Nanomaterials: Difference between revisions
| Line 46: | Line 46: | ||
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk [[copper]] (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same [[malleability]] and [[ductility]] as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their magnetisation direction using room temperature thermal energy, thus making them useless for memory storage. [[suspension (chemistry)|Suspension]]s of nanoparticles are possible because the interaction of the particle surface with the [[solvent]] is strong enough to overcome differences in [[density]], which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visual properties because they are small enough to confine their electrons and produce quantum effects. For example [[gold]] nanoparticles appear deep red to black in solution. |
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk [[copper]] (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same [[malleability]] and [[ductility]] as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their magnetisation direction using room temperature thermal energy, thus making them useless for memory storage. [[suspension (chemistry)|Suspension]]s of nanoparticles are possible because the interaction of the particle surface with the [[solvent]] is strong enough to overcome differences in [[density]], which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visual properties because they are small enough to confine their electrons and produce quantum effects. For example [[gold]] nanoparticles appear deep red to black in solution. |
||
The |
The often very high surface area to volume ratio of nanoparticles provides a tremendous driving force for [[diffusion]], especially at elevated temperatures. [[Sintering]] is possible at lower temperatures and over shorter durations than for larger particles. This theoretically does not affect the density of the final product, though flow difficulties and the tendency of nanoparticles to agglomerate do complicate matters. The surface effects of nanoparticles also reduces the incipient [[melting temperature]]. |
||
==Chemical processing of ceramics== |
==Chemical processing of ceramics== |
||
Revision as of 23:27, 28 September 2009
| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
| Part of a series of articles on |
| Nanotechnology |
|---|
| Impact and applications |
| Nanomaterials |
| Molecular self-assembly |
| Nanoelectronics |
| Nanometrology |
| Molecular nanotechnology |
Nanomaterials is a field which takes a materials science-based approach to nanotechnology. It studies materials with morphological features on the nanoscale, and especially those which have special properties stemming from their nanoscale dimensions. Nanoscale is usually defined as smaller than a one tenth of a micrometer in at least one dimension,[1] though this term is sometimes also used for materials smaller than one micrometer.
Fundamental concepts
An aspect of nanotechnology is the vastly increased ratio of surface area to volume present in many nanoscale materials which makes possible new quantum mechanical effects, for example the “quantum size effect” where the electronic properties of solids are altered with great reductions in particle size. This effect does not come into play by going from macro to micro dimensions. However, it becomes pronounced when the nanometer size range is reached. A certain number of physical properties also alter with the change from macroscopic systems. Novel mechanical properties of nanomaterials is a subject of nanomechanics research. Catalytic activities also reveal new behaviour in the interaction with biomaterials.
Nanotechnology can be thought of as extensions of traditional disciplines towards the explicit consideration of these properties. Additionally, traditional disciplines can be re-interpreted as specific applications of nanotechnology. This dynamic reciprocation of ideas and concepts contributes to the modern understanding of the field. Broadly speaking, nanotechnology is the synthesis and application of ideas from science and engineering towards the understanding and production of novel materials and devices. These products generally make copious use of physical properties associated with small scales.
As mentioned above, materials reduced to the nanoscale can suddenly show very different properties compared to what they exhibit on a macroscale, enabling unique applications. For instance, opaque substances become transparent (copper); inert materials attain catalytic properties (platinum); stable materials turn combustible (aluminum); solids turn into liquids at room temperature (gold); insulators become conductors (silicon). Materials such as gold, which is chemically inert at normal scales, can serve as a potent chemical catalyst at nanoscales. Much of the fascination with nanotechnology stems from these unique quantum and surface phenomena that matter exhibits at the nanoscale.
Nanosize powder particles (a few nanometres in diameter, also called nanoparticles) are potentially important in ceramics, powder metallurgy, the achievement of uniform nanoporosity and similar applications. The strong tendency of small particles to form clumps ("agglomerates") is a serious technological problem that impedes such applications. However, a number of dispersants such as ammonium citrate (aqueous) and imidazoline or oleyl alcohol (nonaqueous) are promising solutions as possible additives for deagglomeration.
Tools and techniques
The first observations and size measurements of nano-particles were made during the first decade of the 20th century. They are mostly associated with the name of Zsigmondy who made detailed studies of gold sols and other nanomaterials with sizes down to 10 nm and less. He published a book in 1914.[2] He used ultramicroscope that employs a dark field method for seeing particles with sizes much less than light wavelength.
There are traditional techniques developed during 20th century in Interface and Colloid Science for characterizing nanomaterials. These are widely used for first generation passive nanomaterials specified in the next section.
These methods include several different techniques for characterizing particle size distribution. This characterization is imperative because many materials that are expected to be nano-sized are actually aggregated in solutions. Some of methods are based on light scattering. Other apply ultrasound, such as ultrasound attenuation spectroscopy for testing concentrated nano-dispersions and microemulsions.[3]
There is also a group of traditional techniques for characterizing surface charge or zeta potential of nano-particles in solutions. This information is required for proper system stabilzation, preventing its aggregation or flocculation. These methods include microelectrophoresis, electrophoretic light scattering and electroacoustics. The last one, for instance colloid vibration current method is suitable for characterizing concentrated systems.
Materials used in nanotechnology
Materials referred to as "nanomaterials" generally fall into two categories: fullerenes, and inorganic nanoparticles. See also Nanomaterials in List of nanotechnology topics
Fullerenes
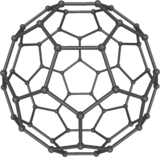
The fullerenes are a class of allotropes of carbon which conceptually are graphene sheets rolled into tubes or spheres. These include the carbon nanotubes which are of interest both because of their mechanical strength and also because of their electrical properties.
For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. In April 2003, fullerenes were under study for potential medicinal use: binding specific antibiotics to the structure of resistant bacteria and even target certain types of cancer cells such as melanoma. The October 2005 issue of Chemistry and Biology contains an article describing the use of fullerenes as light-activated antimicrobial agents. In the field of nanotechnology, heat resistance and superconductivity are among the properties attracting intense research.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
There are many calculations that have been done using ab-initio Quantum Methods applied to fullerenes. By DFT and TDDFT methods one can obtain IR, Raman and UV spectra. Results of such calculations can be compared with experimental results.
Nanoparticles
Nanoparticles or nanocrystals made of metals, semiconductors, or oxides are of particular interest for their mechanical, electrical, magnetic, optical, chemical and other properties. Nanoparticles have been used as quantum dots and as chemical catalysts.
Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and atomic or molecular structures. A bulk material should have constant physical properties regardless of its size, but at the nano-scale this is often not the case. Size-dependent properties are observed such as quantum confinement in semiconductor particles, surface plasmon resonance in some metal particles and superparamagnetism in magnetic materials.
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk copper (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same malleability and ductility as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their magnetisation direction using room temperature thermal energy, thus making them useless for memory storage. Suspensions of nanoparticles are possible because the interaction of the particle surface with the solvent is strong enough to overcome differences in density, which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visual properties because they are small enough to confine their electrons and produce quantum effects. For example gold nanoparticles appear deep red to black in solution.
The often very high surface area to volume ratio of nanoparticles provides a tremendous driving force for diffusion, especially at elevated temperatures. Sintering is possible at lower temperatures and over shorter durations than for larger particles. This theoretically does not affect the density of the final product, though flow difficulties and the tendency of nanoparticles to agglomerate do complicate matters. The surface effects of nanoparticles also reduces the incipient melting temperature.
Chemical processing of ceramics
Microstructural uniformity
In the processing of fine ceramics, the irregular particle sizes and shapes in a typical powder often lead to non-uniform packing morphologies that result in packing density variations in the powder compact. Uncontrolled agglomeration of powders due to attractive van der Waals forces can also give rise to in microstructural inhomogeneities. [4] [5]
Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the solvent can be removed, and thus highly dependent upon the distribution of porosity. Such stresses have been associated with a plastic-to-brittle transition in consolidated bodies, [6] and can yield to crack propagation in the unfired body if not relieved.
In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the sintering process, yielding inhomogeneous densification. [7][8] Some pores and other structural defects associated with density variations have been shown to play a detrimental role in the sintering process by growing and thus limiting end-point densities. [9] Differential stresses arising from inhomogeneous densification have also been shown to result in the propagation of internal cracks, thus becoming the strength-controlling flaws. [10]
It would therefore appear desirable to process a material in such a way that it is physically uniform with regard to the distribution of components and porosity, rather than using particle size distributions which will maximize the green density. The containment of a uniformly dispersed assembly of strongly interacting particles in suspension requires total control over particle-particle interactions. Monodisperse colloids provide this potential. [11][12]
Monodisperse powders of colloidal silica, for example, may therefore be stabilized sufficiently to ensure a high degree of order in the colloidal crystal or polycrystalline colloidal solid which results from aggregation. The degree of order appears to be limited by the time and space allowed for longer-range correlations to be established. [13] [14] Such defective polycrystalline colloidal structures would appear to be the basic elements of submicrometre colloidal materials science, and, therefore, provide the first step in developing a more rigorous understanding of the mechanisms involved in microstructural evolution in inorganic systems such as polycrystalline ceramics.
Sol-gel processing
Safety of manufactured nanomaterials
Nanomaterials behave differently than other similarly-sized particles. It is therefore necessary to develop specialized approaches to testing and monitoring their effects on human health and on the environment. The OECD Chemicals Committee has established the Working Party on Manufactured Nanomaterials to address this issue and to study the practices of OECD member countries in regards to nanomaterial safety.[15]
While nanomaterials and nanotechnologies are expected to yield numerous health and health care advances, such as more targeted methods of delivering drugs, new cancer therapies, and methods of early detection of diseases, they also may have unwanted effects. [16] Increased rate of absorption is the main concern associated with manufactured nanoparticles.
When materials are made into nanoparticles, their surface area to volume ratio increases. The greater specific surface area (surface area per unit weight) may lead to increased rate of absorption through the skin, lungs, or digestive tract and may cause unwanted effects to the lungs as well as other organs. However, the particles must be absorbed in sufficient quantities in order to pose health risks.[16]
As the use of nanomaterials increases worldwide, concerns for worker and user safety are mounting. To address such concerns, the Swedish Karolinska Institute conducted a study in which various nanoparticles were introduced to human lung epithelial cells. The results, released in 2008, showed that iron oxide nanoparticles caused little DNA damage and were non-toxic. Zinc oxide nanoparticles were slightly worse. Titanium dioxide caused only DNA damage. Carbon nanotubes caused DNA damage at low levels. Copper oxide was found to be the worst offender, and was the only nanomaterial identified by the researchers as a clear health risk.[17]
In October 2008, the Department of Toxic Substances Control (DTSC), within the California Environmental Protection Agency, announced its intent to request information regarding analytical test methods, fate and transport in the environment, and other relevant information from manufacturers of carbon nanotubes.[18] The term "manufacturers” includes persons and businesses that produce nanotubes in California, or import carbon nanotubes into California for sale. The purpose of this information request will be to identify information gaps and to develop information about carbon nanotubes, an important emerging nanomaterial.
On January 22, 2009, a formal information request letter [19] was sent to manufacturers[20] who produce or import carbon nanotubes in California, or who may export carbon nanotubes into the State. This letter constitutes the first formal implementation of the authorities placed into statute by AB 289 (2006) and is directed to manufacturers of carbon nanotubes, both industry and academia within the State, and to manufacturers outside California who export carbon nanotubes to California. This request for information must be met by the manufacturers within one year.
See also
References
- ^ C. Buzea (2007). "Nanomaterials and nanoparticles: Sources and toxicity". Biointerphases. 2: MR17.
- ^ Zsigmondy, R. "Colloids and the Ultramicroscope", J.Wiley and Sons, NY, (1914)
- ^ Dukhin, A.S. and Goetz, P.J. (2002). Ultrasound for characterizing colloids. Elsevier.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Onoda, G.Y., Jr. and Hench, L.L. Eds. (1979). Ceramic Processing Before Firing. New York: Wiley & Sons.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Aksay, I.A., Lange, F.F., Davis, B.I. (1983). "Uniformity of Al2O3-ZrO2 Composites by Colloidal Filtration". J. Am. Ceram. Soc. 66: 190. doi:10.1111/j.1151-2916.1983.tb10550.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Franks, G.V. and Lange, F.F. (1996). "Plastic-to-Brittle Transition of Saturated, Alumina Powder Compacts". J. Am. Ceram. Soc. 79: 3161. doi:10.1111/j.1151-2916.1996.tb08091.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Evans, A.G. and Davidge, R.W. (1969). "Strength and fracture of fully dense polycrystalline magnesium oxide". Phil. Mag. 20: 373. doi:10.1080/14786436908228708.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Evans, A.G. and Davidge, R.W. (1970). "Strength and fracture of fully dense polycrystalline magnesium oxide". J. Mat. Sci. 5: 314.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lange, F.F. and Metcalf, M. (1983). "Processing-Related Fracture Origins in A12O3/ZrO2 Composites II: Agglomerate Motion and Crack-like Internal Surfaces Caused by Differential Sintering". J. Am. Ceram. Soc. 66: 398. doi:10.1111/j.1151-2916.1983.tb10069.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Evans, A.G. (1987). "Considerations of Inhomogeneity Effects in Sintering". J. Am. Ceram. Soc. 65: 497.
- ^ Allman III, R.M. and Onoda, G.Y., Jr. (1984). "Ceramic Science Group, IBM T.J. Watson Research Center".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Mangels, J.A. and Messing, G.L., Eds. (1984). "Microstructural Control Through Colloidal Consolidation". Advances in Ceramics: Forming of Ceramics. 9: 94.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Whitesides, G.M.; et al. (1991). "Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures". Science. 254: 1312. doi:10.1126/science.1962191.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Aksay, I.A.; et al. (2000). "Self-Assembled Ceramics". Ann. Rev. Phys. Chem. 51: 601.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "Safety of Manufactured Nanomaterials: About, OECD Environment Directorate". OECD.org. 18 July 2007.
- ^ a b <C. Lauterwasser (18 July 2007). OECD.org http://www.oecd.org/dataoecd/37/19/37770473.pdf.
{{cite news}}: Missing or empty|title=(help) - ^ Chemical & Engineering News Vol. 86 No. 35, 1 Sept. 2008, "Study Sizes up Nanomaterial Toxicity", p. 44
- ^ "Nanotechnology web page". Department of Toxic Substances Control. 2008.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Formal information request letter" (PDF).
- ^ "List of nanotube manufacturers who produce or import carbon nanotubes in California" (PDF). Retrieved 2009-06-06.
Further reading
- Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing by C. Jeffrey Brinker and George W. Scherer, Academic Press (1990)
External links
- Safety of Manufactured Nanomaterials: OECD Environment Directorate
- Assessing health risks of nanomaterials summary by GreenFacts of the European Commission SCENIHR assessment
- International Liposome Society
- Textiles Nanotechnology Laboratory at Cornell University
- IOP.org Article
- Nano Structured Material
- AGAPAC - Advanced GaN Packaging EU FP7 project using nanomaterial composites to enhance the thermal management of GaN electronic devices
