COVID-19 drug repurposing research: Difference between revisions
No edit summary |
No edit summary |
||
| Line 50: | Line 50: | ||
====Controversy==== |
====Controversy==== |
||
{{See also|Surgisphere#COVID-19 controversy}} |
{{See also|Surgisphere#COVID-19 controversy}} |
||
Due to safety concerns and evidence of [[Arrhythmia|heart arrhythmias]] leading to higher death rates, the WHO suspended the hydroxychloroquine arm of the multinational [[Solidarity trial]] in late May 2020.<ref name="who-suspend">{{Cite web|title=WHO Director-General's opening remarks at the media briefing on COVID-19 – 25 May 2020|url=https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---25-may-2020|publisher=World Health Organization|date=2020-05-25|access-date=2020-05-27}}</ref><ref>{{Cite news|title=WHO pauses hydroxychloroquine coronavirus trial over safety concerns|url=https://globalnews.ca/news/6983283/who-hydroxychloroquine-trial/|work=Global News|publisher=The Associated Press|authors=Maria Cheng, Jamey Keaten|date=2020-05-25|access-date=2020-05-27}}</ref><ref>{{Cite news|title=Coronavirus: WHO halts trials of hydroxychloroquine over safety fears|url=https://www.bbc.com/news/health-52799120|work=BBC News Online |date=2020-05-25|access-date=2020-06-04}}</ref> The WHO had enrolled 3,500 patients from 17 countries in the Solidarity trial.<ref name=who-suspend/> The research surrounding this suspension, provided by a company called [[Surgisphere]] based in [[Chicago]], came into question due to errors in the underlying data set.<ref name="stat1">{{cite web |url=https://www.statnews.com/2020/06/02/top-medical-journals-raise-concerns-about-data-in-two-studies-related-to-covid-19/ |title=Top medical journals raise concerns about data in two studies related to Covid-19 | first1 = Matthew | last1 = Herper | first2 = Andrew | last2 = Joseph | name-list-format = vanc |date=June 2, 2020 |website=[[Stat (website)|Stat]] |access-date=June 4, 2020}}</ref><ref>{{cite journal |last1=Servick |first1=Kelly |title=A mysterious company’s coronavirus papers in top medical journals may be unraveling |journal=Science |date=2 June 2020 |doi=10.1126/science.abd1337 }}</ref><ref>{{cite web |url=https://www.theguardian.com/science/2020/may/28/questions-raised-over-hydroxychloroquine-study-which-caused-who-to-halt-trials-for-covid-19 |title=Questions raised over hydroxychloroquine study which caused WHO to halt trials for Covid-19 |author=Melissa Davey |date=May 28, 2020 |website=The Guardian |access-date=June 4, 2020}}</ref> The authors of the study corrected errors in the data later but initially remained firm on their conclusions.<ref name="stat1"/> Subsequently, a retraction of the study by three of its authors was published by ''The Lancet'' on 4{{nbsp}}June, 2020.<ref>{{cite journal |last1=Mehra |first1=Mandeep R |last2=Ruschitzka |first2=Frank |last3=Patel |first3=Amit N |title=Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis |journal=The Lancet |date=June 2020 |volume=395 |issue=10240 |pages=1820 |doi=10.1016/S0140-6736(20)31324-6 |pmid=32511943 |pmc=7274621 }</ref> The authors stated that their reason behind the retraction was because Surgisphere had failed to cooperate with an independent review of the data used for the study by not allowing any such review to take place.<ref>{{cite news |url=https://www.bbc.com/news/health-52929916 |title=Coronavirus: Influential study on hydroxychloroquine withdrawn |date=June 5, 2020 |website=BBC News Online |access-date=June 5, 2020}}</ref><ref>{{cite news | last1=Boseley | first1=Sarah | last2=Davey | first2=Melissa | name-list-format = vanc | title=Covid-19: Lancet retracts paper that halted hydroxychloroquine trials | website=[[The Guardian]] | date=4 June 2020 | url=http://www.theguardian.com/world/2020/jun/04/covid-19-lancet-retracts-paper-that-halted-hydroxychloroquine-trials | access-date=4 June 2020}}</ref> |
Due to safety concerns and evidence of [[Arrhythmia|heart arrhythmias]] leading to higher death rates, the WHO suspended the hydroxychloroquine arm of the multinational [[Solidarity trial]] in late May 2020.<ref name="who-suspend">{{Cite web|title=WHO Director-General's opening remarks at the media briefing on COVID-19 – 25 May 2020|url=https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---25-may-2020|publisher=World Health Organization|date=2020-05-25|access-date=2020-05-27}}</ref><ref>{{Cite news|title=WHO pauses hydroxychloroquine coronavirus trial over safety concerns|url=https://globalnews.ca/news/6983283/who-hydroxychloroquine-trial/|work=Global News|publisher=The Associated Press|authors=Maria Cheng, Jamey Keaten|date=2020-05-25|access-date=2020-05-27}}</ref><ref>{{Cite news|title=Coronavirus: WHO halts trials of hydroxychloroquine over safety fears|url=https://www.bbc.com/news/health-52799120|work=BBC News Online |date=2020-05-25|access-date=2020-06-04}}</ref> The WHO had enrolled 3,500 patients from 17 countries in the Solidarity trial.<ref name=who-suspend/> The research surrounding this suspension, provided by a company called [[Surgisphere]] based in [[Chicago]], came into question due to errors in the underlying data set.<ref name="stat1">{{cite web |url=https://www.statnews.com/2020/06/02/top-medical-journals-raise-concerns-about-data-in-two-studies-related-to-covid-19/ |title=Top medical journals raise concerns about data in two studies related to Covid-19 | first1 = Matthew | last1 = Herper | first2 = Andrew | last2 = Joseph | name-list-format = vanc |date=June 2, 2020 |website=[[Stat (website)|Stat]] |access-date=June 4, 2020}}</ref><ref>{{cite journal |last1=Servick |first1=Kelly |title=A mysterious company’s coronavirus papers in top medical journals may be unraveling |journal=Science |date=2 June 2020 |doi=10.1126/science.abd1337 }}</ref><ref>{{cite web |url=https://www.theguardian.com/science/2020/may/28/questions-raised-over-hydroxychloroquine-study-which-caused-who-to-halt-trials-for-covid-19 |title=Questions raised over hydroxychloroquine study which caused WHO to halt trials for Covid-19 |author=Melissa Davey |date=May 28, 2020 |website=The Guardian |access-date=June 4, 2020}}</ref> The authors of the study corrected errors in the data later but initially remained firm on their conclusions.<ref name="stat1"/> Subsequently, a retraction of the study by three of its authors was published by ''The Lancet'' on 4{{nbsp}}June, 2020.<ref>{{cite journal |last1=Mehra |first1=Mandeep R |last2=Ruschitzka |first2=Frank |last3=Patel |first3=Amit N |title=Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis |journal=The Lancet |date=June 2020 |volume=395 |issue=10240 |pages=1820 |doi=10.1016/S0140-6736(20)31324-6 |pmid=32511943 |pmc=7274621 }}</ref> The authors stated that their reason behind the retraction was because Surgisphere had failed to cooperate with an independent review of the data used for the study by not allowing any such review to take place.<ref>{{cite news |url=https://www.bbc.com/news/health-52929916 |title=Coronavirus: Influential study on hydroxychloroquine withdrawn |date=June 5, 2020 |website=BBC News Online |access-date=June 5, 2020}}</ref><ref>{{cite news | last1=Boseley | first1=Sarah | last2=Davey | first2=Melissa | name-list-format = vanc | title=Covid-19: Lancet retracts paper that halted hydroxychloroquine trials | website=[[The Guardian]] | date=4 June 2020 | url=http://www.theguardian.com/world/2020/jun/04/covid-19-lancet-retracts-paper-that-halted-hydroxychloroquine-trials | access-date=4 June 2020}}</ref> |
||
The WHO decided to resume the trial on 3{{nbsp}}June, after reviewing the safety concerns which had been raised. Speaking at a press briefing, WHO's director-general, [[Tedros Adhanom Ghebreyesus]] stated that the board had reviewed the available mortality data and had found "no reasons to modify the trial".<ref>{{cite web |url=https://www.statnews.com/2020/06/03/who-resuming-hydroxychloroquine-study-for-covid-19/ |title=WHO resumes hydroxychloroquine study for Covid-19, after reviewing safety concerns |author=Andrew Joseph |date=June 3, 2020 |website=[[Stat (website)|Stat]] |access-date=June 4, 2020}}</ref><ref>{{cite web |url=https://www.independent.co.uk/news/health/coronavirus-hydroxychloroquine-trials-trump-who-lancet-a9546836.html |title=Coronavirus: WHO re-starts hydroxychloroquine trials amid controversy over published research |author=Shaun Lintern |date=June 3, 2020 |website=The Independent |access-date=June 4, 2020}}</ref> |
The WHO decided to resume the trial on 3{{nbsp}}June, after reviewing the safety concerns which had been raised. Speaking at a press briefing, WHO's director-general, [[Tedros Adhanom Ghebreyesus]] stated that the board had reviewed the available mortality data and had found "no reasons to modify the trial".<ref>{{cite web |url=https://www.statnews.com/2020/06/03/who-resuming-hydroxychloroquine-study-for-covid-19/ |title=WHO resumes hydroxychloroquine study for Covid-19, after reviewing safety concerns |author=Andrew Joseph |date=June 3, 2020 |website=[[Stat (website)|Stat]] |access-date=June 4, 2020}}</ref><ref>{{cite web |url=https://www.independent.co.uk/news/health/coronavirus-hydroxychloroquine-trials-trump-who-lancet-a9546836.html |title=Coronavirus: WHO re-starts hydroxychloroquine trials amid controversy over published research |author=Shaun Lintern |date=June 3, 2020 |website=The Independent |access-date=June 4, 2020}}</ref> |
||
Revision as of 21:48, 10 September 2020
| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|
Drug repositioning (also known as drug re-purposing, re-profiling, re-tasking, or therapeutic switching) is the re-purposing of an approved drug for the treatment of a different disease or medical condition than that for which it was originally developed.[1] This is one line of scientific research which is being pursued to develop safe and effective COVID-19 treatments.[2][3] Other research directions include the development of a COVID-19 vaccine[4] and convalescent plasma transfusion.[5]
Several existing antiviral medications, previously developed or used as treatments for severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), HIV/AIDS, and malaria, have been researched as COVID‑19 treatments, with some moving into clinical trials.[6][7][8]
In a statement to the journal Nature Biotechnology in February 2020, US National Institutes of Health Viral Ecology Unit chief Vincent Munster said, "The general genomic layout and the general replication kinetics and the biology of the MERS, SARS and [SARS-CoV-2] viruses are very similar, so testing drugs which target relatively generic parts of these coronaviruses is a logical step".[2]
RECOVERY Trial
A large-scale, randomized controlled trial named the RECOVERY Trial was set up in March 2020, in the UK to test possible treatments for COVID-19. It is run by the Nuffield Departments of Public Health and of Medicine at the University of Oxford and is testing five repurposed drugs and also convalescent plasma. The trial enrolled more than 11,500 COVID-19 positive participants in the U.K by June 2020.[9][10][11]
By the end of June 2020, the trial had published findings regarding hydroxychloroquine and dexamethasone.[11][12] It has also announced results for lopinavir/ritonavir which are yet to be published.[13]
Studies
Chloroquine and hydroxychloroquine
Chloroquine is an anti-malarial medication also used against some auto-immune diseases. On 18 March, the World Health Organization (WHO) announced that chloroquine and the related hydroxychloroquine would be among the four drugs studied as part of the multinational Solidarity clinical trial.[14] On 19 March, US President Donald Trump encouraged the use of chloroquine and hydroxychloroquine during a national press conference. These endorsements led to massive increases in public demand for the drugs in the United States.[15] New York Governor Andrew Cuomo announced that New York State trials of chloroquine and hydroxychloroquine would begin on 24 March.[16]
On 28 March, the US Food and Drug Administration (FDA) authorized the use of hydroxychloroquine sulfate and chloroquine phosphate under an Emergency Use Authorization (EUA).[17][18] The treatment has not been approved by the FDA's clinical trials process.[18] However, the drug is authorized under the EUA as an experimental treatment for emergency use in patients who are hospitalized, but are not able to receive treatment in a clinical trial.[18][19][17] The Centers for Disease Control and Prevention (CDC) said that "the use, dosing, or duration of hydroxychloroquine for prophylaxis or treatment of SARS-CoV-2 infection" are not yet established.[20] Doctors have said they are using the drug when "there's no other option".[21] On 9 April, the National Institutes of Health began the first clinical trial to assess whether hydroxychloroquine is safe and effective to treat COVID‑19.[21][22]
On 15 June, the FDA revoked the emergency use authorization for hydroxychloroquine and chloroquine, stating that although the evaluation of both these drugs under clinical trials continues, the FDA (after interagency consultation with the Biomedical Advanced Research and Development Authority (BARDA)) concluded that, based on new information and other information discussed "... it is no longer reasonable to believe that oral formulations of hydroxychloroquine (HCQ) and chloroquine (CQ) may be effective in treating COVID‑19, nor is it reasonable to believe that the known and potential benefits of these products outweigh their known and potential risks".[23][24][25][26]
On 24 April, the FDA cautioned against using the drug outside a hospital setting or clinical trial after reviewing case reports of adverse effects including ventricular tachycardia, ventricular fibrillation and in some cases death.[27] According to Johns Hopkins' ABX Guide for COVID‑19, "Hydroxychloroquine may cause prolonged QT, and caution should be used in critically ill COVID‑19 patients who may have cardiac dysfunction or if combined with other drugs that cause QT prolongation".[28] Caution was also recommended as to the combination of chloroquine and hydroxychloroquine with treatments which might inhibit the CYP3A4 enzyme (by which these drugs are metabolized). As such, combination might indirectly result in higher plasma levels of chloroquine and hydroxychloroquine, and thus enhance the risk for significant QT prolongation. CYP3A4 inhibitors include Azithromycin, ritonavir and lopinavir.[29] A Veterans Affairs study released results on 21 April suggesting COVID‑19-hospitalized patients treated with hydroxychloroquine were more likely to die than those who received no drug treatment at all, after correcting for clinical characteristics.[30][31]
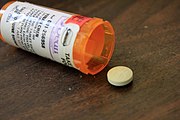
A randomized, double-blind, placebo-controlled trial of hydroxychloroquine in 821 participants by the University of Minnesota Medical School found that it does not treat COVID‑19 infection.[32][33][34]
In June, use of hydroxychloroquine in the UK RECOVERY Trial was discontinued when an interim analysis of 1,542 treatments showed it provided no mortality benefit to people hospitalized with severe COVID‑19 infection over 28 days of observation.[11]
A multicenter, randomized, open-label, three-group, controlled trial of 667 participants in Brazil found no benefit from using hydroxychloroquine, alone or with azithromycin, to treat mild-to-moderate COVID‑19.[35]
In late July, the U.S President Donald Trump once again promoted the use of the drug contradicting various public health officials, including Dr. Fauci.[36]
Combined with zinc and another antibiotic
Due to the properties of zinc as a cofactor in the immune response for producing antibodies during viral infections,[37] it is being included among multiple-agent "cocktails" for investigating potential treatment of people hospitalized with COVID‑19 infection.[38] One such cocktail – hydroxychloroquine combined with a high dose of zinc (as a sulfate, 220 mg per day for five days, a zinc dose twenty times higher than the reference daily intake level)[37] and an approved antibiotic, either azithromycin or doxycycline – began in May as a Phase IV trial in New York State.[39] However, caution was recommended about the combination of chloroquine or hydroxychloroquine with CYP3A4 inhibitors, such as azithromycin,[29] a treatment combination found to be ineffective for preventing death in hospitalized people with COVID‑19.[40] There is preliminary evidence that combining hydroxychloroquine and azithromycin for treating non-hospitalized ("outpatient") people with COVID‑19 infection with multiple comorbidities was effective, but remains under preliminary research.[41]
Zinc deficiency – which decreases immune capacity to defend against pathogens – is common among elderly people, and may be a susceptibility factor in viral infections.[37] The mechanism for any potential benefit of including zinc in a cocktail treatment for recovery from severe COVID‑19 or any viral infection is unknown.[37][38]
Prophylaxis
Drugs used for treatment of infectious diseases may also be considered for use for post-exposure prophylaxis. On 22 May, The Lancet published a response to criticism of the Indian government's decision to allow chemoprophylaxis with hydroxychloroquine for some high risk persons who may have had exposure to COVID. Researchers supporting prophylactic administration of hydroxychloquine note that results from human trials have suggested that hydroxychloroquine may decrease the duration of both viral shedding and symptoms if the drug is administered early.[42]
British researchers are studying whether the drug is effective when used for prevention. 10,000 National Health Service (NHS) workers, along with 30,000 additional volunteers from Asia, South America, Africa, and other parts of Europe are participating in the global study. Results are expected by the end of 2020.[43]
Controversy
Due to safety concerns and evidence of heart arrhythmias leading to higher death rates, the WHO suspended the hydroxychloroquine arm of the multinational Solidarity trial in late May 2020.[44][45][46] The WHO had enrolled 3,500 patients from 17 countries in the Solidarity trial.[44] The research surrounding this suspension, provided by a company called Surgisphere based in Chicago, came into question due to errors in the underlying data set.[47][48][49] The authors of the study corrected errors in the data later but initially remained firm on their conclusions.[47] Subsequently, a retraction of the study by three of its authors was published by The Lancet on 4 June, 2020.[50] The authors stated that their reason behind the retraction was because Surgisphere had failed to cooperate with an independent review of the data used for the study by not allowing any such review to take place.[51][52]
The WHO decided to resume the trial on 3 June, after reviewing the safety concerns which had been raised. Speaking at a press briefing, WHO's director-general, Tedros Adhanom Ghebreyesus stated that the board had reviewed the available mortality data and had found "no reasons to modify the trial".[53][54]
Dexamethasone

Dexamethasone is a corticosteroid medication in use for multiple conditions such as rheumatic problems, skin diseases, asthma and chronic obstructive lung disease among others.[55] A multi-center, randomized controlled trial of dexamethasone in treating acute respiratory distress syndrome (ARDS), published in February 2020, showed reduced need for mechanical ventilation and mortality.[56]
On 16 June, the Oxford University RECOVERY Trial issued a press release announcing preliminary results that the drug could reduce deaths by about a third in participants on ventilators and by about a fifth in participants on oxygen; it did not benefit patients who did not require respiratory support. The researchers estimated that treating 8 patients on ventilators with dexamethasone saved one life, and treating 25 patients on oxygen saved one life.[12] Several experts called for the full dataset to be published quickly to allow wider analysis of the results.[57][58] A preprint was published on June 22[59] and the peer-reviewed article appeared on July 17.[60]
Based on those preliminary results, dexamethasone treatment has been recommended by the US National Institutes of Health (NIH) for patients with COVID-19 who are mechanically ventilated or who require supplemental oxygen but are not mechanically ventilated. The NIH recommends against using dexamethasone in patients with COVID-19 who do not require supplemental oxygen.[61] In July 2020, the World Health Organization (WHO) stated they are in the process of updating treatment guidelines to include dexamethasone or other steroids.[62]
The Infectious Diseases Society of America (IDSA) guideline panel suggests the use of glucocorticoids for patients with severe COVID-19; where severe is defined as patients with oxygen saturation (SpO2) ≤94% on room air, and those who require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).[63] The IDSA recommends against the use of glucocorticoids for those with COVID-19 without hypoxemia requiring supplemental oxygen.[63]
In late July 2020, the European Medicines Agency (EMA) started reviewing results from the RECOVERY study arm that involved the use of dexamethasone in the treatment of patients with COVID-19 admitted to the hospital to provide an opinion on the results. It focused particularly on the potential use of the drug for the treatment of adults with COVID-19.[64]
In September 2020, the WHO released updated guidance on using corticosteroids for COVID-19.[65] The WHO recommends systemic corticosteroids rather than no systemic corticosteroids for the treatment of people with severe and critical COVID-19 (strong recommendation, based on moderate certainty evidence).[65] The WHO suggests not to use corticosteroids in the treatment of people with non-severe COVID-19 (conditional recommendation, based on low certainty evidence).[65]
Favipiravir
Chinese clinical trials in Wuhan and Shenzhen claimed to show that favipiravir was "clearly effective".[66] 35 patients in Shenzhen tested negative in a median of 4 days, while the length of illness was 11 days in the 45 patients who did not receive it.[67] In a study conducted in Wuhan on 240 patients with pneumonia, half were given favipiravir and half received umifenovir. The researchers found that patients recovered from coughs and fevers faster when treated with favipiravir, but there was no change in how many patients in each group progressed to more advanced stages of illness (treatment with a ventilator).[68]
On 22 March, Italy approved the drug for experimental use against COVID‑19 and began conducting trials in the three regions most affected by the disease.[69] The Italian Medicines Agency reminded the public that the existing evidence in support of the drug is scant and preliminary.[70] On 2 April, Germany announced that it would purchase the drug from Japan for its stockpile, and use the military to deliver the drug to university hospitals, where the drug will be used to treat COVID‑19 patients.[71] According to the South China Morning Post, Shinzo Abe has made overtures to the Trump administration about purchasing the drug.[72] On 30 May, the Russian Health Ministry approved a generic version of favipiravir named Avifavir, after the Russian Direct Investment Fund claimed it had proved highly effective in the first phase of clinical trials.[73][74][75]
The drug may be less effective in severe cases where the virus has already multiplied. It may not be safe for use by pregnant women or those trying to conceive.[67]
One study of lopinavir/ritonavir (Kaletra), a combination of the antivirals lopinavir and ritonavir, concluded that "no benefit was observed".[76][77] The drugs were designed to inhibit HIV from replicating by binding to the protease. A team of researchers at the University of Colorado are trying to modify the drugs to find a compound that will bind with the protease of SARS-CoV-2.[78] On 29 June, the chief investigators of the UK RECOVERY Trial reported that there was no clinical benefit from use of lopinavir-ritonavir in 1,596 people hospitalized with severe COVID-19 infection over 28 days of treatment. The results are yet to be published.[13]
There are criticisms within the scientific community about directing resources to repurposing drugs specifically developed for HIV/AIDS because such drugs are unlikely to be effective against a virus lacking the specific HIV-1 protease they target.[2] The WHO included lopinavir/ritonavir in the international Solidarity trial.[14]
Remdesivir
Remdesivir was created and developed by Gilead Sciences as a treatment for hepatitis C,[79] and was subsequently repurposed for Ebola virus disease and Marburg virus infections.[80]
During 2020, several clinical trials were underway.[7] The first randomized, placebo-controlled trial of remdesivir in China showed the drug had no clinical or virological benefits compared to the placebo group and caused adverse effects in the remdesivir-treated people, leading to early termination of the trial.[81][82]
On 1 May, the US Food and Drug Administration granted Gilead Emergency Use Authorization of remdesivir to be distributed and used by licensed health care providers to treat adults and children hospitalized with severe COVID‐19.[83] Severe COVID‐19 is defined as patients with oxygen saturation (SpO2) ≤94% on room air, and those who require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO), a heart‐lung bypass machine.[84][85] Distribution of remdesivir under the EUA will be controlled by the US government for use consistent with the terms and conditions of the EUA.[83] Gilead will supply remdesivir to authorized distributors, or directly to a US government agency, who will distribute to hospitals and other healthcare facilities as directed by the US Government, in collaboration with state and local government authorities, as needed.[83] The agreement between Gilead and five generic pharmaceutical companies in India and Pakistan will help make the medicine for 127 countries.[86] On 15 June, the FDA updated the fact sheets for the emergency use authorization of remdesivir to warn that using chloroquine or hydroxychloroquine with remdesivir may reduce the antiviral activity of remdesivir.[87]
A small trial of remdesivir in rhesus macaque monkeys with COVID‑19 infections reported that early treatment with remdesivir reduced damage and disease progression, but not viral shedding.[88]
On 25 June, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended granting a conditional marketing authorization for remdesivir for the treatment of COVID-19 in adults and adolescents from twelve years of age with pneumonia who require supplemental oxygen.[89][90][91] The brand name will be Veklury.[89][90]
On 29 June, the U.S Department of Health & Human Services announced that it had agreed to buy 500,000 doses of the drug for use in hospitals across the country through September 2020. This represents 100% of Gilead's projected production for July and 90% each for the months of August and September. The department secretary Alex Azar stated in a press release, "President Trump has struck an amazing deal to ensure Americans have access to the first authorized therapeutic for COVID‑19."[92][93]
The European Union granted remdesivir a conditional marketing authorization on 3 July, with an indication for the treatment of COVID‑19 in adults and adolescents (aged twelve years and older with body weight at least 40 kg) with pneumonia requiring supplemental oxygen.[94]
In July 2020, remdesivir was provisionally approved for use in Australia for use in adults and adolescents with severe COVID‑19 symptoms who have been hospitalized.[95]
On 28 August 2020, the FDA broadened the Emergency Use Authorization (EUA) for remdesivir to include all hospitalized patients with suspected or laboratory-confirmed COVID-19, irrespective of the severity of their disease.[96][97][98]
Remdesivir/baricitinib
In May 2020, the National Institute of Allergy and Infectious Diseases (NIAID) started the Adaptive COVID-19 Treatment Trial 2 (ACTT 2) to evaluate the safety and efficacy of a treatment regimen consisting of remdesivir plus baricitinib for treating hospitalized adults who have a laboratory-confirmed SARS-CoV-2 infection with evidence of lung involvement, including a need for supplemental oxygen, abnormal chest X-rays, or illness requiring mechanical ventilation.[99][100][101]
Remdesivir/interferon beta-1a
In August 2020, the NIAID started the Adaptive COVID-19 Treatment Trial 3 (ACTT 3) to evaluate the safety and efficacy of a treatment regimen consisting of remdesivir plus interferon beta-1a for hospitalized adults who have a laboratory-confirmed SARS-CoV-2 infection with evidence of lung involvement, including a need for supplemental oxygen, abnormal chest X-rays, or illness requiring mechanical ventilation.[100][102]
GS-441524
GS-441524 is the nucleoside of the ProTide remdesivir. It has been shown to cure cats infected with Feline infectious peritonitis (FIP), a feline form of coronavirus with a 96% cure rate.[103][104] Studies have shown that even when remdesivir is administered, GS-441524 is the predominant metabolite circulating in serum due to rapid hydrolysis of the remdesivir pro-drugs, followed by dephosphorylation.[105][80][106][107][unreliable medical source?] Some researchers have suggested its utility as a treatment for COVID‑19,[108][109][110][111][112] noting easier synthesis, lack of first-pass metabolism in the liver, greater hydrophilicity and triphosphate formation in cell types irrespective of expression CES1 and CTSA, the enzymes required to bioactivate remdesivir.
Vitamins
Intravenous vitamin C
According to ClinicalTrials.gov, there are six ongoing clinical trials of intravenous vitamin C for people who are hospitalized and severely ill with COVID‑19; three placebo controlled (China, Canada and, the US) and three with no control (Italy, the US and, the US).[113]
Oral vitamin D

According to ClinicalTrials.gov, several Phase II–IV clinical trials are underway to assess the use of oral vitamin D for prevention or treatment of COVID‑19 infection, with most in preliminary stages and none completed, as of May 2020.[114] As of August 27, 2020, a first result of a randomized treatment trial was released, and was favorable.[115]
Most trials have focused on studying infected people who are vitamin D deficient.[114] The rationale for these trials is based on speculation and observational reports that low vitamin D may be associated with a higher incidence and severity of this infection.[116] After adjustments were made for potential confounding factors, such as ethnicity, one study found insufficient evidence to indicate that vitamin D supplementation provided a benefit to reduce susceptibility to COVID‑19 infection.[117]
The first clinical trial of one group randomized to usual care only (hydroxychloroquine with azithromycin and sometimes ceftriaxone), and compared with a group receiving a form of vitamin D (calcifediol) in addition to usual care, found far less admission to intensive care and no deaths in the vitamin D group. The sample size in this pilot study was small, yet the group receiving vitamin D had such better results that the differences were statistically significant.[118]
Other drugs
| Drug | Research | |
|---|---|---|
| Anticoagulants | Several anticoagulants have been tested in Italy, with Low-molecular-weight heparin being widely used to treat patients, prompting the Italian Medicines Agency to publish guidelines on its use.[119] A multicenter study on 300 patients researching the use of enoxaparin sodium at prophylaxis and therapeutic dosages was announced in Italy on 14 April.[120] | |
| APN01 | A form of angiotensin-converting enzyme 2, a Phase II trial is underway with 200 patients to be recruited from severe, hospitalized cases in Denmark, Germany, and Austria to determine the effectiveness of the treatment.[121][122] | |
| Artesunate/pyronaridine | It was announced on 3 April 2020 that pyronaridine and artesunate, the main components of a new ACT antimalarial drug sold under the brand name Pyramax,[123] showed an inhibitory effect on SARS-CoV-2 in vitro tests using Hela cells. Pyramax showed a virus titer inhibition rate of 99% or more after 24 hours, while cytotoxicity was also reduced.[124] A preprint published in July 2020 reported that pyronaridine and artesunate exhibit antiviral activity against SARS-CoV-2 and influenza viruses using human lung epithelial (Calu-3) cells.[125] It is currently in phase II clinical trial in South Korea.[126][127][128] | |
| Azithromycin | New York State began trials for the antibiotic azithromycin on 24 March 2020.[129] | |
| Bucillamine | On 31 July 2020, the U.S. Food and Drug Administration (FDA) authorized Revive Therapeutics to proceed with a randomized, double-blind, placebo-controlled confirmatory Phase III clinical trial protocol to evaluate the safety and efficacy of bucillamine in patients with mild-moderate COVID-19.[130] | |
| Budesonide | Patients already prescribed inhaled corticosteroids have been observed to develop less serious illness when diagnosed with COVID-19, despite often having conditions such as asthma that might be thought to lead to more serious illness.[131][132] UK-Australia trial underway as of June 2020 examining the drug as an early-intervention treatment for COVID-19, results expected in September 2020.[133] | |
| Ciclesonide | Japan's National Center for Global Health and Medicine (NCGM) is planning a clinical trial for Teijin's Alvesco (ciclesonide), an inhaled corticosteroid for asthma, for the treatment of pre-symptomatic patients infected with the novel coronavirus.[134]
Ciclesonide was identified as a candidate antiviral in an in vitro drug screening assay done in South Korea.[135] | |
| Cimetidine | Has been suggested as a treatment for COVID-19.[136][137] | |
| Colchicine | Researchers from the Montreal Heart Institute in Canada are studying the role of colchicine in reducing inflammation and pulmonary complications in patients suffering from mild symptoms of COVID‑19.[138] The study, named COLCORONA, is recruiting 6000 adults 40 and older who were diagnosed with COVID‑19 and experience mild symptoms not requiring hospitalization.[138][139] Women who are pregnant or breastfeeding or who do not have an effective contraceptive method are not eligible.[139] | |
| Dipyridamole | Is proposed as a treatment for COVID‑19,[136][137] and a clinical trial is underway.[140] | |
| EIDD-2801 | A drug developed to treat influenza. It is in Phase II trials as a treatment for COVID-19.[141][142] | |
| Famotidine | Has been suggested as a treatment for COVID-19,[136][137][unreliable source?] and a clinical study is underway.[143] | |
| Fibrates | Fenofibrate and bezafibrate have been suggested for treatment of life-threatening symptoms of COVID-19.[136][137][144] | |
| Heparin | Scientists have identified an ability of Heparin to bind to the spike protein of the SARS-CoV-2 virus, neutralising it, and proposed the drug as a possible antiviral.[145] | |
| Ibuprofen | A trial called "Liberate" has been started in the United Kingdom to determine the effectiveness of ibuprofen in reducing the severity and progression of lung injury which results in breathing difficulties for COVID‑19 patients. Subjects are to receive three doses of a special formulation of the drug – lipid ibuprofen – in addition to usual care.[146][147] | |
| Influenza vaccine | A clinical cohort study in Brazil found that COVID-19 patients who received a recent influenza vaccine needed less intensive care support, less invasive respiratory support, and were less likely to die.[148] | |
| Interferon beta | IFN-β 1b have been shown in an open label randomised controlled trial in combination with lopinavir/ ritonavir and ribavirin to significantly reduce viral load, alleviate symptoms and reduce cytokine responses when compared to lopinavir/ ritonavir alone.<Lancet 2020;395(10238):1695-1704> IFN-β will be included in the international Solidarity Trial in combination with the HIV drugs Lopinavir and Ritonavir.[149] as well as the REMAP-CAP[150]
Finnish biotech firm Faron Pharmaceuticals continues to develop INF-beta for ARDS and is involved in worldwide initiatives[which?] against COVID‑19, including the Solidarity trial.[151] UK biotech firm Synairgen started conducting trials on IFN-β, a drug that was originally developed to treat COPD.[14] | |
| Ivermectin | Ivermectin is one of an increasing number of compounds found to inhibit SARS-CoV-2 in vitro without a defined mechanism of action. Plasma levels following oral administration appear too low to inhibit replication. An early-April preprint claimed benefits of Ivermectin, but it was based on questionable hospital data from Surgisphere and was withdrawn at the end of May. It led to government agencies in Latin America recommending Ivermectin as a treatment of COVID-19; these recommendations were later denounced by the regional WHO office.[152] As of July, ivermectin was being studied in 19 ongoing and 18 planned clinical trials.[153] A June 10 preprint reported on an observational retrospective study of hospitalized COVID-19 patients in Florida and found a significantly lower mortality in those who had received Ivermectin.[154] | |
| Mavrilimumab | Is a human monoclonal antibody that inhibits human granulocyte macrophage colony-stimulating factor receptor (GM-CSF-R).[155][156] It has been studied to see if it can improve the prognosis for patients with COVID-19 pneumonia and systemic hyperinflammation. One small study indicated some beneficial effects of treatment with mavrilimumab compared with those who were not.[157] | |
| nanoFenretinide | nanoFenretinide is nanoparticle sized fenretinide and repurposed oncology drug approved to enter the clinic for a lymphoma indication.[158] It was identified as a candidate antiviral in an in vitro drug screening assay done in South Korea.[135] Fenretinide's clinical safety profile also makes it an ideal candidate in combination regimens.[citation needed] | |
| Niclosamide | It was identified as a candidate antiviral in an in vitro drug screening assay done in South Korea.[135] | |
| Sildenafil | Is proposed as a treatment for COVID-19,[136][137] and a Phase III clinical trial is underway.[159] | |
| Tocilizumab | In March 2020, China approved the drug for the treatment of inflammation in COVID-19 patients but found no conclusive evidence whether the treatment is effective.[160] The Australasian Society for Clinical Immunology and Allergy recommend tocilizumab be considered as an off-label treatment for those with COVID-19 related acute respiratory distress syndrome.[161]
It is part of the RECOVERY Trial in the UK to be tested as a potential treatment,[9] while Hoffmann–La Roche and the WHO have also launched separate trials for its use in severe cases.[162] Roche announced on July 29 that its randomized double-blind trial of tocilizumab for the treatment of pneumonia in Covid patients had shown no benefits.[163] | |
Drugs by class
Antibody
Antivirals
Considerable scientific attention has been focused on re-purposing approved antiviral drugs that have been previously developed against other viruses, such as MERS-CoV, SARS-CoV, and West Nile virus.[164]
Antiparasitics
- Chloroquine[169]
- Hydroxychloroquine[170]
- Mefloquine[171][unreliable medical source?][172]
- Ivermectin[173]
- Atovaquone[174]
Antibiotics
Some antibiotics that have been identified as potentially re-purposable as COVID‑19 treatments:[175][176]
Immunologicals
Drugs with immune modulating effects that may prove useful in COVID‑19 treatment:
- Type I Interferons (Interferon-β, peginterferon alpha-2a and -2b)[149][150]
- Anti-IL-6 agents (Tocilizumab)[9]
- Anti-IL-8 (BMS-986253)[179]
References
- ^ "Repurposing Drugs". National Center for Advancing Translational Sciences (NCATS). U.S. Department of Health & Human Services, National Institutes of Health. 7 November 2017. Retrieved 26 March 2020.
- ^ a b c Harrison, Charlotte (April 2020). "Coronavirus puts drug repurposing on the fast track". Nature Biotechnology. 38 (4): 379–381. doi:10.1038/d41587-020-00003-1. PMID 32205870.
- ^ Sleigh SH, Barton CL (2010). "Repurposing Strategies for Therapeutics". Pharmaceutical Medicine. 24 (3): 151–159. doi:10.1007/BF03256811. S2CID 25267555.
- ^ "COVID-19 Vaccine Frontrunners".
- ^ Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. (April 2020). "Effectiveness of convalescent plasma therapy in severe COVID-19 patients". Proceedings of the National Academy of Sciences of the United States of America. 117 (17): 9490–9496. doi:10.1073/pnas.2004168117. PMC 7196837. PMID 32253318.
- ^ Li G, De Clercq E (March 2020). "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)". Nature Reviews. Drug Discovery. 19 (3): 149–150. doi:10.1038/d41573-020-00016-0. PMID 32127666.
- ^ a b "COVID-19 treatment and vaccines tracker (Choose tab of interest, apply filters to view select data)". Milken Institute. 2 June 2020. Retrieved 3 June 2020.
{{cite web}}: Unknown parameter|lay-url=ignored (help) - ^ Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (April 2020). "Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review". JAMA. doi:10.1001/jama.2020.6019. PMID 32282022.
- ^ a b c "RECOVERY Trial". Retrieved 17 June 2020.
- ^ Walsh, Fergus (20 June 2020). "At last some good news about coronavirus". BBC News. Retrieved 20 June 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c "No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19". Recovery Trial, Nuffield Department of Population Health, University of Oxford, UK. 5 June 2020. Retrieved 7 June 2020.
- ^ a b "Dexamethasone reduces death in hospitalised patients with severe respiratory complications of COVID-19". University of Oxford. 16 June 2020. Retrieved 16 June 2020.
- ^ a b "No clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients studied in RECOVERY" (PDF). RECOVERY Trial: Statement from the Chief Investigators. 29 June 2020. Retrieved 30 June 2020.
- ^ a b c Devlin, Hannah; Sample, Ian (19 March 2020). "What are the prospects for a COVID-19 treatment?". The Guardian.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Liu M, Caputi TL, Dredze M, Kesselheim AS, Ayers JW (April 2020). "Internet Searches for Unproven COVID-19 Therapies in the United States". JAMA Internal Medicine. 180 (8): 1116. doi:10.1001/jamainternmed.2020.1764. PMC 7191468. PMID 32347895.
- ^ "NY COVID-19 cases surge; Javits Center to house temporary hospitals". Fox 5. 23 March 2020.
- ^ a b "Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020" (Press release). U.S. Food and Drug Administration (FDA). 30 March 2020.
- ^ a b c "Chloroquine phosphate and hydroxychloroquine sulfate for treatment of COVID-19 Emergency Use Authorization" (PDF). U.S. Food and Drug Administration (FDA). 28 March 2020. Retrieved 14 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Fact Sheet for Patients and Parent/Caregivers Emergency Use Authorization (EUA) of Chloroquine Phosphate for Treatment of COVID-19 in Certain Hospitalized Patients" (PDF). U.S. Food and Drug Administration (FDA).
- ^ "Information for clinicians on therapeutic options for COVID-19 patients". U.S. Centers for Disease Control and Prevention. 21 March 2020. Retrieved 22 March 2020.
- ^ a b Gross, Samantha J. (9 April 2020). "As CDC drops guidance on chloroquine as COVID-19 therapy, doctors ask for research". Miami Herald.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Clinical trial number NCT04332991 for "Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease (ORCHID)" at ClinicalTrials.gov
- ^ "Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine". U.S. Food and Drug Administration (FDA) (Press release). 15 June 2020. Retrieved 15 June 2020.
- ^ "EUA Archive". U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
On June 15, 2020, based on FDA's continued review of the scientific evidence available for hydroxychloroquine sulfate (HCQ) and chloroquine phosphate (CQ) to treat COVID-19, FDA has determined that the statutory criteria for EUA as outlined in Section 564(c)(2) of the Food, Drug, and Cosmetic Act are no longer met. Specifically, FDA has determined that CQ and HCQ are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA. Additionally, in light of ongoing serious cardiac adverse events and other serious side effects, the known and potential benefits of CQ and HCQ no longer outweigh the known and potential risks for the authorized use. This warrants revocation of the EUA for HCQ and CQ for the treatment of COVID-19.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "HCQ and CQ revocation letter" (PDF). U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Frequently Asked Questions on the Revocation of the Emergency Use Authorization for Hydroxychloroquine Sulfate and Chloroquine Phosphate" (PDF). U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
- ^ "FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems". U.S. Food and Drug Administration (FDA). 24 April 2020. Retrieved 1 May 2020.
- ^ see under Treatment section of Coronavirus COVID‑19 (SARS-CoV-2); Johns Hopkins ABX Guide (Retrieved 18 April 2020)
- ^ a b Wu CI, Postema PG, Arbelo E, Behr ER, Bezzina CR, Napolitano C, et al. (March 2020). "SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes". Heart Rhythm. 17 (9): 1456–1462. doi:10.1016/j.hrthm.2020.03.024. PMC 7156157. PMID 32244059.
- ^ Rowland, Christopher. "Anti-malarial drug Trump touted is linked to higher rates of death in VA coronavirus patients, study says". The Washington Post. Retrieved 22 April 2020.
- ^ Magagnoli, Joseph; Narendran, Siddharth; Pereira, Felipe; Cummings, Tammy; Hardin, James W; Sutton, S Scott; Ambati, Jayakrishna (21 April 2020). "Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19". medRxiv. doi:10.1101/2020.04.16.20065920. PMC 7276049. PMID 32511622.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. (June 2020). "A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19". The New England Journal of Medicine. 383 (6): 517–525. doi:10.1056/NEJMoa2016638. PMC 7289276. PMID 32492293.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ Cohen MS (June 2020). "Hydroxychloroquine for the Prevention of Covid-19 – Searching for Evidence". The New England Journal of Medicine. 383 (6): 585–586. doi:10.1056/NEJMe2020388. PMC 7289275. PMID 32492298.
- ^ McGinley, Laurie; Cha, Ariana Eunjung (3 June 2020). "Hydroxychloroquine, a drug promoted by Trump, failed to prevent healthy people from getting covid-19 in trial". The Washington Post. Retrieved 5 June 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LC, Veiga VC, Avezum A, et al. (July 2020). "Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19". New England Journal of Medicine. doi:10.1056/nejmoa2019014. PMC 7397242. PMID 32706953.
- ^ "Coronavirus: Hydroxychloroquine ineffective says Fauci". BBC. 29 July 2020. Retrieved 30 July 2020.
- ^ a b c d "Zinc". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 May 2019. Retrieved 20 May 2020.
- ^ a b Brian P. Dunleavy (13 May 2020). "Zinc might boost effectiveness of malaria drug against COVID-19, experts say". United Press International. Retrieved 20 May 2020.
- ^ Clinical trial number NCT04370782 for "Hydroxychloroquine and Zinc With Either Azithromycin or Doxycycline for Treatment of COVID-19 in Outpatient Setting" at ClinicalTrials.gov
- ^ Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. (May 2020). "Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State". JAMA. 323 (24): 2493. doi:10.1001/jama.2020.8630. PMC 7215635. PMID 32392282.
- ^ Risch HA (May 2020). "Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis". American Journal of Epidemiology: kwaa093. doi:10.1093/aje/kwaa093. PMID 32458969.
- ^ Tilangi P, Desai D, Khan A, Soneja M (May 2020). "Hydroxychloroquine prophylaxis for high-risk COVID-19 contacts in India: a prudent approach". The Lancet. Infectious Diseases. doi:10.1016/S1473-3099(20)30430-8. PMC 7255125. PMID 32450054.
- ^ "Hydroxychloroquine: NHS staff to take drug as part of global trial". The Guardian. 21 May 2020.
- ^ a b "WHO Director-General's opening remarks at the media briefing on COVID-19 – 25 May 2020". World Health Organization. 25 May 2020. Retrieved 27 May 2020.
- ^ "WHO pauses hydroxychloroquine coronavirus trial over safety concerns". Global News. The Associated Press. 25 May 2020. Retrieved 27 May 2020.
{{cite news}}: Cite uses deprecated parameter|authors=(help) - ^ "Coronavirus: WHO halts trials of hydroxychloroquine over safety fears". BBC News Online. 25 May 2020. Retrieved 4 June 2020.
- ^ a b Herper, Matthew; Joseph, Andrew (2 June 2020). "Top medical journals raise concerns about data in two studies related to Covid-19". Stat. Retrieved 4 June 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Servick, Kelly (2 June 2020). "A mysterious company's coronavirus papers in top medical journals may be unraveling". Science. doi:10.1126/science.abd1337.
- ^ Melissa Davey (28 May 2020). "Questions raised over hydroxychloroquine study which caused WHO to halt trials for Covid-19". The Guardian. Retrieved 4 June 2020.
- ^ Mehra, Mandeep R; Ruschitzka, Frank; Patel, Amit N (June 2020). "Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis". The Lancet. 395 (10240): 1820. doi:10.1016/S0140-6736(20)31324-6. PMC 7274621. PMID 32511943.
- ^ "Coronavirus: Influential study on hydroxychloroquine withdrawn". BBC News Online. 5 June 2020. Retrieved 5 June 2020.
- ^ Boseley, Sarah; Davey, Melissa (4 June 2020). "Covid-19: Lancet retracts paper that halted hydroxychloroquine trials". The Guardian. Retrieved 4 June 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Andrew Joseph (3 June 2020). "WHO resumes hydroxychloroquine study for Covid-19, after reviewing safety concerns". Stat. Retrieved 4 June 2020.
- ^ Shaun Lintern (3 June 2020). "Coronavirus: WHO re-starts hydroxychloroquine trials amid controversy over published research". The Independent. Retrieved 4 June 2020.
- ^ "Dexamethasone". The American Society of Health-System Pharmacists. Archived from the original on 31 August 2017. Retrieved 29 July 2015.
- ^ Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. (March 2020). "Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial". The Lancet. Respiratory Medicine. 8 (3): 267–276. doi:10.1016/s2213-2600(19)30417-5. PMID 32043986.
Early administration of dexamethasone could reduce duration of mechanical ventilation and overall mortality in patients with established moderate-to-severe ARDS.
- ^ "Steroid drug hailed as 'breakthrough' for seriously ill COVID-19 patients". Reuters. 17 June 2020. Retrieved 18 June 2020.
- ^ Ducharme, Jamie. "A Low-Cost Steroid Shows Promise for Treating COVID-19. But Take the News With a Grain of Salt". Time. Retrieved 18 June 2020.
- ^ Horby, Peter; Lim, Wei Shen; Emberson, Jonathan; Mafham, Marion; Bell, Jennifer; Linsell, Louise; Staplin, Natalie; Brightling, Christopher; Ustianowski, Andrew; Elmahi, Einas; Prudon, Benjamin; Green, Christopher; Felton, Timothy; Chadwick, David; Rege, Kanchan; Fegan, Christopher; Chappell, Lucy C; Faust, Saul N; Jaki, Thomas; Jeffery, Katie; Montgomery, Alan; Rowan, Kathryn; Juszczak, Edmund; Baillie, J Kenneth; Haynes, Richard; Landray, Martin J (22 June 2020). "Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report". doi:10.1101/2020.06.22.20137273. S2CID 219965377.
{{cite journal}}: Cite journal requires|journal=(help) - ^ The RECOVERY Collaborative Group (17 July 2020). "Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report". New England Journal of Medicine. doi:10.1056/NEJMoa2021436. PMC 7383595. PMID 32678530.
- ^ "Corticosteroids (Including Dexamethasone)". COVID-19 Treatment Guidelines. National Institutes of Health. Retrieved 12 July 2020.
- ^ "Q&A: Dexamethasone and COVID-19". World Health Organization (WHO). Retrieved 12 July 2020.
- ^ a b "COVID-19 Guideline, Part 1: Treatment and Management". Infectious Diseases Society of America. Retrieved 22 July 2020.
Recommendation 4. Among hospitalized patients with severe* COVID-19, the IDSA guideline panel suggests glucocorticoids rather than no glucocorticoids. (Conditional recommendation, Moderate certainty of evidence)
Remark: Dexamethasone 6 mg IV or PO for 10 days (or until discharge if earlier) or equivalent glucocorticoid dose may be substituted if dexamethasone unavailable. Equivalent total daily doses of alternative glucocorticoids to dexamethasone 6 mg daily are methylprednisolone 32 mg and prednisone 40 mg.
Recommendation 5. Among hospitalized patients with COVID-19 without hypoxemia requiring supplemental oxygen, the IDSA guideline panel suggests against the use of glucocorticoids. (Conditional recommendation, Low certainty of evidence) - ^ "EMA starts review of dexamethasone for treating adults with COVID-19 requiring respiratory support". European Medicines Agency (EMA) (Press release). 24 July 2020. Retrieved 27 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c World Health Organization (2020). Corticosteroids for COVID-19: living guidance, 2 September 2020 (Report). World Health Organization. hdl:10665/334125. WHO/2019-nCoV/Corticosteroids/2020.1.
{{cite report}}: Unknown parameter|lay-url=ignored (help) - ^ McCurry, Justin (18 March 2020). "Japanese flu drug 'clearly effective' in treating coronavirus, says China".
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Gregory, Andy (18 March 2020). "Coronavirus: Japanese anti-viral drug effective in treating patients, Chinese official says". The Independent.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Regalado, Antonio (23 March 2020). "Which Covid-19 drugs work best?". MIT Technology Review.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ di Pietrobelli, Giuseppe (22 March 2020). "Coronavirus, il Veneto sperimenta l'antivirale giapponese Favipiravir. Ma l'Aifa: "Ci sono scarse evidenze scientifiche su efficacia"". Il Fatto Quotidiano (in Italian). Retrieved 23 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "AIFA precisa, uso favipiravir per COVID-19 non autorizzato in Europa e USA, scarse evidenze scientifiche sull'efficacia". aifa.gov.it (in Italian). 22 March 2020. Retrieved 23 March 2020.
- ^ Fukao, Kosei (3 April 2020). "Berlin to stockpile millions of doses of Fujifilm's anti-flu drug". Nikkei Asian Review.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Jeong-ho, Lee. "China and South Korea split over Japanese anti-flu drug Avigan in fight against coronavirus". South China Morning Post.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Russian Ministry of Health approves the first COVID-19 drug Avifavir produced by JV of RDIF and ChemRar". RDIF. 30 May 2020. Retrieved 31 May 2020.
- ^ "Russian Health Ministry approves anti-coronavirus drug Avifavir". BNN Bloomberg. 31 May 2020. Retrieved 31 May 2020.
- ^ "Russia plans coronavirus vaccine clinical trials in two weeks – report". Reuters. 30 May 2020. Retrieved 31 May 2020.
- ^ "Antiviral Drug Combo Ineffective Vs. Coronavirus". WebMD. 20 March 2020.
- ^ Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. (May 2020). "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19". The New England Journal of Medicine. 382 (19): 1787–1799. doi:10.1056/NEJMoa2001282. PMC 7121492. PMID 32187464.
- ^ Brown, Jennifer (20 March 2020). "Colorado researchers are racing to find an antiviral drug that could save people with the new coronavirus".
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Stephens, Bret (18 April 2020). "The Story of Remdesivir". The New York Times. p. A23. Retrieved 11 May 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. (March 2016). "Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys". Nature. 531 (7594): 381–5. Bibcode:2016Natur.531..381W. doi:10.1038/nature17180. PMC 5551389. PMID 26934220.
- ^ a b Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. (May 2020). "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial". Lancet. 395 (10236): 1569–1578. doi:10.1016/S0140-6736(20)31022-9. PMC 7190303. PMID 32423584.
- ^ Boseley, Sarah (23 April 2020). "First trial for potential Covid-19 drug shows it has no effect". The Guardian. ISSN 0261-3077. Retrieved 25 April 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c "Frequently Asked Questions on the Emergency Use Authorization for Remdesivir for Certain Hospitalized COVID‐19 Patients" (PDF). U.S. Food and Drug Administration (FDA). 1 May 2020. Retrieved 1 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Emergency Use Authorization (EUA) of Remdesivir for Coronavirus Disease 2019 (COVID-19)" (PDF). U.S. Food and Drug Administration (FDA). 1 May 2020. Retrieved 1 May 2020.
{{cite web}}: Unknown parameter|lay-url=ignored (help) This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Holland, Steve; Mason, Jeff; Maler, Sandra (1 May 2020). "FDA Authorizes Remdesivir Drug for COVID-19". The New York Times.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Silverman, Ed (12 May 2020). "Gilead signs deals for generic companies to make and sell remdesivir". Stat. Retrieved 30 May 2020.
- ^ "Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use". U.S. Food and Drug Administration (FDA) (Press release). 15 June 2020. Retrieved 15 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. (June 2020). "Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2". Nature. doi:10.1038/s41586-020-2423-5. PMID 32516797.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ a b "First COVID-19 treatment recommended for EU authorisation" (Press release). European Medicines Agency (EMA). 25 June 2020. Retrieved 25 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Veklury: Pending EC decision". European Medicines Agency (EMA). 25 June 2020. Retrieved 25 June 2020.
{{cite web}}: Unknown parameter|lay-url=ignored (help) This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Veklury product information" (PDF). European Medicines Agency (EMA). Retrieved 25 June 2020.
- ^ "Trump Administration Secures New Supplies of Remdesivir for the United States" (Press release). United States Department of Health & Human Services (HHS). 29 June 2020. Retrieved 3 July 2020.
- ^ "Coronavirus: US buys nearly all of Gilead's Covid-19 drug remdesivir". BBC. 1 July 2020. Retrieved 3 July 2020.
- ^ "Veklury EPAR". European Medicines Agency (EMA). 23 June 2020. Retrieved 6 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Australia's first COVID treatment approved". Therapeutic Goods Administration (TGA) (Press release). 10 July 2020. Retrieved 11 July 2020.
- ^ "COVID-19 Update: FDA Broadens Emergency Use Authorization for Veklury (remdesivir) to Include All Hospitalized Patients for Treatment of COVID-19". U.S. Food and Drug Administration (FDA) (Press release). 28 August 2020. Retrieved 28 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Gilead's Investigational Antiviral Veklury (Remdesivir) Receives U.S. Food and Drug Administration Emergency Use Authorization for the Treatment of Patients With Moderate COVID-19" (Press release). Gilead Sciences. 28 August 2020. Retrieved 28 August 2020 – via Business Wire.
- ^ "FDA EUA Remdesivir Fact Sheet for Health Care Providers" (PDF). U.S. Food and Drug Administration (FDA). 28 August 2020. Retrieved 28 August 2020.
{{cite web}}: Unknown parameter|lay-url=ignored (help) This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "NIH Clinical Trial Testing Antiviral Remdesivir Plus Anti-Inflammatory Drug Baricitinib for COVID-19 Begins". National Institute of Allergy and Infectious Diseases (NIAID) (Press release). 8 May 2020. Retrieved 5 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "NIH Clinical Trial Testing Remdesivir Plus Interferon Beta-1a for COVID-19 Treatment Begins". National Institute of Allergy and Infectious Diseases (NIAID) (Press release). 30 July 2020. Retrieved 5 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Clinical trial number NCT04401579 for "Adaptive COVID-19 Treatment Trial 2 (ACTT-2)" at ClinicalTrials.gov
- ^ Clinical trial number NCT04492475 for "Adaptive COVID-19 Treatment Trial 3 (ACTT-3)" at ClinicalTrials.gov
- ^ Zhang, Sarah (8 May 2020). "A Much-Hyped COVID-19 Drug Is Almost Identical to a Black-Market Cat Cure". The Atlantic.
- ^ Pedersen NC, Perron M, Bannasch M, Montgomery E, Murakami E, Liepnieks M, Liu H (April 2019). "Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis". Journal of Feline Medicine and Surgery. 21 (4): 271–281. doi:10.1177/1098612X19825701. PMC 6435921. PMID 30755068.
- ^ Yan VC, Muller FL (July 2020). "Advantages of the Parent Nucleoside GS-441524 over Remdesivir for Covid-19 Treatment". ACS Medicinal Chemistry Letters. 11 (7): 1361–1366. doi:10.1021/acsmedchemlett.0c00316. PMC 7315846. PMID 32665809.
- ^ Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. (June 2017). "Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses". Science Translational Medicine. 9 (396): eaal3653. doi:10.1126/scitranslmed.aal3653. PMC 5567817. PMID 28659436.
- ^ Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. (April 2020). "Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2". bioRxiv: 2020.04.15.043166. doi:10.1101/2020.04.15.043166. PMC 7239049. PMID 32511319.
- ^ "Gilead should ditch remdesivir and focus on its simpler ancestor". Stat. 14 May 2020. Retrieved 5 June 2020.
- ^ Yan VC, Muller FL (July 2020). "Advantages of the Parent Nucleoside GS-441524 over Remdesivir for Covid-19 Treatment". ACS Medicinal Chemistry Letters. 11 (7): 1361–1366. doi:10.1021/acsmedchemlett.0c00316. PMID 32665809. S2CID 220056568.
- ^ Yan VC, Muller FL (14 May 2020). "Gilead should ditch remdesivir and focus on its simpler and safer ancestor". Statnews.
- ^ Westgate, James (7 May 2020). "Vet science 'being ignored' in quest for COVID-19 drug". Vet Times. Retrieved 28 July 2020.
- ^ GmbH, Avoxa-Mediengruppe Deutscher Apotheker. "Verwandtschaft ersten Grades: Remdesivir-Metabolit noch schärfere Waffe gegen Covid-19?". Pharmazeutische Zeitung online (in German).
- ^ "clinicaltrials.gov Vitamin C COVID-19". 26 March 2020. Retrieved 28 April 2020.
- ^ a b "International clinical trials assessing vitamin D in people with COVID-19". ClinicalTrials.gov, U.S. National Library of Medicine. May 2020. Retrieved 20 May 2020.
- ^ Castillo, Marta Entrenas; Entrenas Costa, Luis Manuel; Vaquero Barrios, José Manuel; Alcalá Díaz, Juan Francisco; Miranda, José López; Bouillon, Roger; Quesada Gomez, José Manuel (29 August 2020). "Effect of Calcifediol Treatment and best Available Therapy versus best Available Therapy on Intensive Care Unit Admission and Mortality Among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical study". The Journal of Steroid Biochemistry and Molecular Biology. doi:10.1016/j.jsbmb.2020.105751. PMC 7456194.
- ^ Rhodes JM, Subramanian S, Laird E, Kenny RA (June 2020). "Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity". Alimentary Pharmacology & Therapeutics. 51 (12): 1434–1437. doi:10.1111/apt.15777. PMC 7264531. PMID 32311755.
- ^ Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, et al. (May 2020). "Vitamin D concentrations and COVID-19 infection in UK Biobank". Diabetes & Metabolic Syndrome. 14 (4): 561–565. doi:10.1016/j.dsx.2020.04.050. PMC 7204679. PMID 32413819.
- ^ Castillo ME, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, Miranda JL, Bouillon R, et al. (August 2020). ""Effect of Calcifediol Treatment and best Available Therapy versus best Available Therapy on Intensive Care Unit Admission and Mortality Among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical study"". J. Steroid Biochem. Mol. Biol.: 105751. doi:10.1016/j.jsbmb.2020.105751. PMC 7456194. PMID 32871238.
- ^ "Farmaci utilizzabili per il trattamento della malattia COVID19 | Agenzia Italiana del Farmaco". aifa.gov.it (in Italian). Retrieved 15 April 2020.
- ^ "Coronavirus, al via studio su eparina per 300 pazienti". Adnkronos. Retrieved 15 April 2020.
- ^ Ansede, Manuel (3 April 2020). "Doscientos enfermos probarán un fármaco que ha bloqueado el coronavirus en minirriñones humanos". El País (in Spanish). Retrieved 3 April 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Apeiron Biologics moves forward with APN01 for treatment of COVID-19". www.thepharmaletter.com. Retrieved 3 April 2020.
- ^ "Pyramax® (pyronaridine-artesunate)". www.mmv.org. Retrieved 26 June 2020.
- ^ "'Pyramax' drug repurposing?... targets COVID-19 treatment". Doctor's News. Retrieved 25 June 2020.
- ^ Bae, Joon-Yong; Lee, Gee Eun; Park, Heedo; Cho, Juyoung; Kim, Yung-Eui; Lee, Joo-Yeon; Ju, Chung; Kim, Won-Ki; Kim, Jin Il; Park, Man-Seong (2020). "Pyronaridine and artesunate are potential antiviral drugs against COVID-19 and influenza". bioRxiv. doi:10.1101/2020.07.28.225102. S2CID 220885187. Retrieved 29 July 2020.
- ^ "COVID-19: Pyramax Enters Phase II Clinical Trial in South Korea". www.pharmanewsonline.com. Retrieved 17 June 2020.
- ^ "A Multi-center, Randomized, Double-blind, Parallel, Placebo-Controlled, Phase Ⅱ Clinical Trial to Evaluate Efficacy and Safety of Pyramax in Mild to Moderate COVID-19 Patients". nedrug.mfds.go.kr. Retrieved 25 June 2020.
- ^ Clinical trial number NCT04475107 for "The Efficacy and Safety of Pyramax in Mild to Moderate COVID-19 Patients" at ClinicalTrials.gov
- ^ a b "Amid Ongoing COVID-19 Pandemic, Governor Cuomo Accepts Recommendation of Army Corps of Engineers for Four Temporary Hospital Sites in New York". governor.ny.gov. 22 March 2020.
- ^ "Revive Therapeutics Announces U.S. FDA Approval of Confirmatory Phase 3 Clinical Trial for Bucillamine in COVID-19" (Press release). Revive Therapeutics Ltd. 31 July 2020. Retrieved 5 August 2020 – via GlobeNewswire.
- ^ Mandal, Anaya (7 July 2020). "Asthma inhalers being trialed for treatment of COVID-19". News Medical. Retrieved 13 July 2020.
- ^ "QUT and Oxford researchers collaborate on new COVID-19 asthma drug trial". Queensland University of Technology. Retrieved 13 July 2020.
- ^ "Use of inhaled corticosteroids as treatment of early COVID-19 infection to prevent clinical deterioration and hospitalisation". EU Clinical Trials Register. Retrieved 13 July 2020.
- ^ "Japan Plans Alvesco Clinical Trial for Coronavirus". pj.jiho.jp. 31 March 2020.
- ^ a b c Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, et al. (May 2020). "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs". Antimicrobial Agents and Chemotherapy. 64 (7). American Society for Microbiology. doi:10.1128/aac.00819-20. PMC 7318052. PMID 32366720.
Drug repositioning is the only feasible option to address the COVID‑19 global challenge immediately. We screened a panel of 48 FDA-approved drugs against SARS-CoV-2 which were pre-selected by an assay of SARS-CoV and identified 24 potential antiviral drug candidates against SARS-CoV-2 infection. Some drug candidates showed very low micromolar IC50s and in particular, two FDA-approved drugs – niclosamide and ciclesonide – were notable in some respects.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ a b c d e Rogosnitzky M, Berkowitz E, Jadad AR (May 2020). "Delivering Benefits at Speed Through Real-World Repurposing of Off-Patent Drugs: The COVID-19 Pandemic as a Case in Point". JMIR Public Health and Surveillance. 6 (2): e19199. doi:10.2196/19199. PMC 7224168. PMID 32374264.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e Rogosnitzky M, Berkowitz E, Jadad AR. "No Time to Waste: Real-world Repurposing of Generic Drugs as a Multifaceted Strategy against COVID-19" (Document).
{{cite document}}: Cite document requires|publisher=(help); Unknown parameter|url=ignored (help) - ^ a b ICI.Radio-Canada.ca, Zone Santé-. "Début d'une étude clinique pour tester un médicament contre les effets de la COVID-19 | Coronavirus". Radio-Canada.ca.
- ^ a b "COLCORONA Clinical Trial | Stop COVID-19". Colcorona.
- ^ "Chinese Clinical Trial Register (ChiCTR) – The world health organization international clinical trials registered organization registered platform". www.chictr.org.cn.
- ^ Clinical trial number NCT04405570 for "A Safety, Tolerability and Efficacy of EIDD-2801 to Eliminate Infectious Virus Detection in Persons With COVID-19" at ClinicalTrials.gov
- ^ Clinical trial number NCT04405739 for "The Safety of EIDD-2801 and Its Effect on Viral Shedding of SARS-CoV-2 (END-COVID)" at ClinicalTrials.gov
- ^ "New York clinical trial quietly tests heartburn remedy against coronavirus". Science. 26 April 2020.
- ^ Ehrlich A, Uhl S, Ioannidis K, Hofree M, tenOever BR, Nahmias Y (14 July 2020). "The SARS-CoV-2 Transcriptional Metabolic Signature in Lung Epithelium". Rochester, NY. SSRN 3650499.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Weintraub, Arlene. "How COVID-19 could be crippled by an age-old blood thinner". Fierce Biotech. Retrieved 22 July 2020.
- ^ Clinical trial number NCT04334629 for "LIBERATE Trial in COVID-19 (LIBERATE) " at ClinicalTrials.gov
- ^ "Coronavirus: Ibuprofen tested as a treatment". BBC. 3 June 2020. Retrieved 4 June 2020.
- ^ Fink G, Orlova-Fink N, Schindler T, Grisi S, Ferrer AP, Daubenberger C, et al. (1 July 2020). "Inactivated trivalent influenza vaccine is associated with lower mortality among Covid-19 patients in Brazil (preprint)". MedRxiv. doi:10.1101/2020.06.29.20142505. S2CID 220267530.
Covid-19 patients with recent influenza vaccination experience better health outcomes than non-vaccinated patients in Brazil.
- ^ a b "WHO officials enroll first patients from Norway and Spain in 'historic' coronavirus drug trial". CNBC. 27 March 2020.
- ^ a b "REMAP-CAP Trial". REMAP-CAP.
- ^ "Traumakine to be a part of WHO's Solidarity trial investigating potential COVID-19 treatments". Faron Pharmaceuticals (Press release). 27 April 2020. Retrieved 8 May 2020.
- ^ "Surgisphere Sows Confusion About Another Unproven COVID-19 Drug". The Scientist Magazine. 16 June 2020. Retrieved 8 July 2020.
- ^ Stanford, University. "SARS-COV-2 ANTIVIRAL THERAPY". CORONAVIRUS ANTIVIRAL RESEARCH DATABASE. Retrieved 20 August 2020.
- ^ Rajter JC, Sherman M, Fatteh N, Vogel F, Sacks J, Rajter JJ (10 June 2020). "ICON (Ivermectin in COvid Nineteen) study: Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID19 (preprint)". MedRxiv. doi:10.1101/2020.06.06.20124461. S2CID 219544840.
- ^ "Statement On A Nonproprietary Name Adopted By The USAN Council: Mavrilimumab" (PDF). American Medical Association. Archived from the original (PDF) on 28 September 2012.
- ^ Burmester GR, Feist E, Sleeman MA, Wang B, White B, Magrini F (September 2011). "Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study". Annals of the Rheumatic Diseases. 70 (9): 1542–9. doi:10.1136/ard.2010.146225. PMC 3147227. PMID 21613310.
- ^ a b De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. (16 June 2020). "GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study". The Lancet Rheumatology. 2 (8): e465–e473. doi:10.1016/S2665-9913(20)30170-3. PMC 7430344. PMID 32835256.
- ^ Clinical trial number NCT04234048 for "Phase 1 Trial of ST-001 nanoFenretinide in Relapsed/Refractory T-cell Non-Hodgkin Lymphoma" at ClinicalTrials.gov
- ^ Clinical trial number NCT04304313 for "A Pilot Study of Sildenafil in COVID-19" at ClinicalTrials.gov
- ^ "China approves use of Roche arthritis drug for coronavirus patients". Reuters. 4 March 2020.
- ^ Grainger, Suzanne. "ASCIA Position Statement: Specific Treatments for COVID-19". Australasian Society of Clinical Immunology and Allergy (ASCIA). Retrieved 2 May 2020.
- ^ "WHO and Roche launch trials of potential coronavirus treatments". SwissInfo.Ch. 20 March 2020. Retrieved 8 August 2020.
- ^ "Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia". www.roche.com. 29 July 2020. Retrieved 18 August 2020.
- ^ Dong L, Hu S, Gao J (2020). "Discovering drugs to treat coronavirus disease 2019 (COVID-19)". Drug Discoveries & Therapeutics. 14 (1): 58–60. doi:10.5582/ddt.2020.01012. PMID 32147628.
- ^ Dong L, Hu S, Gao J (29 February 2020). "Discovering drugs to treat coronavirus disease 2019 (COVID-19)". Drug Discoveries & Therapeutics. 14 (1): 58–60. doi:10.5582/ddt.2020.01012. PMID 32147628.
- ^ Lu CC, Chen MY, Lee WS, Chang YL. Potential therapeutic agents against COVID-19: What we know so far. J Chin Med Assoc. 2020;83(6):534-536. doi:10.1097/JCMA.0000000000000318 PMID 32243270
- ^ Wu X, et al. The Efficacy and Safety of Triazavirin for COVID-19: A Trial Protocol. Engineering 2020 July 3. doi:10.1016/j.eng.2020.06.011
- ^ Lu H (March 2020). "Drug treatment options for the 2019-new coronavirus (2019-nCoV)". Bioscience Trends. 14 (1): 69–71. doi:10.5582/bst.2020.01020. PMID 31996494.
- ^ Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. (March 2020). "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro". Cell Research. 30 (3): 269–271. doi:10.1038/s41422-020-0282-0. PMC 7054408. PMID 32020029.
- ^ Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. (March 2020). "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial". International Journal of Antimicrobial Agents. 56 (1): 105949. doi:10.1016/j.ijantimicag.2020.105949. PMC 7102549. PMID 32205204.
- ^ Weston S, Haupt R, Logue J, Matthews K, Frieman MB (27 March 2020). "FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro". bioRxiv. doi:10.1101/2020.03.25.008482.
- ^ "ФМБА России: доказана противовирусная активность "Мефлохина" в отношении возбудителя COVID-19". fmbaros.ru (in Russian). Federal Biomedical Agency. 10 April 2020. Retrieved 11 April 2020.
- ^ Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (April 2020). "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro". Antiviral Research. 178: 104787. doi:10.1016/j.antiviral.2020.104787. PMC 7129059. PMID 32251768.
- ^ Clinical trial number NCT04339426 for "Atovaquone and Azithromycin Combination for Confirmed COVID-19 Infection" at ClinicalTrials.gov
- ^ "Coronavirus: Scientists could repurpose drugs to treat infection". Medical News Today. Retrieved 20 March 2020.
- ^ "Existing Drugs May Offer a First-Line Treatment for Coronavirus Outbreak". Global Health News Wire. Retrieved 20 March 2020.
- ^ Baron SA, Devaux C, Colson P, Raoult D, Rolain JM (April 2020). "Teicoplanin: an alternative drug for the treatment of COVID-19?". International Journal of Antimicrobial Agents. 55 (4): 105944. doi:10.1016/j.ijantimicag.2020.105944. PMC 7102624. PMID 32179150.
- ^ a b c Andersen PI, Ianevski A, Lysvand H, Vitkauskiene A, Oksenych V, Bjørås M, et al. (April 2020). "Discovery and development of safe-in-man broad-spectrum antiviral agents". International Journal of Infectious Diseases. 93: 268–276. doi:10.1016/j.ijid.2020.02.018. PMC 7128205. PMID 32081774.
- ^ Clinical trial number NCT04347226 for "Anti-Interleukin-8 (Anti-IL-8) for Patients With COVID-19" at ClinicalTrials.gov
Further reading
- McCreary EK, Pogue JM (April 2020). "Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options". Open Forum Infectious Diseases. 7 (4): ofaa105. doi:10.1093/ofid/ofaa105. PMC 7144823. PMID 32284951.
- Zimmer, Carl (30 April 2020). "Old Drugs May Find a New Purpose: Fighting the Coronavirus". The New York Times.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Guy RK, DiPaola RS, Romanelli F, Dutch RE (May 2020). "Rapid repurposing of drugs for COVID-19". Science. 368 (6493): 829–830. Bibcode:2020Sci...368..829G. doi:10.1126/science.abb9332. PMID 32385101.
- Kotecha P, Light A, Checcucci E, Amparore D, Fiori C, Porpiglia F, et al. (June 2020). "Repurposing of drugs for Covid-19: a systematic review and meta-analysis". MedRxiv. doi:10.1101/2020.06.07.20124677.
- Velasquez-Manoff, Moises (11 August 2020). "How Covid Sends Some Bodies to War With Themselves". The New York Times.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
External links
- "COVID-19 therapeutics tracker". Regulatory Affairs Professionals Society.
- "STAT's Covid-19 Drugs and Vaccines Tracker". Stat.
- "Coronavirus Drug and Treatment Tracker". The New York Times.
- "JHMI Clinical Recommendations for Available Pharmacologic Therapies for COVID-19". Johns Hopkins.
