman. P., Balasubramanian, P. 2009. Proximate Composition of Different Group of Seaweeds from Vedalai Coastal Waters (Gulf of Mannar): Southeast Coast of India. Middle-East J. Scientific Res., 4: 72-77.</ref> In East and Southeast Asia, [[agar]] is most commonly produced from ''[[Gelidium amansii]]''. |
||
== Gallery == |
== Gallery == |
||
Revision as of 15:53, 16 November 2019
| Red algae Temporal range:
| |
|---|---|
| |
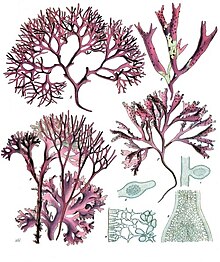
| A-D : Chondrus crispus Stackhouse, E-F : Mastocarpus stellatus J.Ag. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| (unranked): | Archaeplastida |
| Division: | Rhodophyta Wettstein, 1922 Classification is currently disputed. See Taxonomy. |
Red algae, or Rhodophyta (/roʊˈdɒfɪtə/ roh-DOF-it-ə, /ˌroʊdəˈfaɪtə/ ROH-də-FY-tə; from Ancient Greek ῥόδον (rhodon) 'rose', and φυτόν (phyton) 'plant'), are one of the oldest groups of eukaryotic algae.[2] The Rhodophyta also comprises one of the largest phyla of algae, containing over 7,000 currently recognized species with taxonomic revisions ongoing.[3] The majority of species (6,793) are found in the Florideophyceae (class), and mostly consist of multicellular, marine algae, including many notable seaweeds.[3][4] Approximately 5% of the red algae occur in freshwater environments with greater concentrations found in warmer areas.[5] Except for two coastal cave dwelling species in the asexual class Cyanidiophyceae, that diverged from other red algae about 1.3 billion years ago,[6] there are no terrestrial species, which may be due to an evolutionary bottleneck where the last common ancestor lost about 25% of its core genes and much of its evolutionary plasticity.[7]
The red algae form a distinct group characterized by having eukaryotic cells without flagella and centrioles, chloroplasts that lack external endoplasmic reticulum and contain unstacked (stroma) thylakoids, and use phycobiliproteins as accessory pigments, which give them their red color.[8] Red algae store sugars as floridean starch, which is a type of starch that consists of highly branched amylopectin without amylose,[9] as food reserves outside their plastids. Most red algae are also multicellular, macroscopic, marine, and reproduce sexually. The red algal life history is typically an alternation of generations that may have three generations rather than two.[10] The coralline algae, which secrete calcium carbonate and play a major role in building coral reefs, belong here. Red algae such as dulse (Palmaria palmata) and laver (nori/gim) are a traditional part of European and Asian cuisines and are used to make other products such as agar, carrageenans and other food additives.[11]
Evolution
Chloroplasts evolved following an endosymbiotic event between an ancestral, photosynthetic cyanobacterium and an early eukaryotic phagotroph.[12] This event (termed primary endosymbiosis) resulted in the origin of the red and green algae, and the glaucophytes, which make up the oldest evolutionary lineages of photosynthetic eukaryotes.[13] A secondary endosymbiosis event involving an ancestral red alga and a heterotrophic eukaryote resulted in the evolution and diversification of several other photosynthetic lineages such as Cryptophyta, Haptophyta, Stramenopiles (or Heterokontophyta), and Alveolata.[13] In addition to multicellular brown algae, it is estimated that more than half of all known species of microbial eukaryotes harbor red-algal-derived plastids.[14]
Red algae are divided into the Cyanidiophyceae, a class of unicellular and thermoacidophilic extremophiles found in sulphuric hot springs and other acidic environments,[15] an adaptation partly made possible by horizontal gene transfers from prokaryotes,[16] with about 1% of their genome having this origin,[17] and two sister clades called SCRP (Stylonematophyceae, Compsopogonophyceae, Rhodellophyceae and Porphyridiophyceae) and BF (Bangiophyceae and Florideophyceae), which are found in both marine and freshwater environments. The SCRP clade are microalgae, consisting of both unicellular forms and multicellular microscopic filaments and blades. The BF are macroalgae, seaweed that usually do not grow to more than about 50 cm in length, but a few species can reach lengths of 2 m.[18][19] Most rhodophytes are marine with a worldwide distribution, and are often found at greater depths compared to other seaweeds. While this was formerly attributed to the presence of pigments (such as phycoerythrin) that would permit red algae to inhabit greater depths than other macroalgae by chromatic adaption, recent evidence calls this into question (e.g. the discovery of green algae at great depth in the Bahamas).[20] Some marine species are found on sandy shores, while most others can be found attached to rocky substrata.[21] Freshwater species account for 5% of red algal diversity, but they also have a worldwide distribution in various habitats;[5] they generally prefer clean, high-flow streams with clear waters and rocky bottoms, but with some exceptions.[22] A few freshwater species are found in black waters with sandy bottoms [23] and even fewer are found in more lentic waters.[24] Both marine and freshwater taxa are represented by free-living macroalgal forms and smaller endo/epiphytic/zoic forms, meaning they live in or on other algae, plants, and animals.[8] In addition, some marine species have adopted a parasitic lifestyle and may be found on closely or more distantly related red algal hosts.[25][26]
Taxonomy
In the system of Adl et al. 2005, the red algae are classified in the Archaeplastida, along with the glaucophytes and green algae plus land plants (Viridiplantae or Chloroplastida). The authors use a hierarchical arrangement where the clade names do not signify rank; the class name Rhodophyceae is used for the red algae. No subdivisions are given; the authors say, "Traditional subgroups are artificial constructs, and no longer valid."[27]
Many studies published since Adl et al. 2005 have provided evidence that is in agreement for monophyly in the Archaeplastida (including red algae).[28][29][30][31] However, other studies have suggested Archaeplastida is paraphyletic.[32][33] As of January 2011[update], the situation appears unresolved.
Below are other published taxonomies of the red algae using molecular and traditional alpha taxonomic data; however, the taxonomy of the red algae is still in a state of flux (with classification above the level of order having received little scientific attention for most of the 20th century).[34]
- If one defines the kingdom Plantae to mean the Archaeplastida, the red algae will be part of that kingdom
- If Plantae are defined more narrowly, to be the Viridiplantae, then the red algae might be considered their own kingdom, or part of the kingdom Protista.
A major research initiative to reconstruct the Red Algal Tree of Life (RedToL) using phylogenetic and genomic approach is funded by the National Science Foundation as part of the Assembling the Tree of Life Program.
Classification comparison
Some sources (such as Lee) place all red algae into the class "Rhodophyceae". (Lee's organization is not a comprehensive classification, but a selection of orders considered common or important.[36])
A subphylum - Proteorhodophytina - has been proposed to encompass the existing classes Compsopogonophyceae, Porphyridiophyceae, Rhodellophyceae and Stylonematophyceae.[37] This proposal was made on the basis of the analysis of the plastid genomes.
Species of red algae
Over 7,000 species are currently described for the red algae,[3] but the taxonomy is in constant flux with new species described each year.[34][35] The vast majority of these are marine with about 200 that live only in fresh water.
Some examples of species and genera of red algae are:
- Cyanidioschyzon merolae, a primitive red alga
- Atractophora hypnoides
- Gelidiella calcicola
- Lemanea, a freshwater genus
- Palmaria palmata, dulse
- Schmitzia hiscockiana
- Chondrus crispus, Irish moss
- Mastocarpus stellatus
- Vanvoorstia bennettiana, became extinct in the early 20th century
- Acrochaetium efflorescens
- Audouinella, with freshwater as well as marine species
- Polysiphonia ceramiaeformis, banded siphon weed
- Vertebrata simulans
Morphology
Red algal morphology is diverse ranging from unicellular forms to complex parenchymatous and non- parenchymatous thallus.[38] Red algae have double cell walls.[39] The outer layers contain the polysaccharides agarose and agaropectin that can be extracted from the cell walls by boiling as agar.[39] The internal walls are mostly cellulose.[39] They also have the most gene-rich plastid genomes known.[40]
Cell Structure
Red algae do not have flagella and centrioles during their entire life cycle. Presence of normal spindle fibres, microtubules, un-stacked photosynthetic membranes, presence of phycobilin pigment granules.,[41] presence of pit connection between cells filamentous genera, absence of chloroplast endoplasmic reticulum are the distinguishing characters of red algal cell structure.[42]
Chloroplasts
Presence of the water-soluble pigments called phycobilins (phycocyanobilin, phycoerythrobilin, phycourobilin and phycobiliviolin), which are localized into phycobilisomes, gives red algae their distinctive color.[43] Chloroplast contains evenly spaced and ungrouped thylakoids.[44] Double membrane of chloroplast envelope surrounds the chloroplast. Absence of grana and attachment of phycobilisomes on the stromal surface of the thylakoid membrane are other distinguishing characters of red algal chloroplast.[45]
Storage products
The major photosynthetic products include floridoside (major product), D‐isofloridoside, digeneaside, mannitol, sorbitol, dulcitol etc.[46] Floridean starch (similar to amylopectin in land plants), a long term storage product, is deposited freely (scattered) in the cytoplasm.[47] The concentration of photosynthetic products are altered by the environmental conditions like change in pH, the salinity of medium, change in light intensity, nutrient limitation etc.[48][49] When the salinity of the medium increases the production of floridoside is increased in order to prevent water from leaving the algal cells.
Pit connections and pit plugs
Pit connections
Pit connections and pit plugs are unique and distinctive features of red algae that form during the process of cytokinesis following mitosis.[50][51] In red algae, cytokinesis is incomplete. Typically, a small pore is left in the middle of the newly formed partition. The pit connection is formed where the daughter cells remain in contact.
Shortly after the pit connection is formed, cytoplasmic continuity is blocked by the generation of a pit plug, which is deposited in the wall gap that connects the cells.
Connections between cells having a common parent cell are called primary pit connections. Because apical growth is the norm in red algae, most cells have two primary pit connections, one to each adjacent cell.
Connections that exist between cells not sharing a common parent cell are labelled secondary pit connections. These connections are formed when an unequal cell division produced a nucleated daughter cell that then fuses to an adjacent cell. Patterns of secondary pit connections can be seen in the order Ceramiales.[51]
Pit plugs
After a pit connection is formed, tubular membranes appear. A granular protein called the plug core then forms around the membranes. The tubular membranes eventually disappear. While some orders of red algae simply have a plug core, others have an associated membrane at each side of the protein mass, called cap membranes. The pit plug continues to exist between the cells until one of the cells dies. When this happens, the living cell produces a layer of wall material that seals off the plug.
Function
The pit connections have been suggested to function as structural reinforcement, or as avenues for cell-to-cell communication and transport in red algae, however little data supports this hypothesis.[52]
Reproduction
The reproductive cycle of red algae may be triggered by factors such as day length.[2]
Fertilization
Red algae lack motile sperm. Hence, they rely on water currents to transport their gametes to the female organs – although their sperm are capable of "gliding" to a carpogonium's trichogyne.[2]
The trichogyne will continue to grow until it encounters a spermatium; once it has been fertilized, the cell wall at its base progressively thickens, separating it from the rest of the carpogonium at its base.[2]
Upon their collision, the walls of the spermatium and carpogonium dissolve. The male nucleus divides and moves into the carpogonium; one half of the nucleus merges with the carpogonium's nucleus.[2]
The polyamine spermine is produced, which triggers carpospore production.[2]
Spermatangia may have long, delicate appendages, which increase their chances of "hooking up".[2]
Life cycle
They display alternation of generations; in addition to gametophyte generation, many have two sporophyte generations, the carposporophyte-producing carpospores, which germinate into a tetrasporophyte – this produces spore tetrads, which dissociate and germinate into gametophytes.[2] The gametophyte is typically (but not always) identical to the tetrasporophyte.[53]
Carpospores may also germinate directly into thalloid gametophytes, or the carposporophytes may produce a tetraspore without going through a (free-living) tetrasporophyte phase.[53] Tetrasporangia may be arranged in a row (zonate), in a cross (cruciate), or in a tetrad.[2]
The carposporophyte may be enclosed within the gametophyte, which may cover it with branches to form a cystocarp.[53]
These case studies may be helpful to understand some of the life histories algae may display:
In a simple case, such as Rhodochorton investiens:
In the Carposporophyte: a spermatium merges with a trichogyne (a long hair on the female sexual organ), which then divides to form carposporangia – which produce carpospores.
Carpospores germinate into gametophytes, which produce sporophytes. Both of these are very similar; they produce monospores from monosporangia "just below a cross-wall in a filament"[2] and their spores are "liberated through the apex of sporangial cell."[2]
The spores of a sporophyte produce either tetrasporophytes. Monospores produced by this phase germinates immediately, with no resting phase, to form an identical copy of the parent. Tetrasporophytes may also produce a carpospore, which germinates to form another tetrasporophyte.[verification needed][2]
The gametophyte may replicate using monospores, but produces sperm in spermatangia, and "eggs"(?) in carpogonium.[2]
A rather different example is Porphyra gardneri:
In its diploid phase, a carpospore can germinate to form a filamentous "conchocelis stage", which can also self-replicate using monospores. The conchocelis stage eventually produces conchosporangia. The resulting conchospore germinates to form a tiny prothallus with rhizoids, which develops to a cm-scale leafy thallus. This too can reproduce via monospores, which are produced inside the thallus itself.[2] They can also reproduce via spermatia, produced internally, which are released to meet a prospective carpogonium in its conceptacle.[2]
Chemistry
| Algal group | δ13C range[54] |
|---|---|
| HCO3-using red algae | −22.5‰ to −9.6‰ |
| CO2-using red algae | −34.5‰ to −29.9‰ |
| Brown algae | −20.8‰ to −10.5‰ |
| Green algae | −20.3‰ to −8.8‰ |
The δ13C values of red algae reflect their lifestyles. The largest difference results from their photosynthetic metabolic pathway: algae that use HCO3 as a carbon source have less negative δ13C values than those that only use CO2.[54] An additional difference of about 1.71‰ separates groups intertidal from those below the lowest tide line, which are never exposed to atmospheric carbon. The latter group uses the more 13C-negative CO2 dissolved in sea water, whereas those with access to atmospheric carbon reflect the more positive signature of this reserve.
Photosynthetic pigments of Rhodophyta are chlorophylls a and d. Red algae are red due to phycoerythrin. They contain the sulfated polysaccharide carrageenan in the amorphous sections of their cell walls, although red algae from the genus Porphyra contain porphyran. They also produce a specific type of tannin called phlorotannins, but in a lower amount than brown algae do.
Genomes and transcriptomes of red algae
As enlisted in realDB,[55] 27 complete transcriptomes and 10 complete genomes sequences of red algae are available. Listed below are the 10 complete genomes of red algae.
- Cyanidioschyzon merolae, Cyanidiophyceae[56][57]
- Galdieria sulphuraria, Cyanidiophyceae[58]
- Pyropia yezoensis, Bangiophyceae[59]
- Chondrus crispus, Florideophyceae[60]
- Porphyridium purpureum, Porphyridiophyceae[61]
- Porphyra umbilicalis, Bangiophyceae[62]
- Gracilaria changii, Gracilariales [63]
- Galdieria phlegrea, Cyanidiophytina [64]
- Gracilariopsis lemaneiformis, Gracilariales [65]
- Gracilariopsis chorda, Gracilariales [66]
Fossil record
One of the oldest fossils identified as a red alga is also the oldest fossil eukaryote that belongs to a specific modern taxon. Bangiomorpha pubescens, a multicellular fossil from arctic Canada, strongly resembles the modern red alga Bangia despite occurring in rocks dating to 1.2 billion years ago.[1]
Two kinds of fossils resembling red algae were found sometime between 2006 and 2011 in well-preserved sedimentary rocks in Chitrakoot, central India. The presumed red algae lie embedded in fossil mats of cyanobacteria, called stromatolites, in 1.6 billion-year-old Indian phosphorite — making them the oldest plant-like fossils ever found by about 400 million years.[67]
Red algae are important builders of limestone reefs. The earliest such coralline algae, the solenopores, are known from the Cambrian period. Other algae of different origins filled a similar role in the late Paleozoic, and in more recent reefs.
Calcite crusts that have been interpreted as the remains of coralline red algae, date to the terminal Proterozoic.[68] Thallophytes resembling coralline red algae are known from the late Proterozoic Doushantuo formation.[69]
Relationship to other algae
Chromista and Alveolata algae (e.g., chrysophytes, diatoms, phaeophytes, dinophytes) seem to have evolved from bikonts that have acquired red algae as endosymbionts. According to this theory, over time these endosymbiont red algae have evolved to become chloroplasts. This part of endosymbiotic theory is supported by various structural and genetic similarities.[70]
Human consumption
Red algae has a long history of being utilized as an important source of nutritional, functional food ingredients and pharmaceutical substances.[71] Many of the edible red algae are a rich source of antioxidants, have high amount of protein content, minerals, trace elements, vitamins and essential fatty acids.[72] Traditionally red algae are eaten raw, in salads, soups, meal and condiments. Several species are important food crops, in particular members of the genus Porphyra, variously known as nori (Japan), gim (Korea), 紫菜 (China), or laver (Britain). Dulse (Palmaria palmata)[73] is another important British species.[74] These rhodophyte foods are high in vitamins and protein and are easily grown; for example, nori cultivation in Japan goes back more than three centuries. Some of the red algal species like Gracilaria and Laurencia are found to be rich in polyunsaturated fatty acids (eicopentaenoic acid, docohexaenoic acid, arachidonic acid)[75] and have protein content up to 47% of total biomass.[71] Where a big portion of world population is not getting insufficient amount of daily iodine intake, a 150 ug/day requirement of iodine is obtained from a single gram of red algae.[76] Red algae, like Gracilaria, Gelidium, Euchema, Porphyra, Acanthophora, and Palmaria are primarily known for their industrial use for phycocolloids (agar, align, furcellaran and carrageenan) as thickening agent, textiles, food, anticoagulants, water-binding agents etc.[77] Dulse (Palmaria palmata) is one of the most consumed red algae and is a good source of iodine, protein, magnesium and calcium.[78] China, Japan, Republic of Korea are the top producers of seaweeds.[79] In East and Southeast Asia, agar is most commonly produced from Gelidium amansii.
Gallery
-
Cyanidium sp. (Cyanidiophyceae)
-
Porphyra sp., haploid and diploid (Bangiophyceae)
-
Gracilaria sp. (Florideophyceae: Gracilariales)
-
Corallina officinalis sp. (Florideophyceae: Corallinales)
-
Laurencia sp. (Florideophyceae: Ceramiales)
-
Some red algae are iridescent when not covered with water
See also
References
- ^ a b N. J. Butterfield (2000). "Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology. 26 (3): 386–404. doi:10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. ISSN 0094-8373.
- ^ a b c d e f g h i j k l m n o Lee, R.E. (2008). Phycology (4th ed.). Cambridge University Press. ISBN 978-0-521-63883-8.
- ^ a b c Guiry, M.D.; Guiry, G.M. (2016). "Algaebase". www.algaebase.org. Retrieved November 20, 2016.
- ^ D. Thomas (2002). Seaweeds. Life Series. Natural History Museum, London. ISBN 978-0-565-09175-0.
- ^ a b Sheath, Robert G. (1984). "The biology of freshwater red algae". Progress Phycological Research. 3: 89–157.
- ^ Azua-Bustos, A; González-Silva, C; Arenas-Fajardo, C; Vicuña, R (2012). "Extreme environments as potential drivers of convergent evolution by exaptation: the Atacama Desert Coastal Range case". Front Microbiol. 3: 426. doi:10.3389/fmicb.2012.00426. PMC 3526103. PMID 23267354.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Why don't we live on a red planet?
- ^ a b W. J. Woelkerling (1990). "An introduction". In K. M. Cole; R. G. Sheath (eds.). Biology of the Red Algae. Cambridge University Press, Cambridge. pp. 1–6. ISBN 978-0-521-34301-5.
- ^ Viola, R.; Nyvall, P.; Pedersén, M. (2001). "The unique features of starch metabolism in red algae". Proceedings of the Royal Society of London B. 268 (1474): 1417–1422. doi:10.1098/rspb.2001.1644. PMC 1088757. PMID 11429143.
- ^ "Algae". autocww.colorado.edu.
- ^ M. D. Guiry. "Rhodophyta: red algae". National University of Ireland, Galway. Archived from the original on 2007-05-04. Retrieved 2007-06-28.
- ^ Gould, S.B.; Waller, R.F.; McFadden, G.I. (2008). "Plastid Evolution". Annual Review of Plant Biology. 59: 491–517. doi:10.1146/annurev.arplant.59.032607.092915. PMID 18315522.
- ^ a b McFadden, G.I. (2001). "Primary and Secondary Endosymbiosis and the Evolution of Plastids". Journal of Phycology. 37 (6): 951–959. doi:10.1046/j.1529-8817.2001.01126.x.
- ^ Steal My Sunshine | The Scientist Magazine
- ^ Ciniglia, C.; Yoon, H.; Pollio, A.; Bhattacharya, D. (2004). "Hidden biodiversity of the extremophilic Cyanidiales red algae". Molecular Ecology. 13 (7): 1827–1838. doi:10.1111/j.1365-294X.2004.02180.x. PMID 15189206.
- ^ Plants and animals sometimes take genes from bacteria, study of algae suggests - Sciencemag.org
- ^ The genomes of polyextremophilic cyanidiales contain 1% horizontally transferred genes with diverse adaptive functions
- ^ Brawley, SH (2017). "Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta)". Proc Natl Acad Sci U S A. 114: E6361–E6370. doi:10.1073/pnas.1703088114. PMC 5547612. PMID 28716924.
- ^ Brawley, SH (2017). "Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta)". Proc Natl Acad Sci U S A. 114: E6361–E6370. doi:10.1073/pnas.1703088114. PMC 5547612. PMID 28716924.
- ^ Norris, J. N.; Olsen, J. L. (1991). "Deep-water green algae from the Bahamas, including Cladophora vandenhoekii sp. nov. (Cladophorales)". Phycologia. 30 (4): 315–328. doi:10.2216/i0031-8884-30-4-315.1. ISSN 0031-8884.
- ^ Kain, J.M.; Norton, T.A. (1990). "Marine Ecology". In Cole, J.M.; Sheath, R.G. (eds.). Biology of the Red Algae. Cambridge, U.K.: Cambridge University Press. pp. 377–423. ISBN 978-0521343015.
- ^ Eloranta, P.; Kwandrans, J. (2004). "Indicator value of freshwater red algae in running waters for water quality assessment" (PDF). International Journal of Oceanography and Hydrobiology. XXXIII (1): 47–54. ISSN 1730-413X. Archived from the original (PDF) on 2011-07-27.
- ^ Vis, M.L.; Sheath, R.G.; Chiasson, W.B. (2008). "A survey of Rhodophyta and associated macroalgae from coastal streams in French Guiana". Cryptogamie Algologie. 25: 161–174.
- ^ Sheath, R.G.; Hambrook, J.A. (1990). "Freshwater Ecology". In Cole, K.M.; Sheath, R.G. (eds.). Biology of the Red Algae. Cambridge, U.K.: Cambridge University Press. pp. 423–453. ISBN 978-0521343015.
- ^ Goff, L.J. (1982). "The biology of parasitic red algae". Progress Phycological Research. 1: 289–369.
- ^ Salomaki, E.D.; Lane, C.E. (2014). "Are all red algal parasites cut from the same cloth?". Acta Societatis Botanicorum Poloniae. 83 (4): 369–375. doi:10.5586/asbp.2014.047.
- ^ Adl, Sina M.; et al. (2005). "The New Higher Level Classification of Eukaryotes with Emphasis on the Taxonomy of Protists". Journal of Eukaryotic Microbiology. 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873.
- ^ Fabien Burki; Kamran Shalchian-Tabrizi; Marianne Minge; Åsmund Skjæveland; Sergey I. Nikolaev; Kjetill S. Jakobsen; Jan Pawlowski (2007). Butler, Geraldine (ed.). "Phylogenomics Reshuffles the Eukaryotic Supergroups". PLoS ONE. 2 (8): e790. Bibcode:2007PLoSO...2..790B. doi:10.1371/journal.pone.0000790. PMC 1949142. PMID 17726520.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Burki, Fabien; Inagaki, Yuji; Bråte, Jon; Archibald, John M.; Keeling, Patrick J.; Cavalier-Smith, Thomas; Sakaguchi, Miako; Hashimoto, Tetsuo; Horak, Ales; Kumar, Surendra; Klaveness, Dag; Jakobsen, Kjetill S.; Pawlowski, Jan; Shalchian-Tabrizi, Kamran (2009). "Large-Scale Phylogenomic Analyses Reveal That Two Enigmatic Protist Lineages, Telonemia and Centroheliozoa, Are Related to Photosynthetic Chromalveolates". Genome Biology and Evolution. 1: 231–8. doi:10.1093/gbe/evp022. PMC 2817417. PMID 20333193.
- ^ Cavalier-Smith, Thomas (2009). "Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree". Biology Letters. 6 (3): 342–5. doi:10.1098/rsbl.2009.0948. PMC 2880060. PMID 20031978.
- ^ Rogozin, I.B.; Basu, M.K.; Csürös, M.; Koonin, E.V. (2009). "Analysis of Rare Genomic Changes Does Not Support the Unikont–Bikont Phylogeny and Suggests Cyanobacterial Symbiosis as the Point of Primary Radiation of Eukaryotes". Genome Biology and Evolution. 1: 99–113. doi:10.1093/gbe/evp011. PMC 2817406. PMID 20333181.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Kim, E.; Graham, L.E.; Graham, Linda E. (2008). Redfield, Rosemary Jeanne (ed.). "EEF2 analysis challenges the monophyly of Archaeplastida and Chromalveolata". PLoS ONE. 3 (7): e2621. Bibcode:2008PLoSO...3.2621K. doi:10.1371/journal.pone.0002621. PMC 2440802. PMID 18612431.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Nozaki, H.; Maruyama, S.; Matsuzaki, M.; Nakada, T.; Kato, S.; Misawa, K. (2009). "Phylogenetic positions of Glaucophyta, green plants (Archaeplastida) and Haptophyta (Chromalveolata) as deduced from slowly evolving nuclear genes". Molecular Phylogenetics and Evolution. 53 (3): 872–880. doi:10.1016/j.ympev.2009.08.015. PMID 19698794.
- ^ a b c G. W. Saunders; M. H. Hommersand (2004). "Assessing red algal supraordinal diversity and taxonomy in the context of contemporary systematic data". American Journal of Botany. 91 (10): 1494–1507. doi:10.3732/ajb.91.10.1494. PMID 21652305.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ a b Hwan Su Yoon; K. M. Müller; R. G. Sheath; F. D. Ott; D. Bhattacharya (2006). "Defining the major lineages of red algae (Rhodophyta)" (PDF). Journal of Phycology. 42 (2): 482–492. doi:10.1111/j.1529-8817.2006.00210.x.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Robert Edward Lee (2008). Phycology. Cambridge University Press. p. 107. ISBN 978-0-521-68277-0. Retrieved 31 January 2011.
- ^ Muñoz-Gómez, SA; Mejía-Franco, FG; Durnin, K; Colp, M; Grisdale, CJ; Archibald, JM; Ch, Slamovits (2017). "The new red algal subphylum Proteorhodophytina comprises the largest and most divergent plastid genomes known". Curr Biol. 27 (11): 1677–1684.
- ^ Goff, L. J.; Coleman, A. W. (1986). "A Novel Pattern of Apical Cell Polyploidy, Sequential Polyploidy Reduction and Intercellular Nuclear Transfer in the Red Alga Polysiphonia". American Journal of Botany. 73 (8): 1109–1130. doi:10.1002/j.1537-2197.1986.tb08558.x.
- ^ a b c Fritsch, F. E. (1945), The structure and reproduction of the algae, Cambridge: Cambridge Univ. Press, ISBN 0521050421, OCLC 223742770
- ^ Evolution of Red Algal Plastid Genomes: Ancient Architectures, Introns, Horizontal Gene Transfer, and Taxonomic Utility of Plastid Markers - PLOS
- ^ W. J. Woelkerling (1990). "An introduction". In K. M. Cole; R. G. Sheath (eds.). Biology of the Red Algae. Cambridge University Press, Cambridge. pp. 1–6. ISBN 978-0-521-34301-5.
- ^ Scott, J.; Cynthia, B.; Schornstein, K.; Thomas, J. (1980). "Ultrastructure of Cell Division and Reproductive Differentiation of Male Plants in the Florideophyceae (Rhodophyta): Cell Division in Polysiphonia1". Journal of Phycology. 16 (4): 507–524. doi:10.1111/j.1529-8817.1980.tb03068.x.
- ^ Gantt, E (1969). "Properties and Ultrastructure of Phycoerythrin From Porphyridium cruentum12". Plant Physiology. 44 (11): 1629–1638.
- ^ The Fine Structure of Algal Cells—1st Edition. (n.d.). Retrieved October 16, 2019, from https://www.elsevier.com/books/the-fine-structure-of-algal-cells/dodge/978-0-12-219150-3
- ^ Tsekos, I.; Reiss, H.-D.; Orfanidis, S.; Orologas, N. (1996). "Ultrastructure and supramolecular organization of photosynthetic membranes of some marine red algae". New Phytologist. 133 (4): 543–551. doi:10.1111/j.1469-8137.1996.tb01923.x.
- ^ Karsten, U.; West, J. A.; Zuccarello, G. C.; Engbrodt, R.; Yokoyama, A.; Hara, Y.; Brodie, J. (2003). "Low Molecular Weight Carbohydrates of the Bangiophycidae (Rhodophyta)1". Journal of Phycology. 39 (3): 584–589. doi:10.1046/j.1529-8817.2003.02192.x.
- ^ Lee, R. E. (1974). Chloroplast structure and starch grain production as phylogenetic indicators in the lower Rhodophyceae. British Phycological Journal, 9(3), 291–295. https://doi.org/10.1080/00071617400650351
- ^ Joniyas, A., Surif, M., & Dehgahi, R. (2016). Effect of Nutrient and Light Intensity on Nutrient Uptakes of Gracilaria manilaensis. https://doi.org/10.12983/ijsres-2016-p0173-0185
- ^ Low Molecular Weight Carbohydrates in Red Algae – an Ecophysiological and Biochemical Perspective | SpringerLink. (n.d.). Retrieved October 16, 2019, from https://link.springer.com/chapter/10.1007/978-90-481-3795-4_24
- ^ Clinton JD, Scott FM, Bowler E (November–December 1961). "A Light- and Electron-Microscopic Survey of Algal Cell Walls. I. Phaeophyta and Rhodophyta". American Journal of Botany. 48 (10): 925–934. doi:10.2307/2439535. JSTOR 2439535.
- ^ a b Lee RE (2008). Phycology (4th ed.). Cambridge University Press. ISBN 978-0-521-63883-8.
- ^ "Pit Plugs". FHL Marine Botany. Retrieved 2016-06-30.
- ^ a b c Kohlmeyer, J. (February 1975). "New Clues to the Possible Origin of Ascomycetes". BioScience. 25 (2): 86–93. Bibcode:1985BioSc..35..499W. doi:10.2307/1297108. JSTOR 1297108.
- ^ a b Maberly, S. C.; Raven, J. A.; Johnston, A. M. (1992). "Discrimination between 12C and 13C by marine plants". Oecologia. 91 (4): 481. doi:10.1007/BF00650320. JSTOR 4220100.
- ^ Chen, F., Zhang, J., Chen, J., Li, X., Dong, W., Hu, J., … Zhang, L. (2018). realDB: A genome and transcriptome resource for the red algae (phylum Rhodophyta). Database, 2018. https://doi.org/10.1093/database/bay072
- ^ Matsuzaki; et al. (April 2004). "Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D". Nature. 428 (6983): 653–7. Bibcode:2004Natur.428..653M. doi:10.1038/nature02398. PMID 15071595.
- ^ Nozaki; et al. (2007). "A 100%-complete sequence reveals unusually simple genomic features in the hot-spring red alga Cyanidioschyzon merolae". BMC Biology. 5: 28. doi:10.1186/1741-7007-5-28. PMC 1955436. PMID 17623057.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Schönknecht; et al. (March 2013). "Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote". Science. 339 (6124): 1207–1210. Bibcode:2013Sci...339.1207S. doi:10.1126/science.1231707. PMID 23471408.
- ^ Nakamura; et al. (2013). "The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis)". PLoS ONE. 8 (3): e57122. Bibcode:2013PLoSO...857122N. doi:10.1371/journal.pone.0057122. PMC 3594237. PMID 23536760.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Collen; et al. (2013). "Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida". PNAS. 110 (13): 5247–5252. Bibcode:2013PNAS..110.5247C. doi:10.1073/pnas.1221259110. PMC 3612618. PMID 23503846.
- ^ Bhattacharya; et al. (2013). "Genome of the red alga Porphyridium purpureum". Nature Communications. 4: 1941. Bibcode:2013NatCo...4.1941B. doi:10.1038/ncomms2931. PMC 3709513. PMID 23770768.
- ^ Brawley, SH; Blouin, NA; Ficko-Blean, E; Wheeler, GL; et al. (1 August 2017). "Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta)". Proceedings of the National Academy of Sciences of the United States of America. 114 (31): E6361–E6370. doi:10.1073/pnas.1703088114. PMC 5547612. PMID 28716924.
- ^ Ho, C.-L.; Lee, W.-K.; Lim, E.-L. (2018). "Unraveling the nuclear and chloroplast genomes of an agar producing red macroalga, Gracilaria changii (Rhodophyta, Gracilariales)". Genomics. 110 (2): 124–133. doi:10.1016/j.ygeno.2017.09.003.
- ^ Qiu, H.; Price, D. C.; Weber, A. P. M.; Reeb, V.; Yang, E. C.; Lee, J. M.; Bhattacharya, D. (2013). "Adaptation through horizontal gene transfer in the cryptoendolithic red alga Galdieria phlegrea". Current Biology. 23 (19): R865–R866. doi:10.1016/j.cub.2013.08.046.
- ^ Zhou, W.; Hu, Y.; Sui, Z.; Fu, F.; Wang, J.; Chang, L.; Li, B. (2013). "Genome Survey Sequencing and Genetic Background Characterization of Gracilariopsis lemaneiformis (Rhodophyta) Based on Next-Generation Sequencing". PLOS ONE. 8 (7): e69909. doi:10.1371/journal.pone.0069909.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ JunMo Lee, Eun Chan Yang, Louis Graf, Ji Hyun Yang, Huan Qiu, Udi Zelzion, Cheong Xin Chan, Timothy G Stephens, Andreas P M Weber, Ga Hun Boo, Sung Min Boo, Kyeong Mi Kim, Younhee Shin, Myunghee Jung, Seung Jae Lee, Hyung-Soon Yim, Jung-Hyun Lee, Debashish Bhattacharya, Hwan Su Yoon, Analysis of the Draft Genome of the Red Seaweed Gracilariopsis chorda Provides Insights into Genome Size Evolution in Rhodophyta, Molecular Biology and Evolution, Volume 35, Issue 8, August 2018, Pages 1869–1886, https://doi.org/10.1093/molbev/msy081
- ^ Bengtson, S; Sallstedt, T; Belivanova, V; Whitehouse, M (2017). "Three-dimensional preservation of cellular and subcellular structures suggests 1.6 billion-year-old crown-group red algae". PLoS Biol. 15 (3): e2000735. doi:10.1371/journal.pbio.2000735. PMC 5349422. PMID 28291791.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Grant, S. W. F.; Knoll, A. H.; Germs, G. J. B. (1991). "Probable Calcified Metaphytes in the Latest Proterozoic Nama Group, Namibia: Origin, Diagenesis, and Implications". Journal of Paleontology. 65 (1): 1–18. doi:10.1017/S002233600002014X. JSTOR 1305691. PMID 11538648.
- ^ Yun, Z.; Xun-lal, Y. (1992). "New data on multicellular thallophytes and fragments of cellular tissues from Late Proterozoic phosphate rocks, South China". Lethaia. 25 (1): 1–18. doi:10.1111/j.1502-3931.1992.tb01788.x.
- ^ Summarised in Cavalier-Smith, Thomas (April 2000). "Membrane heredity and early chloroplast evolution". Trends in Plant Science. 5 (4): 174–182. doi:10.1016/S1360-1385(00)01598-3. PMID 10740299.
- ^ a b Wang, T., Jónsdóttir, R., Kristinsson, H. G., Hreggvidsson, G. O., Jónsson, J. Ó., Thorkelsson, G., & Ólafsdóttir, G. (2010). Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT - Food Science and Technology, 43(9), 1387–1393. https://doi.org/10.1016/j.lwt.2010.05.010
- ^ MacArtain, P.; Gill, C. I. R.; Brooks, M.; Campbell, R.; Rowland, I. R. (2007). "Nutritional Value of Edible Seaweeds". Nutrition Reviews. 65 (12): 535–543. doi:10.1111/j.1753-4887.2007.tb00278.x.
- ^ "Dulse: Palmaria palmata". Quality Sea Veg. Retrieved 2007-06-28.
- ^ T. F. Mumford; A. Muira (1988). "Porphyra as food: cultivation and economics". In C. A. Lembi; J. Waaland (eds.). Algae and Human Affairs. Cambridge University Press, Cambridge. ISBN 978-0-521-32115-0.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Gressler, V., Yokoya, N. S., Fujii, M. T., Colepicolo, P., Filho, J. M., Torres, R. P., & Pinto, E. (2010). Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chemistry, 120(2), 585–590. https://doi.org/10.1016/j.foodchem.2009.10.028
- ^ Hoek, C. van den, Mann, D.G. and Jahns, H.M. 1995. Algae An Introduction to Phycology. Cambridge University Press, Cambridge. ISBN 0 521 30419 9
- ^ Dhargalkar VK, Verlecar XN. Southern Ocean Seaweeds: a resource for exploration in food and drugs. Aquaculture 2009; 287: 229-42.
- ^ Musa Jibril, Sauban; Hassan Jakada, Bello; Yunusa Umar, Harisu; Abdul-qadir Ahmad, Tahir (2016). "Importance of Some Algal Species as a Source of Food and Supplement. Int.J.Curr.Microbiol.App". Sci. 5 (5): 186–193.
- ^ Manivannan, K., Thirumaran, G., Karthikai, D.G., Anantharaman. P., Balasubramanian, P. 2009. Proximate Composition of Different Group of Seaweeds from Vedalai Coastal Waters (Gulf of Mannar): Southeast Coast of India. Middle-East J. Scientific Res., 4: 72-77.







