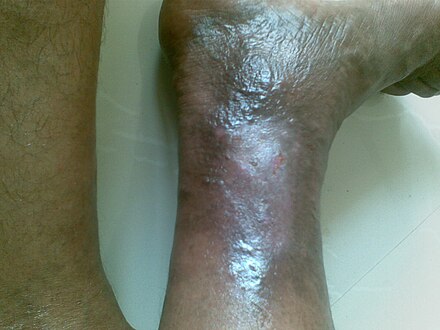
Venous ulcer
| Venous ulcer | |
|---|---|
| Other names | Venous insufficiency ulceration, stasis ulcer, stasis dermatitis, varicose ulcer, ulcus cruris, crural ulceration |
 | |
| Venous ulcer on the back of the right leg | |
| Specialty | Dermatology |
Venous ulcer is defined by the American Venous Forum as "a full-thickness defect of skin, most frequently in the ankle region, that fails to heal spontaneously and is sustained by chronic venous disease, based on venous duplex ultrasound testing."[1] Venous ulcers are wounds that are thought to occur due to improper functioning of venous valves, usually of the legs (hence leg ulcers).[2]: 846 They are an important cause of chronic wounds, affecting 1% of the population.[3] Venous ulcers develop mostly along the medial distal leg, and can be painful with negative effects on quality of life.[4]
Exercise, together with compression stockings, increases healing.[5] The NICE guideline recommends that everyone with a venous leg ulcer, even if healed, should be referred to a vascular specialist for venous duplex ultrasound and assessment for endovenous surgery.[6]
Signs and symptoms[edit]
Signs and symptoms of venous ulcers include:[citation needed]
- Moderate pain, which improves on elevation (unlike arterial ulcers which worsen with elevation)
- Irregular, sloping edges
- Associated oedema, due to increased hydrostatic pressure, which contributes to 'atrophie blanche'
- 'Atrophie blanche', localised loss of skin pigmentation due to death of erythrocytes and scarring
- Lipodermatosclerosis, a hardening of the skin which can lead to an "inverted champagne bottle" appearance to the leg
- Associated superficial varicose veins or "ankle flare", a collection of small, dark, engorged superficial veins[7]
-
Venous ulcer before surgery
-
Healing process of a chronic venous stasis ulcer of the lower leg
-
Healing venous ulcer after one month

Pathophysiology[edit]
The exact cause of venous ulcers is not certain, but a common denominator is generally venous stasis, which may be caused by chronic venous insufficiency,[8] and/or congestive heart failure.[9] Venous stasis causes the pressure in veins to increase.[10][11][12][13] The body needs the pressure gradient between arteries and veins in order for the heart to pump blood forward through arteries and into veins. When venous hypertension exists, arteries no longer have significantly higher pressure than veins, and blood is not pumped as effectively into or out of the area.[10][11][12][13]
Venous hypertension may also stretch veins and allow blood proteins to leak into the extravascular space, isolating extracellular matrix (ECM) molecules and growth factors, preventing them from helping to heal the wound.[10][13] Leakage of fibrinogen from veins as well as deficiencies in fibrinolysis may also cause fibrin to build up around the vessels, preventing oxygen and nutrients from reaching cells.[10] Venous insufficiency may also cause white blood cells (leukocytes) to accumulate in small blood vessels, releasing inflammatory factors and reactive oxygen species (ROS, free radicals) and further contributing to chronic wound formation.[10][13] Buildup of white blood cells in small blood vessels may also plug the vessels, further contributing to ischemia.[14] This blockage of blood vessels by leukocytes may be responsible for the "no reflow phenomenon", in which ischemic tissue is never fully reperfused.[14] Allowing blood to flow back into the limb, for example by elevating it, is necessary but also contributes to reperfusion injury.[11] Other comorbidities may also be the root cause of venous ulcers.[12]
It is in the crus that the classic venous stasis ulcer occurs. Venous stasis results from damage to the vein valvular system in the lower extremity and in extreme cases allows the pressure in the veins to be higher than the pressure in the arteries. This pressure results in transudation of inflammatory mediators into the subcutaneous tissues of the lower extremity and subsequent breakdown of the tissue including the skin.[citation needed]
Wounds of the distal lower extremities arising from causes not directly related to venous insufficiency (e.g., scratch, bite, burn, or surgical incision) may ultimately fail to heal if underlying (often undiagnosed) venous disease is not properly addressed.[citation needed]
Diagnosis[edit]

Classification[edit]
A clinical severity score has been developed to assess chronic venous ulcers. It is based on the CEAP (clinical, etiology, anatomy, and pathophysiology) classification system developed by an expert panel. A high score gives a poor prognosis.[15]
Distinction from arterial ulcer[edit]
A venous ulcer tends to occur on the medial side of the leg, typically around the medial malleolus in the 'gaiter area' whereas arterial ulcer tends to occur on lateral side of the leg and over bony prominences. A venous ulcer is typically shallow with irregular sloping edges whereas an arterial ulcer can be deep and has a 'punched out' appearance. Venous ulcers are typically 'wet' with a moderate to heavy exudate, whereas arterial ulcers are typically 'dry' and scabbed. The skin surrounding a venous ulcer may be edematous (swollen) and there may be evidence of varicose veins; the skin surrounding an arterial ulcer may be pale, cold, shiny and hairless. Both venous and arterial ulcers may be painful, however arterial ulcers tend to be more painful, especially with elevation of the leg, for example when in bed.[citation needed]
Differential diagnosis[edit]
Leg ulcerations may result from various pathologic processes. Common causes of leg ulcerations include inadequate blood flow and oxygen delivery to tissues as seen in peripheral arterial disease and venous stasis ulcerations. Additional causes include neutrophilic skin conditions such as pyoderma gangrenosum or Sweet's syndrome; vasculitic processes such as cryoglobulinemia; calciphylaxis (often seen in people with end-stage kidney disease but may also occur with medications such as warfarin); cancers such as squamous cell carcinoma (Marjolin's ulcer) or myelodysplastic syndrome; neuropathy (e.g., diabetic peripheral neuropathy); or atypical infections such as nocardiosis, sporotrichosis, or mycobacterial infections.[citation needed]
Prevention[edit]
Compression stockings appear to prevent the formation of new ulcers in people with a history of venous ulcers.[16]
Treatment[edit]
The main aim of the treatment is to create such an environment that allows skin to grow across an ulcer. In the majority of cases this requires finding and treating underlying venous reflux. The National Institute for Health and Care Excellence (NICE) recommends referral to a vascular service for anyone with a leg ulcer that has not healed within two weeks or anyone with a healed leg ulcer.[17]
Most venous ulcers respond to patient education, elevation of foot, elastic compression, and evaluation (known as the Bisgaard regimen).[18] Exercise together with compression stocking increases healing.[5] There is no evidence that antibiotics, whether administered intravenously or by mouth, are useful.[19] Silver products are also not typically useful, while there is some evidence of benefit from cadexomer iodine creams.[19] There is a lack of quality evidence regarding the use of medical grade honey for venous leg ulcers.[20]
The recommendations of dressings to treat venous ulcers vary between the countries. Antibiotics are often recommended to be used only if so advised by the physician due to emergence of resistance of bacteria to antibiotics. This is an issue on venous ulcers as they tend to heal slower than acute wounds for example. Natural alternatives that are suitable for the longer term use exists on the market such as honey and resin salve. These products are considered as Medical Devices in EU and the products have to be CE marked.[21][22]
There is uncertain evidence whether alginate dressing is effective in the healing of venous ulcer when compared to hydrocolloid dressing or plain non-adherent dressing.[23]
It is uncertain whether therapeutic ultrasound improve the healing of venous ulcer.[24]
Compression therapy[edit]
Non-elastic, ambulatory, below knee (BK) compression counters the impact of reflux on venous pump failure. Compression therapy is used for venous leg ulcers and can decrease blood vessel diameter and pressure, which increases their effectiveness, preventing blood from flowing backwards.[10] Compression is also used[10][25] to decrease release of inflammatory cytokines, lower the amount of fluid leaking from capillaries and therefore prevent swelling, and prevent clotting by decreasing activation of thrombin and increasing that of plasmin.[3] Compression is applied using elastic bandages or boots specifically designed for the purpose.[10]
A 2021 systematic review found that compression dressings probably reduce pain and help ulcers to heal more quickly (usually within 12 months) and may also improve quality of life.[26][27] However, it is not clear whether or not compression bandages have any unwanted effects or if the potential health benefits of using compression outweigh its costs.[26] It is not clear whether non-elastic systems are better than a multilayer elastic system.[27] Patients should wear as much compression as is comfortable.[28] In treating an existing ulcer, the type of dressing applied beneath the compression does not seem to matter, and hydrocolloid is not better than simple low adherent dressings.[29] Good outcomes in ulcer treatment were shown after the application of double compression stockings, e.g. ulcer stockings. These systems contain two different stockings, one often of white colour. This one is to be put on first, is also worn overnight and exerts a basic pressure of 20 mmHg or less. Also it keeps the wound dressing in place. A second stocking, often brown, sometimes black, achieves a pressure of 20–30 mmHg and is applied over the other stocking during the daytime.[30]
Intermittent pneumatic compression devices may be used, but it is not clear that they are superior to simple compression dressings.[31]
It is not clear if interventions that are aimed to help people adhere to compression therapy are effective.[32] More research is needed in this field.
Medications[edit]
Pentoxifylline is a useful add on treatment to compression stockings and may also help by itself.[33] It works by reducing platelet aggregation and thrombus formation. Gastrointestinal disturbances were reported as a potential adverse effect.[33]
Sulodexide, which reduces the formation of blood clots and reduces inflammation, may improve the healing of venous ulcers when taken in conjunction with proper local wound care.[34] Further research is necessary to determine potential adverse effects, the effectiveness, and the dosing protocol for sulodexide treatment.[citation needed]
An oral dose of aspirin is being investigated as a potential treatment option for people with venous ulcers. A 2016 Cochrane systematic review concluded that further research is necessary before this treatment option can be confirmed to be safe and effective.[35]
Oral zinc supplements have not been proven to be effective in aiding the healing of venous ulcers, however more research is necessary to confirm these results.[36]
Treatments aimed at decreasing protease activity to promote healing in chronic wounds have been suggested, however, the benefit remains uncertain.[37] There is also lack of evidence on effectiveness on testing for elevated proteases in venous ulcers and treating them with protease modulating treatment.[38] There is low certainty evidence that protease modulating matrix treatment is helpful in the healing of venous ulcer.[39]
Flavonoids may be useful for treating venous ulcers but the evidence needs to be interpreted cautiously.[40]
Wound Cleansing Solutions[edit]
There is insufficient evidence to determine if cleaning wounds is beneficial or whether wound cleaning solutions (polyhexamethylene biguanide, aqueous oxygen peroxide, etc.) are better than sterile water or saline solutions to help venous leg ulcers heal.[41] It is uncertain whether the choice of cleaning solution or method of application makes any difference to venous leg ulcer healing.[41]
Skin grafts and artificial skin[edit]
Two layers of skin created from animal sources as a skin graft has been found to be useful in venous leg ulcers.[42]
Artificial skin, made of collagen and cultured skin cells, is also used to cover venous ulcers and excrete growth factors to help them heal.[43] A systematic review found that bilayer artificial skin with compression bandaging is useful in the healing of venous ulcers when compared to simple dressings.[42]
Surgery[edit]
A randomized controlled trial found that surgery "reduces the recurrence of ulcers at four years and results in a greater proportion of ulcer free time".[44]
Local anaesthetic endovenous surgery using the thermoablation (endovenous laser ablation or radiofrequency), perforator closure (TRLOP) and foam sclerotherapy showed an 85% success rate of healing, with no recurrence of healed ulcers at an average of 3.1 years, and a clinical improvement in 98% in a selected group of venous leg ulcers.[45]
Endovenous ablation, in combination with compression, on superficial venous incompetence has been shown (high quality evidence) to improve leg ulcer healing when compared to compression alone.[46] The use of subfascial endoscopic perforator surgery is uncertain in the healing of venous ulcer.[47]
Dressings[edit]
It is not certain which dressings and topical agents are most effective for healing venous leg ulcers.[48][49] Silver-containing dressings may increase the probability of healing for venous leg ulcers.[48] A clinical trial was successfully performed with a mixture of 60% sugar or glucose powder and 40% vaseline.[50] A 2013 Cochrane systematic review aimed to determine the effectiveness of foam dressings for helping to heal venous leg ulcers. The authors concluded that is uncertain whether or not foam dressings are more effective than other dressing types and that more randomized controlled trials are needed to help answer this research question.[51] However, there is some evidence that ibuprofen dressings may offer pain relief to people with venous leg ulcers.[52]
Prognosis[edit]
Venous ulcers are costly to treat, and there is a significant chance that they will recur after healing;[3][10] one study found that up to 48% of venous ulcers had recurred by the fifth year after healing.[10] However treatment with local anaesthetic endovenous techniques suggests a reduction of this high recurrence rate is possible.[45]
Without proper care, the ulcer may get infected leading to cellulitis or gangrene and eventually may need amputation of the part of limb in future.[citation needed]
Some topical drugs used to treat venous ulcer may cause venous eczema.[53]
Research[edit]
The current 'best' practice in the UK is to treat the underlying venous reflux once an ulcer has healed. It is questionable as to whether endovenous treatment should be offered before ulcer healing, as current evidence would not support this approach as standard care. The EVRA (Early Venous Reflux Ablation) ulcer trial, a randomised clinical trial funded by the National Institute for Health and Care Research (NIHR) to compare early versus delayed endovenous treatment of superficial venous reflux in patients with chronic venous ulceration, opened for recruitment in October 2013. The study hopes to show an increase in healing rates from 60% to 75% at 24 weeks.[54]
Research from the University of Surrey and funded by the Leg Ulcer Charity looked at the psychological impact of having a leg ulcer, on the relatives and friends of the affected person, and the influence of treatment.[55]
References[edit]
- ^ O'Donnell TF, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, et al. (August 2014). "Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum". Journal of Vascular Surgery. 60 (2 Suppl): 3S–59S. doi:10.1016/j.jvs.2014.04.049. PMID 24974070.
- ^ James WD, Berger TG, Elston DM (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 978-0-7216-2921-6. OCLC 968428064.
- ^ a b c Lal BK (March 2015). "Venous ulcers of the lower extremity: Definition, epidemiology, and economic and social burdens". Seminars in Vascular Surgery. 28 (1): 3–5. doi:10.1053/j.semvascsurg.2015.05.002. PMID 26358303.
- ^ Phillips P, Lumley E, Duncan R, Aber A, Woods HB, Jones GL, Michaels J (March 2018). "A systematic review of qualitative research into people's experiences of living with venous leg ulcers" (PDF). Journal of Advanced Nursing. 74 (3): 550–563. doi:10.1111/jan.13465. PMID 28960514. S2CID 206018724.
- ^ a b Jull A, Slark J, Parsons J (November 2018). "Prescribed Exercise With Compression vs Compression Alone in Treating Patients With Venous Leg Ulcers: A Systematic Review and Meta-analysis". JAMA Dermatology. 154 (11): 1304–1311. doi:10.1001/jamadermatol.2018.3281. PMC 6248128. PMID 30285080.
- ^ NICE (July 23, 2013). "Varicose veins in the legs: The diagnosis and management of varicose veins. 1.2 Referral to a vascular service". National Institute for Health and Care Excellence. Retrieved June 15, 2019.
- ^ Hugo F, Norris-Cervetto E, Warbrick-Smith J (2015). Oxford cases in medicine and surgery (Second ed.). Oxford: Oxford University Press. ISBN 978-0198716228. OCLC 923846134.
- ^ "Chronic Venous Insufficiency (CVI)". Cleveland Clinic. Last reviewed by a Cleveland Clinic medical professional on 05/14/2019.
- ^ Zhu R, Hu Y, Tang L (2017). "Reduced cardiac function and risk of venous thromboembolism in Asian countries". Thrombosis Journal. 15 (1): 12. doi:10.1186/s12959-017-0135-3. PMC 5404284. PMID 28450810.
- ^ a b c d e f g h i j Brem H, Kirsner RS, Falanga V (July 2004). "Protocol for the successful treatment of venous ulcers". American Journal of Surgery. 188 (1A Suppl): 1–8. doi:10.1016/S0002-9610(03)00284-8. PMID 15223495.
- ^ a b c Mustoe T (May 2004). "Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy". American Journal of Surgery. 187 (5A): 65S–70S. doi:10.1016/S0002-9610(03)00306-4. PMID 15147994.
- ^ a b c Moreo K (2005). "Understanding and overcoming the challenges of effective case management for patients with chronic wounds". The Case Manager. 16 (2): 62–3, 67. doi:10.1016/j.casemgr.2005.01.014. PMID 15818347.
- ^ a b c d Stanley AC, Lounsbury KM, Corrow K, Callas PW, Zhar R, Howe AK, Ricci MA (September 2005). "Pressure elevation slows the fibroblast response to wound healing". Journal of Vascular Surgery. 42 (3): 546–551. doi:10.1016/j.jvs.2005.04.047. PMID 16171604.
- ^ a b "eMedicine - Reperfusion Injury in Stroke : Article by Wayne M Clark, MD". Retrieved 2007-08-05.
- ^ Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. (December 2004). "Revision of the CEAP classification for chronic venous disorders: consensus statement". Journal of Vascular Surgery. 40 (6): 1248–1252. doi:10.1016/j.jvs.2004.09.027. PMID 15622385.
- ^ de Moraes Silva, Melissa Andreia; Nelson, Andrea; Bell-Syer, Sally Em; Jesus-Silva, Seleno G. de; Miranda, Fausto (2024-03-07). "Compression for preventing recurrence of venous ulcers". The Cochrane Database of Systematic Reviews. 2024 (3): CD002303. doi:10.1002/14651858.CD002303.pub4. ISSN 1469-493X. PMC 10919450. PMID 38451842.
- ^ NICE (July 23, 2013). "Varicose veins in the legs: The diagnosis and management of varicose veins. 1.2 Referral to a vascular service". National Institute for Health and Care Excellence. Retrieved August 25, 2014.
- ^ van Gent WB, Wilschut ED, Wittens C (November 2010). "Management of venous ulcer disease". BMJ. 341: c6045. doi:10.1136/bmj.c6045. PMID 21075818. S2CID 5218584.
- ^ a b O'Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R (January 2014). "Antibiotics and antiseptics for venous leg ulcers". The Cochrane Database of Systematic Reviews. 1 (1): CD003557. doi:10.1002/14651858.CD003557.pub5. PMC 10580125. PMID 24408354.
- ^ Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N (March 2015). "Honey as a topical treatment for wounds". The Cochrane Database of Systematic Reviews. 3 (3): CD005083. doi:10.1002/14651858.CD005083.pub4. PMC 9719456. PMID 25742878.
- ^ Lohi J, Sipponen A, Jokinen JJ (March 2010). "Local dressings for pressure ulcers: what is the best tool to apply in primary and second care?". Journal of Wound Care. 19 (3): 123–127. doi:10.12968/jowc.2010.19.3.47282. PMID 20559190.
- ^ "Regulation (Eu) 2017/745 of the European Parliament and of the Council on medical devices". Official Journal of the European Union. 5 April 2017.
- ^ O'Meara S, Martyn-St James M, Adderley UJ, et al. (Cochrane Wounds Group) (August 2015). "Alginate dressings for venous leg ulcers". The Cochrane Database of Systematic Reviews. 2015 (8): CD010182. doi:10.1002/14651858.CD010182.pub3. PMC 7087437. PMID 26286189.
- ^ Cullum N, Liu Z, et al. (Cochrane Wounds Group) (May 2017). "Therapeutic ultrasound for venous leg ulcers". The Cochrane Database of Systematic Reviews. 2017 (5): CD001180. doi:10.1002/14651858.CD001180.pub4. PMC 6481488. PMID 28504325.
- ^ Taylor JE, Laity PR, Hicks J, Wong SS, Norris K, Khunkamchoo P, et al. (October 2005). "Extent of iron pick-up in deforoxamine-coupled polyurethane materials for therapy of chronic wounds". Biomaterials. 26 (30): 6024–6033. doi:10.1016/j.biomaterials.2005.03.015. PMID 15885771.
- ^ a b Shi C, Dumville JC, Cullum N, Connaughton E, Norman G, et al. (Cochrane Wounds Group) (July 2021). "Compression bandages or stockings versus no compression for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 2021 (7): CD013397. doi:10.1002/14651858.CD013397.pub2. PMC 8407020. PMID 34308565.
- ^ a b Nelson EA, Cullum N, Jones J (June 2006). "Venous leg ulcers". Clinical Evidence (15): 2607–2626. PMID 16973096.
- ^ Nelson EA, Harper DR, Prescott RJ, Gibson B, Brown D, Ruckley CV (October 2006). "Prevention of recurrence of venous ulceration: randomized controlled trial of class 2 and class 3 elastic compression". Journal of Vascular Surgery. 44 (4): 803–808. doi:10.1016/j.jvs.2006.05.051. PMID 17012004.
- ^ Palfreyman S, Nelson EA, Michaels JA (August 2007). "Dressings for venous leg ulcers: systematic review and meta-analysis". BMJ. 335 (7613): 244. doi:10.1136/bmj.39248.634977.AE. PMC 1939774. PMID 17631512.
- ^ Partsch H, Mortimer P (August 2015). "Compression for leg wounds". The British Journal of Dermatology. 173 (2): 359–369. doi:10.1111/bjd.13851. PMID 26094638.
- ^ Nelson EA, Hillman A, Thomas K (May 2014). "Intermittent pneumatic compression for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 5 (5): CD001899. arXiv:quant-ph/0403227. doi:10.1002/14651858.CD001899.pub4. PMC 10788769. PMID 24820100.
- ^ Weller CD, Buchbinder R, Johnston RV (March 2016). "Interventions for helping people adhere to compression treatments for venous leg ulceration". The Cochrane Database of Systematic Reviews. 2016 (3): CD008378. doi:10.1002/14651858.CD008378.pub3. PMC 6823259. PMID 26932818.
- ^ a b Jull AB, Arroll B, Parag V, Waters J (December 2012). "Pentoxifylline for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 12 (12): CD001733. doi:10.1002/14651858.CD001733.pub3. PMC 7061323. PMID 23235582.
- ^ Wu B, Lu J, Yang M, Xu T (June 2016). "Sulodexide for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 2016 (6): CD010694. doi:10.1002/14651858.CD010694.pub2. PMC 9308373. PMID 27251175.
- ^ de Oliveira Carvalho PE, Magolbo NG, De Aquino RF, Weller CD (February 2016). "Oral aspirin for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 2016 (2): CD009432. doi:10.1002/14651858.CD009432.pub2. PMC 8627253. PMID 26889740.
- ^ Wilkinson EA (September 2014). "Oral zinc for arterial and venous leg ulcers". The Cochrane Database of Systematic Reviews. 2014 (9): CD001273. doi:10.1002/14651858.CD001273.pub3. PMC 6486207. PMID 25202988.
- ^ Westby MJ, Dumville JC, Stubbs N, Norman G, Wong JK, Cullum N, Riley RD (September 2018). "Protease activity as a prognostic factor for wound healing in venous leg ulcers". The Cochrane Database of Systematic Reviews. 2018 (9): CD012841. doi:10.1002/14651858.CD012841.pub2. PMC 6513613. PMID 30171767.
- ^ Norman G, Westby MJ, Stubbs N, Dumville JC, Cullum N, et al. (Cochrane Wounds Group) (January 2016). "A 'test and treat' strategy for elevated wound protease activity for healing in venous leg ulcers". The Cochrane Database of Systematic Reviews. 2016 (1): CD011753. doi:10.1002/14651858.CD011753.pub2. PMC 8627254. PMID 26771894.
- ^ Westby MJ, Norman G, Dumville JC, Stubbs N, Cullum N, et al. (Cochrane Wounds Group) (December 2016). "Protease-modulating matrix treatments for healing venous leg ulcers". The Cochrane Database of Systematic Reviews. 12 (4): CD011918. doi:10.1002/14651858.CD011918.pub2. PMC 6463954. PMID 27977053.
- ^ Scallon C, Bell-Syer SE, Aziz Z, et al. (Cochrane Wounds Group) (May 2013). "Flavonoids for treating venous leg ulcers". The Cochrane Database of Systematic Reviews (5): CD006477. doi:10.1002/14651858.CD006477.pub2. PMID 23728661.
- ^ a b McLain NE, Moore ZE, Avsar P, et al. (Cochrane Wounds Group) (March 2021). "Wound cleansing for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 2021 (3): CD011675. doi:10.1002/14651858.CD011675.pub2. PMC 8092712. PMID 33734426.
- ^ a b Jones JE, Nelson EA, Al-Hity A (January 2013). "Skin grafting for venous leg ulcers". The Cochrane Database of Systematic Reviews. 1 (1): CD001737. doi:10.1002/14651858.CD001737.pub4. PMC 7061325. PMID 23440784.
- ^ Mustoe T (March 17–18, 2005). Dermal ulcer healing: Advances in understanding. Tissue repair and ulcer/wound healing: molecular mechanisms, therapeutic targets and future directions. Paris, France.
- ^ Gohel MS, Barwell JR, Taylor M, Chant T, Foy C, Earnshaw JJ, et al. (July 2007). "Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial". BMJ. 335 (7610): 83. doi:10.1136/bmj.39216.542442.BE. PMC 1914523. PMID 17545185.
- ^ a b Thomas CA, Holdstock JM, Harrison CC, Price BA, Whiteley MS (April 2013). "Healing rates following venous surgery for chronic venous leg ulcers in an independent specialist vein unit". Phlebology. 28 (3): 132–139. doi:10.1258/phleb.2012.011097. PMID 22833505. S2CID 9186619.
- ^ Cai PL, Hitchman LH, Mohamed AH, Smith GE, Chetter I, Carradice D (July 2023). "Endovenous ablation for venous leg ulcers". The Cochrane Database of Systematic Reviews. 2023 (7): CD009494. doi:10.1002/14651858.CD009494.pub3. PMC 10373122. PMID 37497816.
- ^ Lin ZC, Loveland PM, Johnston RV, Bruce M, Weller CD, et al. (Cochrane Wounds Group) (March 2019). "Subfascial endoscopic perforator surgery (SEPS) for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 2019 (3): CD012164. doi:10.1002/14651858.CD012164.pub2. PMC 6397791. PMID 30827037.
- ^ a b Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC (June 2018). "Dressings and topical agents for treating venous leg ulcers". The Cochrane Database of Systematic Reviews. 2018 (6): CD012583. doi:10.1002/14651858.CD012583.pub2. PMC 6513558. PMID 29906322.
- ^ Ribeiro CT, Dias FA, Fregonezi GA, et al. (Cochrane Wounds Group) (August 2022). "Hydrogel dressings for venous leg ulcers". The Cochrane Database of Systematic Reviews. 2022 (8): CD010738. doi:10.1002/14651858.CD010738.pub2. PMC 9354941. PMID 35930364.
- ^ Anti-Infective Effects of Sugar-Vaseline Mixture on Leg Ulcers
- ^ O'Meara S, Martyn-St James M (May 2013). "Foam dressings for venous leg ulcers". The Cochrane Database of Systematic Reviews (5): CD009907. doi:10.1002/14651858.cd009907.pub2. PMID 23728697.
- ^ Briggs M, Nelson EA, Martyn-St James M, et al. (Cochrane Wounds Group) (November 2012). "Topical agents or dressings for pain in venous leg ulcers". The Cochrane Database of Systematic Reviews. 11 (11): CD001177. doi:10.1002/14651858.CD001177.pub3. PMC 7054838. PMID 23152206.
- ^ Marks R (2003-04-30). Roxburgh's Common Skin Diseases (17th ed.). CRC Press. p. 127. ISBN 978-0-340-76232-5.
- ^ Davies A, Heatley F. "EVRA (Early Venous Reflux Ablation) Ulcer Trial". Faculty of Medicine Imperial College London.
- ^ Tollow P (April 2014). "Impact of Leg Ulcers on Relatives and Carers of Affected Patients - A PhD Study funded by The Leg Ulcer Charity". The Leg Ulcer Charity. Archived from the original on August 26, 2014. Retrieved August 25, 2014.