Tiomesterone
This is an old revision of this page, as edited by Vaccinationist (talk | contribs) at 19:19, 7 February 2014. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.

| |
| Names | |
|---|---|
| IUPAC name
S-[(1S,7R,8S,9S,10R,13S,14S,17S)-1-acetylsulfanyl-17-hydroxy-10,13,17-trimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-7-yl] ethanethioate
| |
| Other names
Thiomesterone; Thiomestrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.016.923 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C24H34O4S2 | |
| Molar mass | 450.65 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tiomesterone is an anabolic steroid.
Synthesis
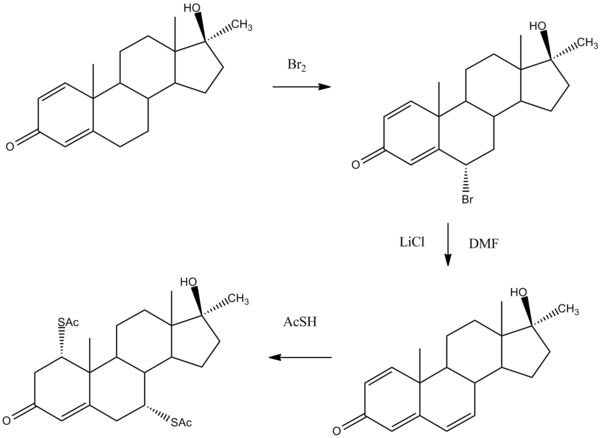
Extension of the chain of double bonds in androstenes provides yet another site for modifying the basic structure.
Bromination of the intermediate () that features the 1,4-dien-3-one system proceed on the allylic position at C6 (). Dehydrobromination by means of lithium carbonate in DMF leads to formation of the 1,4,6-trien-3-one system (). Treatment of that intermediate with thioacetic acid proceeds to add one thiol group to each end of the conjugated systems to afford the bisthiolated product thiomestrone ().
| ARTooltip Androgen receptor |
| ||||||
|---|---|---|---|---|---|---|---|
| GPRC6A |
| ||||||
- Articles without InChI source
- Chemical pages without ChemSpiderID
- Articles without EBI source
- Articles without KEGG source
- Articles without UNII source
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Pages using Chembox with unknown parameters
- Articles with short description
- Short description matches Wikidata
- All stub articles