Neuroendocrine tumor: Difference between revisions
FeatherPluma (talk | contribs) |
FeatherPluma (talk | contribs) |
||
| Line 301: | Line 301: | ||
* achaete-scute homolog 1 (ASCL1) |
* achaete-scute homolog 1 (ASCL1) |
||
{{Div col end}} |
{{Div col end}} |
||
According to "CIS Chromagranin [sic] Monography: Chromogranin: A circulating neuroendocrine marker: biology, pathology, assay technology and clinical applications" by Luca C. Giovanella, at http://www.eoc.ch/allegati/mednuc_cromogranina%20A.pdf, markers include |
|||
* Cytoplasmic markers |
|||
** Neuron-specific enolase (NSE, gamma-gamma dimer) |
|||
** Synaptophysin (P38) |
|||
** Synaptobrevin (VAMP-1) |
|||
** Synapsin (1A, 1B, 2A, 2B) |
|||
** SV2 |
|||
** Protein P65 |
|||
** Protein S-100 |
|||
** Protein gene product (PGP 9.5) |
|||
** Intermediate filaments (cytokeratins, vimentin, neurofilaments) |
|||
** Protein 7B2 |
|||
* Granular markers |
|||
** Chromogranin A (protein secretory I) |
|||
** Chromogranin B (secretogranin I) |
|||
** Chromogranin C (secretogranin II) |
|||
** Pancreastatin |
|||
** Vasostatin |
|||
** Cytochrome b561 |
|||
** Leu-7 (HNK-1) |
|||
According to "Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours" by Ramage JK et al., markers include |
|||
* chromogranin A (CgA) |
|||
* serum pancreatic polypeptide |
|||
* serum calcitonin |
|||
* and serum HCG ({alpha} and ß) |
|||
* 5-hydroxy indole acetic acid (5-HIAA) (grade C) |
|||
* thyroid function tests (TFTs) |
|||
* parathyroid hormone (PTH) |
|||
* calcium |
|||
* calcitonin |
|||
* prolactin |
|||
* {alpha}-fetoprotein |
|||
* carcinoembryonic antigen (CEA) |
|||
* ß-human chorionic gonadotrophin (ß-HCG) (grade D) |
|||
According to http://www.med.umich.edu/lrc/presentation/endo/islet.htm, markers include |
|||
* Pancreatic polypeptide |
|||
* Human chorionic gonadotropin-alpha |
|||
* Human chorionic gonadotropin-beta |
|||
According to Warner, markers include |
|||
* serotonin |
|||
* neurotensin |
|||
* motilin |
|||
* substance P |
|||
* histamine |
|||
* catecholamines |
|||
* dopa |
|||
* various rarer peptide hormones |
|||
According to Öberg, at http://pubmed.org PMID 15933475, new markers include |
|||
* N-terminally truncated variant of heat shock protein 70 (Hsp70) |
|||
* CDX-2, a homeobox gene product |
|||
* neuroendocrine secretory protein-55 |
|||
http://www.medscape.com/viewarticle/420088?src=search: |
|||
* protein gene product 9.5 |
|||
* synaptophysin, synapsin, and synaptotagmin |
|||
* chromogranin A and HISL-19 |
|||
Aside from their use in diagnosis, some markers can track the progress of therapy while the patient avoids the detrimental side-effects of CT-scan contrast. |
Aside from their use in diagnosis, some markers can track the progress of therapy while the patient avoids the detrimental side-effects of CT-scan contrast. |
||
Revision as of 12:06, 2 June 2011
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (May 2011) |
| Neuroendocrine tumor | |
|---|---|
| Specialty | Endocrine oncology |
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms that arise from the endocrine and nervous systems, characterized by the presence of secretory granules as well as the ability to produce biogenic amines and polypeptide hormones.[1] Many neuroendocrine tumors are usually benign, but some are malignant. NETs include gastroenteropancreatic neuroendocrine tumors (GEP-NETs) of the pancreatic islet cells and neuroendocrine gastroenteric tissue,[1] certain thymus and lung tumors, as well as medullary carcinoma of the parafollicular cells of the thyroid.[1] Tumors with similar cellular characteristics that arise in the pituitary, parathyroid, and adrenomedullary gland are sometimes included[2] or excluded[1] within the general category of neuroendocrine tumors.
NETs can arise within specific glands, such as the thyroid (and the pituitary gland, pancreas, and adrenal gland if included in NETs) of the neuroendocrine system, or from neuroendocrine Kulchitsky cells that are diffusely distributed throughout the body, particularly the gastrointestinal and pulmonary systems.[3]
Background
Neuroendocrine system
NETs are believed to be neoplasms of various different cells whose normal function is to serve at the neuroendocrine interface. Neuroendocrine cells are present not only in endocrine glands throughout the body that produce hormones, but also diffusely in all body tissues.
Some neuroendocrine tumor cells possess especially strong hormone receptors, such as somatostatin receptors (SSTRs) and uptake hormones strongly. This avidity for somatostatin can assist in diagnosis and may make some tumors vulnerable to hormone targeted therapies.
History
Small intestinal neuroendocrine tumors were first distinguished from other tumors in 1907.[4][5] They were named carcinoid tumors because their slow growth was considered to be "cancer-like" rather than truly cancerous.[5] However, in 1929 it was recognized that some of these small bowel tumors could actually be malignant,[4][5] a category that would now be called neuroendocrine carcinoma (NEC). Despite the differences between these two categories, and further complexities due to the subsequent inclusion of NETs of pancreas and pulmonary origin into the overall broad category of neuroendocrine tumors, all NETs are sometimes (incorrectly) subsumed into the term "carcinoid."
Enterochromaffin cells, which give rise to carcinoid tumors, had been identified in 1897 by Kulchitsky[4] but their secretion of serotonin was not established until 1953[4] when the “flushing” effect of serotonin had become clinically recognized. Carcinoid heart disease was identified in 1952[4] and carcinoid fibrosis in 1961.[4]
Neuroendocrine tumors were sometimes called APUDomas, referring to these cells often showing amine precursor (DOPA and 5-hydroxytryptophan) uptake and decarboxylation to produce biogenic amines such as catecholamines and serotonin. Although the concept that these cells embryologically arise from the neural crest has proven incorrect[6][7] it has continuing merit in referring to the capacity of neuroendocrine cells to produce an extensive array of hormones and amines.[7]
There have been multiple nomenclature systems for these tumors,[8] and the differences between these schema have often been confusing.[8] Nonetheless, all these sytems distinguish between well-differentiated (low and intermediate-grade) and poorly differentiated (high-grade) NETs.[8] Cellular proliferative rate is of considerable significance in this prognostic assessment.[8]
Incidence
Although estimates vary, the annual incidence of clinically significant neuroendocrine tumors is approximately 2.5-5 per 100,000;[9] two thirds are carcinoid tumors and one third are other NETs.
The prevalence has been estimated as 35 per 100,000,[9] and may be considerably higher if clinically silent tumors are included. An autopsy study of the pancreas in people who died from unrelated causes discovered a remarkably high incidence of tiny asymptomatic NETs. Routine microscopic study of three random sections of the pancreas found NETs in 1.6%, and multiple sections identified NETs in 10%.[10] As diagnostic imaging increases in sensitivity, such as endoscopic ultrasonography, very small, clinically insignificant NETs may be coincidental discovered; being unrelated to symptoms, such neoplasms may not require surgical excision.
Categories
The broad category of neuroendocrine tumors, encompassing many tumor types,[11] is presented here to facilitate retrieving information. It is quite clear that neuroendocrine tumors are uncommon in many of these areas, and frequently represent only a very small proportion of the tumors or cancers at these locations.
- Pituitary
- neuroendocrine tumor of the anterior pituitary
- Thyroid tumors, particulary medullary carcinoma
- Parathyroid tumors
- Thymus and mediastinal carcinoid tumors[12][13]
- Pulmonary neuroendocrine tumors[14][15]
- bronchus[13]
- pulmonary carcinoid tumors: typical carcinoid (TC; low-grade); atypical carcinoid (AC; intermediate-grade)
- small-cell lung cancer (SCLC)
- large-cell neuroendocrine carcinoma (LCNEC of the lung)[16]
- Extrapulmonary small cell carcinomas (ESCC or EPSCC)
- Gastroenteropancreatic neuroendocrine tumors (GEP-NET)
- Foregut GEP-NET (foregut tumors can conceptually encompasses not only NETs of the stomach and proximal duodenum, but also the pancreas, and even thymus, lung and bronchus)
{{citation}}: Empty citation (help) - Midgut GEP-NET (from distal half of 2nd part of the duodenum to the proximal two-thirds of the transverse colon)
- Hindgut GEP-NET[17][18]
- Pancreatic endocrine tumors (if considered separately from foregut GEP-NET)[19]
- Foregut GEP-NET (foregut tumors can conceptually encompasses not only NETs of the stomach and proximal duodenum, but also the pancreas, and even thymus, lung and bronchus)
- Liver[20][21][22] and gallbladder[23]
- Adrenal tumors, particularly adrenomedullary tumors
- Pheochromocytoma
- Peripheral nervous system tumors, such as:
- Breast[24]
- Genitourinary tract
- Merkel cell tumors of skin (trabecular cancer)
- Several inherited conditions:
- multiple endocrine neoplasia type 1 (MEN1)
- multiple endocrine neoplasia type 2 (MEN2)
- neurofibromatosis type 1[28]
- tuberous sclerosis
- von Hippel-Lindau (VHL) disease
- Carney complex
Histopathology
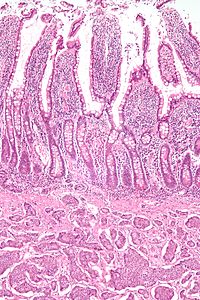
Features in common
Neuroendocrine tumors, despite differing embryological origin, have common phenotypic characteristics.
NETs show tissue immunoreactivity for markers of neuroendocrine differentiation (pan-neuroendocrine tissue markers) and may secrete various peptides and hormones. There is a lengthy list of potential markers in neuroendocrine tumors; several reviews provide assistance in understanding these markers.[29][30] Widely used neuroendocrine tissue markers are various chromogranins, synaptophysin and PGP9.5. Neuron-specific enolase (NSE) is less specific. {{citation}}: Empty citation (help) 2 citations
NETs are often small, yellow or tan masses, often located in the submucosa or more deeply intramurally, and they can be very firm due to an accompanying intense desmoplastic reaction. The overlying mucosa may be either intact or ulcerated. Some GEP-NETs invade deeply to involve the mesentery.
Histologically, NETs are an example of "small blue cell tumors," showing uniform cells which have a round to oval stippled nucleus and scant, pink granular cytoplasm. The cells may align variously in islands, glands or sheets. High power examination shows bland cytopathology. Electron microscopy can identify secretory granules. There is usually minimal pleomorphism but less commonly there can be anaplasia, mitotic activity, and necrosis.
Argentaffin and hormone secretion
NETs from a particular anatomical origin often show similar behavior as a group, such as the foregut (which conceptually includes pancreas, and even thymus, airway and lung NETs), midgut and hindgut; individual tumors within these sites can differ from these group benchmarks:
- Foregut NETs are argentaffin negative. Despite low serotonin content, they often secrete 5-hydroxytryptophan (5-HTP), histamine, and several polypeptide hormones. There may be associated atypical carcinoid syndrome, acromegaly, Cushing disease, other endocrine disorders, telangiectasia, or hypertrophy of the skin in the face and upper neck.[31] These tumors can metastasize to bone.
- Midgut NETs are argentaffin positive, can produce high levels of serotonin 5-hydroxytryptamine (5-HT), kinins, prostaglandins, substance P (SP), and other vasoactive peptides, and sometimes produce corticotropic hormone (previously adrenocorticotropic hormone [ACTH]). Bone metastasis is uncommon.
- Hindgut NETs are argentaffin negative and rarely secrete 5-HT, 5-HTP, or any other vasoactive peptides. Bone metastases are not uncommon.
GEP-NET
The vast majority of GEP-NETs fall into two nearly distinct categories: "carcinoids", and pancreatic endocrine tumors (PETs). Despite great behavioral differences between the two, they are grouped together as GEP-NETs because of similarities in cell structure.[32][33]
The histological and prognostic diversity of GEP-NETs, as well as evolving terminology over time, has given rise to some confusion. In general, GEP-NET secretion of hormones tends to correlate closely but inversely to the aggressiveness of the tumor, as reflected by both its potential for malignancy and the degree of histological differentiation such as anaplasia (dissimilarity between tumor cells and normal cells); however, there are many exceptions, which has further contributed to the confusion in terminology. For example, the term atypical carcinoid is sometimes used to indicate an aggressive tumor without secretions, whether anaplastic or well-differentiated.
Although malignant and metastatic are interchangeable terms in many settings, this has been identified as the underlyng source of considerable controversy in GEP-NETs.Template:Add 3 citations Although the majority of GEP-NETs are usually designated as benign they may have substantial intrinsic malignant potential. The primary site often eludes detection for years, sometimes decades, during which time the tumor has the opportunity to metastasize. Researchers differ widely in their estimates of malignancy rates, especially in regards to the secretory subtypes (the various "-omas"). The most common metastatic sites are the liver, the lymph nodes, and the bones. For those tumors which metastasize, liver lesions are frequent, and can massively enlarge, and can dominate the symptomatic course of the cancer. For a patient with a nonsecretory PET, for example, the primary threat to life may be the sheer bulk of the tumor in the liver.
In 2000, the World Health Organization (WHO) revised the classification of GEP-NETs: carcinoid was updated to neuroendocrine tumor (NET), and islet cell tumor or pancreatic endocrine tumor was renamed neuroendocrine carcinoma (NEC). The medical community has adopted the new terminology slowly. Of note, the WHO scheme labels the least aggressive subclass of neuroendocrine neoplasm as neuroendocrine tumor, a name that had wide previous use to indicate either the entire superclass, or the aggressive noncarcinoid subclass. Four schema[34][35][36] are compared in the table. This table is a faithful transposition of material from an earlier version of this article but it may NOT correspond to the actual citations which have not yet been rechecked carefully. In any case, the material is substantially incomplete and to some extent outdated by further schema: use with caution (the general ideas are OK in outline) or consult an up to date source.[8]
| Category | description | Öberg | Klöppel[35] | WHO | here |
|---|---|---|---|---|---|
| Superclass | the broad category of all of these tumors | gastroenteropancreatic neuroendocrine tumor (GEP-NET) | gastroenteropancreatic neuroendocrine tumor (GEP-NET) | gastroenteropancreatic neuroendocrine tumor (GEP-NET) | gastroenteropancreatic neuroendocrine tumor (GEP-NET) |
| Subclass 1 | less malignant | carcinoid | well-differentiated neuroendocrine tumor (NET) (carcinoid) | neuroendocrine tumor (NET) | carcinoid |
| Subclass 2 | more malignant | endocrine pancreatic tumor | well-differentiated neuroendocrine carcinoma (NEC) (malignant carcinoid) | neuroendocrine carcinoma (NEC) | pancreatic endocrine tumor (PET) or endocrine pancreatic tumor (EPT) or islet cell tumor; also, noncarcinoid GEP-NET (not of pancreas) |
| Subclass 3 | most malignant | poorly-differentiated neuroendocrine carcinoma (high-grade malignant carcinoid) | poorly-differentiated neuroendocrine carcinoma | ||
| Subclass 4 | mixed | mixed endocrine/exocrine tumor | |||
| Subclass 5 | miscellaneous | rare neuroendocrine-like lesions |
There are numerous types within these categories:
Carcinoid
(about two thirds of GEP-NETs)
- with carcinoid syndrome (about 10 percent of carcinoids)
- without carcinoid syndrome (about 90 percent of carcinoids)
PETs
(about one third of GEP-NETs)
- nonfunctioning (15 to 30 percent of PETs)
- functioning (70 to 85 percent of PETs)
- gastrinoma, producing excessive gastrin and causing Zollinger-Ellison Syndrome (ZES)
- insulinoma
- glucagonoma
- VIPoma, producing excessive vasoactive intestinal peptide, which may cause profound chronic watery diarrhea and resultant dehydration, hypokalemia, and achlorhydria (WDHA or pancreatic cholera syndrome)
- PPoma (often classed with nonfunctioning PETs)
- somatostatinoma
- CRHoma
- calcitoninoma
- GHRHoma
- neurotensinoma
- ACTHoma
- GRFoma
- parathyroid hormone–related peptide tumor
Other NETs
Thymus and pulmonary in particular.[discuss]
Symptoms
Carcinoid tumors
The mildest of the carcinoids are discovered only upon surgery for unrelated causes. These coincidental carcinoids are common; one study found that one person in ten has them.
According to Arnold et alia, "many tumors are asymptomatic even in the presence of metastases" (Arnold et al. 2004, 197).
Even very small tumors are not necessarily harmless, if they produce adverse hormones.[38]
Approximately xpercent of carcinoids are symptomatic; Carcinoid tumors tend to grow much more slowly than PETs. A carcinoid tumor may produce serotonin (5-HT), a biogenic amine that causes a specific set of symptoms,T or substance P,[39] or many others, and give rise to:
- flushing
- diarrhea or increase in number of bowel movements
- congestive heart failure (CHF)
- asthma
- weight loss
- weight gain
- heart palpitations
- acromegaly
- Cushing's syndrome
This set of symptoms is called the carcinoid syndrome. Although this serotonin secretion is entirely different from a secretory PET's hormone secretion, carcinoid tumors with carcinoid syndrome are nevertheless sometimes called functioning, adding to the frequent confusion of carcinoids with PETs. Carcinoid syndrome is primarily associated with midgut carcinoids. A severe episode of carcinoid syndrome is called carcinoid crisis; it can be triggered by factors such as diet, alcohol, surgery or chemotherapy.[39]
Serotonin causes thickening of the heart valves, particularly the tricuspid and the pulmonic valves and this can lead to the third feature of the carcinoid syndrome, CHF, congestive heart failure.[39]
The intense desmoplastic reaction at the tumor site, or of the mesentery as a result of the fibrosing effects of NET secretory products[38] may cause kinking of the bowel and obstruction.[38]
Pancreatic endocrine tumors (PETs)
Pancreatic neuroendocrine tumors (PETs or PNETs) are believed to originate generally in the islets of Langerhans within the pancreas or from similar neuroendocrine cells outside of the pancreas. Islet cell tumors refer to well or intermediately differentiated PNETs, while neuroendocrine cancer (NEC) is more aggressive.
PNETs are quite distinct from the usual form of pancreatic cancer, adenocarcinoma, which arises in the exocrine pancreas. About 95 percent of pancreatic tumors are adenocarcinoma; only 1 or 2% of clinically significant pancreas neoplasms are GEP-NETs.
PNETs may secrete hormones that can cause symptoms. Nonsecretory (nonfunctional) PETs do not secrete hormones. Secretory tumors are classified by the hormone most strongly secreted; for example, insulinoma, which produces excessive insulin, and gastrinoma, which produces excessive gastrin.
Other
In addition to the two main categories, there are rarer forms of GEP-NETs, including neuroendocrine lung tumors.
Animals also suffer from NETs, including neuroendocrine cancer of the liver in dogs, and Devil facial tumor disease in Tasmanian Devils. [40][41][42]
Diagnosis
CT-scans, MRIs, sonography (ultrasound), and endoscopy (including endoscopic ultrasound) are common diagnostic tools. Symptoms from hormone secretions or from carcinoid syndrome, or measurement of the corresponding hormones in the blood, can aid in diagnosis. CT-scans using contrast medium can detect 95 percent of tumors over 3 cm in size, but generally not tumors under 1 cm.
Since the half-life of somatostatin in circulation is under three minutes makes it useless for diagnosis and targeted therapies, synthetic forms of somatostatin, with longer half-lives, are used instead; the earliest was octreotide, first marketed, by Sandoz as Sandostatin, in 1988. The synthetic forms are often called somatostatin analogs, although the US Food and Drug Administration (FDA) uses the term somatostatin congeners. OctreoScan, also called somatostatin receptor scintigraphy(SRS or SSRS), utilizes a somatostatin analog: a patient is injected with octreotide chemically bound to a radioactive substance, often indium-111; for those patients whose tumor cells are avid for octreotide, a radiation-sensitive scan can then indicate the locations of the larger lesions.
A gallium-68 receptor PET-CT, integrating a PET image with a CT image, is much more senstitive than an OctreoScan.[citation needed]
Standard PET scans using fluorodeoxyglucose (FDG) are not useful in diagnosis of GEP-NETs[43] but C-5-hydroxy-L-tryptophan PET has very high sensitivity.[44]
Markers
The circulating blood levels or their associated urinary products can conceptually be followed, either in initial diagnosis or to reassess the interval change in the tumor. Secretory activity of the tumor cells is not always a reflection of the tissue immunoreactivity to particular hormones.[30]
Aside from the hormones of secretory tumors, the most important markers are :
- chromogranin A (CgA)
- urine 5-hydroxyindoleacetic acid (5-HIAA)
- neuron-specific enolase (NSE, gamma-gamma dimer)
- synaptophysin (P38)
Several new findings in NET have been reviewed.[45][discuss]
Other potential markers include:
- synaptobrevin (VAMP-1)
- synapsin (1A, 1B, 2A, 2B)
- SV2
- protein P65
- protein S-100
- protein gene product (PGP) 9.5
- intermediate filaments (cytokeratins, vimentin, neurofilaments)
- protein 7B2
- chromogranin B (secretogranin I)
- chromogranin C (secretogranin II)
- pancreastatin
- vasostatin
- cytochrome b561
- leu-7 (HNK-1)
- calcitonin
- human chorionic gonadotropin-alpha (HCG-α)
- human chorionic gonadotropin-beta (HCG-β)
- thyroid function tests (TFTs)
- parathyroid hormone (PTH)
- calcium
- prolactin
- alpha-fetoprotein
- carcinoembryonic antigen (CEA)
- β-human chorionic gonadotrophin (β-HCG) (grade D)
- CGRP
- GRP
- PYY
- hCGα
- N Peptide K
- neurokinin A
- serotonin
- neurotensin
- motilin
- substance P
- histamine
- catecholamines
- dopa
- various rarer peptide hormones
- synaptotagmin
- HISL-19
- N-terminally truncated variant of heat shock protein 70 (Hsp70)
- CDX-2, a homeobox gene product
- neuroendocrine secretory protein-55
- achaete-scute homolog 1 (ASCL1)
According to "CIS Chromagranin [sic] Monography: Chromogranin: A circulating neuroendocrine marker: biology, pathology, assay technology and clinical applications" by Luca C. Giovanella, at http://www.eoc.ch/allegati/mednuc_cromogranina%20A.pdf, markers include
- Cytoplasmic markers
- Neuron-specific enolase (NSE, gamma-gamma dimer)
- Synaptophysin (P38)
- Synaptobrevin (VAMP-1)
- Synapsin (1A, 1B, 2A, 2B)
- SV2
- Protein P65
- Protein S-100
- Protein gene product (PGP 9.5)
- Intermediate filaments (cytokeratins, vimentin, neurofilaments)
- Protein 7B2
- Granular markers
- Chromogranin A (protein secretory I)
- Chromogranin B (secretogranin I)
- Chromogranin C (secretogranin II)
- Pancreastatin
- Vasostatin
- Cytochrome b561
- Leu-7 (HNK-1)
According to "Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours" by Ramage JK et al., markers include
- chromogranin A (CgA)
- serum pancreatic polypeptide
- serum calcitonin
- and serum HCG ({alpha} and ß)
- 5-hydroxy indole acetic acid (5-HIAA) (grade C)
- thyroid function tests (TFTs)
- parathyroid hormone (PTH)
- calcium
- calcitonin
- prolactin
- {alpha}-fetoprotein
- carcinoembryonic antigen (CEA)
- ß-human chorionic gonadotrophin (ß-HCG) (grade D)
According to http://www.med.umich.edu/lrc/presentation/endo/islet.htm, markers include
- Pancreatic polypeptide
- Human chorionic gonadotropin-alpha
- Human chorionic gonadotropin-beta
According to Warner, markers include
- serotonin
- neurotensin
- motilin
- substance P
- histamine
- catecholamines
- dopa
- various rarer peptide hormones
According to Öberg, at http://pubmed.org PMID 15933475, new markers include
- N-terminally truncated variant of heat shock protein 70 (Hsp70)
- CDX-2, a homeobox gene product
- neuroendocrine secretory protein-55
http://www.medscape.com/viewarticle/420088?src=search:
- protein gene product 9.5
- synaptophysin, synapsin, and synaptotagmin
- chromogranin A and HISL-19
Aside from their use in diagnosis, some markers can track the progress of therapy while the patient avoids the detrimental side-effects of CT-scan contrast.
Genetics
Pancreatic neuroendocrine tumors
DNA mutation analysis in well-differentiated pancreatic neuroendocrine tumors identified four important findings:[46][47]
- as expected, the genes mutated in NETs, MEN1, ATRX, DAXX, TSC2, PTEN and PIK3CA, are different from the mutated genes previously found in pancreatic adenocarcinoma: KRAS, TP53, SMAD4 and p16.[48]
- one in six well-differentiated pancreatic NETs have mutations in mTOR pathway genes, such as TSC2, PTEN and PIK3CA. A number of medications target the mTOR pathway; such rapalogues include everolimus. The sequencing discovery might allow selection of which NETs would benefit from an mTOR inhibitor, rather than treating all NETs, but this awaits validation in a clinical trial.
- a new cancer pathway was found: two completely new mutations were identified, in the ATRX and DAXX genes.
- ATRX/DAXX mutations and mutations in MEN1 were associated with significantly better prognosis.
Treatment
Overview
Several issues help define appropriate treatment of a neuroendocrine tumor, including its location, invasiveness, hormone secretion, and metastasis. Treatments may be aimed at curing the disease or at relieving symptoms (palliation).
Observation may be feasible for non-functioning low grade neuroendocrine tumors.[citation needed]
If the tumor is locally advanced or has metastasized, but is nonetheless slowly growing, treatment that relieves symptoms may often be preferred over immediate challenging surgeries.[citation needed]
Intermediate and high grade tumors (noncarcinoids) are usually best treated by various early interventions (active therapy) rather than observation (wait-and-see approach).[49]
Treatments for these tumors have improved over the past several decades, and a sense of these improved outcomes can be found in considering malignant carcinoid tumors and the carcinoid syndrome, in which the median survival has improved from two years to more than eight years for the same category. [7] Also quote Pommier.
Detailed guidelines for managing neuroendocrine tumors are available from ESMO,[50] NCCN[51] and a UK panel.[1] The NCI has guidelines for several categories of NET: islet cell tumors of the pancreas,[52] gastrointestinal carcinoids,[53] Merkel cell tumors[54] and pheochromocytoma and paraganglioma.[55]
Surgery
Surgery is a curative treatment for some neuroendocrine tumors.
Even if the tumor has advanced and metastasized, making curative surgery infeasible, surgery often has a role in neuroendocrine cancers for relief of symptoms (palliation) and possibly improved survival.[49]
Symptomatic relief
Specific counter-hormones or other hormone-blocking medications are sometimes used to provide symptomatic relief.
Somatostatin-analog-based targeted therapies can provide symptomatic relief in secretory tumors. The somatostatin, given subcutaneously or intramuscularly "clogs up" the receptors, blocking the secretion of hormones from the tumor cells.
Somatostatin analogs have been used for about two decades to alleviate symptoms by blocking the production of hormones from secretory tumors. There has been some controversy about whether they stabilize or shrink tumors, including nonsecretory tumors. However, the recent PROMID study (Placebo-controlled prospective randomized study on the antiproliferative efficacy of Octreotide LAR in patients with metastatic neuroendocrine MIDgut tumors) showed that Sandostatin® LAR® in metastatic midgut neuroendocrine tumors resulted in increased tumor stabilization compared to placebo of 14.3 months compared to six months.[56]
A consensus review has reported on the use of somatostatin analogs for GEP-NETs.[57]
Other medications sometimes relieve symptoms.[citation needed]
Chemotherapy
The most common nonsurgical therapy for all GEP-NETs is chemotherapy.[citation needed]
Chemotherapy is reported to be largely ineffective for carcinoids, not particularly durable (long-lasting) for PETs, and inappropriate for PETs of nonpancreatic origin.[1] [58][note 1]
Unresectable islet-cell carcinomas may have a longer response to doxorubicin combinations than 5-FU combination chemotherapy.[citation needed]
When chemotherapy fails, the most common therapy in the United States is more chemotherapy, with a different set of agents.[citation needed] Some studies have shown that the benefit from one agent is not highly predictive of the benefit from another agent, except that the long-term benefit of any agent is likely to be low.
Strong uptake of somatostatin analogs is a negative indication for chemotherapy.[1]
Targeted chemotherapy agents have recently been approved by the FDA based on improved progression-free survival (PFS).
- Everolimus (Afinitor) is labeled for treatment of progressive neuroendocrine tumors of pancreatic origin in patients with unresectable, locally advanced or metastatic disease.[59][60] The safety and effectiveness of everolimus in carcinoid tumors have not been established.[59][60]
- Sunitinib (Sutent) is labeled for treatment of progressive, well-differentiated pancreatic neuroendocrine tumors (pNET) in patients with unresectable locally advanced or metastatic disease.[61][62] Sutent also has approval from the European Commission for the treatment of 'unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumors with disease progression in adults'.[63]
Hormone-delivered radiotherapy
In this type of radioisotope therapy (RIT) for GEP-NETs,[2] a particular peptide or neuroamine hormone that has previously shown good uptake of a tracer dose by the particular tumor within the individual is treated intravenously with the peptide or hormone conjugated to a radionuclide or radioligand. This type of RIT is sometimes called peptide receptor radionuclide therapy (PRRT), or hormone-delivered radiotherapy, and can attack all lesions in the body, not just liver metastases.
The radioactively labelled hormones enter the tumor cells, and these and nearby cells are damaged by the attached radiation. Not all cells are immediately killed this way as cell death can go on for up to two years. With strongly overexpressing tumor cells nearly all the radiation either gets into the tumors or is excreted in urine.
Welsh[64]
Hepatic artery
Metastases to the liver can be treated by several types of hepatic artery treatments based on the observation that tumor cells get nearly all their nutrients from the hepatic artery, while the normal cells of the liver get about 70-80 percent of their nutrients and 50% their oxygen supply from the portal vein, and thus can survive with the hepatic artery effectively blocked. [38][65]
Hepatic artery embolization (HAE) occludes the blood flow to the tumors,[39] achieving significant tumor shrinkage in over 80%.[39]
In hepatic artery chemotherapy, the chemotherapy agents are given into the hepatic artery, often by steady infusion over hours or even days. Compared with systemic chemotherapy, a higher proportion of the chemotherapy agents are (in theory) delivered to the lesions in the liver. [65]
Hepatic artery chemoembolization (HACE), sometimes called transarterial chemoembolization (TACE), combines hepatic artery embolization with hepatic artery chemoinfusion: embospheres bound with chemotherapy agents, injected into the hepatic artery, lodge in downstream capillaries. The spheres not only block blood flow to the lesions, but by halting the chemotherapy agents in the neighborhood of the lesions, they provide a much better targeting leverage than chemoinfusion provides.
The use of radioactive microsphere therapy (RMT), also called selective internal radiation therapy (SIRT), for hepatic metastases in neuroendocrine tumors has been reviewed.[66] This technique combines hepatic artery embolization with radiation therapy delivered by way of microspheres bound with radionuclides, injected into the hepatic artery, that lodge (as with HAE and HACE) in downstream capillaries. In contrast to hormone-delivered radiotherapy, the lesions need not overexpress peptide receptors. Due to the mechanical targeting, the yttrium-labeled microspheres "are selectively taken up by the tumors, thus preserving normal liver" [67] This type of treatment is FDA approved for liver metastases secondary to colorectal carcinoma and is under investigation for treatment of other liver malignancies, including neuroendocrine malignancies.[68]
Other therapies
Radiofrequency ablation (RFA) is used when a patient has relatively few metastases. In RFA, a needle is inserted into the center of the lesion and current is applied to generate heat; the tumor cells are killed by cooking.
Cryoablation is similar to RFA; an endothermic substance is injected into the tumors to kill by freezing. Cryoablation has been considerably less successful for GEP-NETs than RFA.[citation needed]
Interferon is sometimes used to treat GEP-NETs.[69] The dose is titrated within each person, often considering the effect on the blood leukocyte count.[69] interferon is often used at low doses and in combination with other agents (especially somatostatin analogs such as octreotide); some researchers claim that interferon provides little value aside from symptom control.[citation needed]
People with neuoendocrine cancer
Steve Jobs, Apple Inc.[70]
Paul Hunter, Professional World Snooker player
External links
- http://www.netipedia.org
- http://www.carcinoid.org
- http://unicornfoundation.org.au
- Neuroendocrine tumor at Curlie
- The Caring for Carcinoid Foundation
- Cancer.Net: Neuroendocrine Tumor
Template:Gonadal tumors, paraganglioma, glomus, nevi and melanomas
Notes and references
Notes
- ^ "response to chemotherapy in patients with strongly positive carcinoid tumours was of the order of only 10% whereas patients with SSRS negative tumours had a response rate in excess of 70%. The highest response rates with chemotherapy are seen in the poorly differentiated and anaplastic NETs: response rates of 70% or more have been seen with cisplatin and etoposide based combinations. These responses may be relatively short lasting in the order of only 8–10 months. Response rates for pancreatic islet cell tumours vary between 40% and 70% and usually involve combinations of streptozotocin (or lomustine), dacarbazine, 5-fluorouracil, and adriamycin. However, the best results have been seen from the Mayo clinic where up to 70% response rates with remissions lasting several years have been seen by combining chemoembolisation of the hepatic artery with chemotherapy. The use of chemotherapy for midgut carcinoids has a much lower response rate, with 15–30% of patients deriving benefit, which may only last 6–8 months[1]
References
This article has an unclear citation style. (September 2010) |
- ^ a b c d e f g h Ramage JK, Davies AH, Ardill J; et al. (2005). "Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours". Gut. 54. Suppl 4 (suppl_4): iv1–16. doi:10.1136/gut.2004.053314. PMC 1867801. PMID 15888809.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Rufini V, Calcagni ML, Baum RP (2006). "Imaging of neuroendocrine tumors". Semin Nucl Med. 36 (3): 228–47. doi:10.1053/j.semnuclmed.2006.03.007. PMID 16762613.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Liu Y, Sturgis CD, Grzybicki DM; et al. (2001). "Microtubule-associated protein-2: a new sensitive and specific marker for pulmonary carcinoid tumor and small cell carcinoma". Mod Pathol. 14 (9): 880–5. doi:10.1038/modpathol.3880406. PMID 11557784.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.humpath.2004.09.018, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.humpath.2004.09.018instead. - ^ a b c Arnold R, Göke R, Wied M, Behr T (2003). "Chapter 15 Neuroendocrine Gastro-Entero-Pancreatic (GEP) Tumors". In Scheppach W, Bresalier RS, Tytgat GNJ (ed.). Gastrointestinal and Liver Tumors. Berlin: Springer. pp. 195–233. ISBN 3-540-43462-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "The APUD concept led to " (Warner 2005, 2).
- ^ a b c Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10388123, please use {{cite journal}} with
|pmid=10388123instead. - ^ a b c d e Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1097/MPA.0b013e3181ec124e, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1097/MPA.0b013e3181ec124einstead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/s10555-011-9292-1, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/s10555-011-9292-1instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/BF01297144, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/BF01297144instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15053292, please use {{cite journal}} with
|pmid=15053292instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10550713, please use {{cite journal}} with
|pmid=10550713instead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/annonc/mdn116, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/annonc/mdn116instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15898407, please use {{cite journal}} with
|pmid=15898407instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/cncr.23542, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/cncr.23542instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 1317668, please use {{cite journal}} with
|pmid=1317668instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20380002, please use {{cite journal}} with
|pmid=20380002instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21160865, please use {{cite journal}} with
|pmid=21160865instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1053/j.gastro.2008.05.047, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1053/j.gastro.2008.05.047instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12636090, please use {{cite journal}} with
|pmid=12636090instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1080/13651820310017228, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1080/13651820310017228instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8348490, please use {{cite journal}} with
|pmid=8348490instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12725316, please use {{cite journal}} with
|pmid=12725316instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11890336, please use {{cite journal}} with
|pmid=11890336instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17076534, please use {{cite journal}} with
|pmid=17076534instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7511278, please use {{cite journal}} with
|pmid=7511278instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11718210, please use {{cite journal}} with
|pmid=11718210instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/bja/86.4.555, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/bja/86.4.555instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.2741/s68, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.2741/s68instead. - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18401212, please use {{cite journal}} with
|pmid=18401212instead. - ^ Tebbi CK. Carcinoid Tumor. http://emedicine.medscape.com/article/986050-overview
- ^ Öberg 2005a, 90 The main two groups of neuroendocrine GEP tumours are so-called carcinoid tumours and endocrine pancreatic tumours"
- ^ Warner 2005, 9 "Less than 1% of carcinoids arise in the pancreas"
- ^ Öberg
- ^ a b Klöppel G, Perren A, Heitz PU (2004). "The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification". Ann N Y Acad Sci. 1014 (1): 13–27. doi:10.1196/annals.1294.002. PMID 15153416.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ WHO
- ^ Kimura W, Kuroda A, Morioka Y (1991). "Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases". Dig Dis Sci. 36 (7): 933–42. doi:10.1007/BF01297144. PMID 2070707.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)"[In] 800 autopsy cases, ... incidence of tumor was 10% (6/60) in individuals having histological studies of all sections of the pancreas" - ^ a b c d Pommier R. 2003. The role of surgery and chemoembolization in the management of carcinoid. California Carcinoid Fighters Conference. October 25, http://www.carcinoid.org/content/role-surgery-and-chemoembolization-management-carcinoid
- ^ a b c d e Kvols LK. 2002. Carcinoid Tumors and the Carcinoid Syndrome: What's New in the Therapeutic Pipeline. (The Carcinoid Cancer Foundation: Carcinoid Symposium 2002) http://www.carcinoid.org/content/carcinoid-tumors-and-carcinoid-syndrome-whats-new-therapeutic-pipeline
- ^ Bostanci A. Wildlife biology. A devil of a disease. Science 2005; 307:1035 pmid=15718445 doi=10.1126/science.307.5712.1035 "The tumors [of Devil facial tumor disease] have been characterized as a neuroendocrine cancer"
- ^ Kinver, Mark (1 January 2010). "Tasmanian devil facial cancer origins 'identified'". BBC.
- ^ Walsh, Bryan (1 January 2010). "Decoding the Tasmanian Devil's Deadly Cancer". Time.
- ^ As Warner says, "Most GEP NETs do not image with fluorodeoxyglucose PET" (Warner 2005, 7).
- ^ According to Öberg, new PET "tracers such as C-5-hydroxy-L-tryptophan show very high sensitivity for detection of tumors, higher than for somatostatin receptor scintigraphy" (Öberg 2005b, [1])
- ^ Oberg K (2005). "Neuroendocrine tumors of the gastrointestinal tract: recent advances in molecular genetics, diagnosis, and treatment". Curr Opin Oncol. 17 (4): 386–91. doi:10.1097/01.cco.0000167739.56948.a9. PMID 15933475.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ This may need a better WP:MEDS ref but for now [2])
- ^ not the right WP:MEDRS but will work to update Jiao et al
- ^ Jones et al
- ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1053/j.gastro.2005.03.078, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1053/j.gastro.2005.03.078instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/annonc/mdq192, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/annonc/mdq192instead. - ^ http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- ^ National Cancer Institute. Islet Cell Tumors (Endocrine Pancreas) http://www.cancer.gov/cancertopics/types/isletcell
- ^ National Cancer Institute. Gastrointestinal Carcinoid Tumors Treatmenthttp://www.cancer.gov/cancertopics/pdq/treatment/gastrointestinalcarcinoid/HealthProfessional
- ^ National Cancer Institute. Merkel cell tumors http://www.cancer.gov/cancertopics/pdq/treatment/merkelcell/Patient
- ^ National Cancer Institute. Pheochromocytoma and Paragangliomahttp://www.cancer.gov/cancertopics/types/pheochromocytoma
- ^ http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=63&abstractID=10363
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/annonc/mdh216, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/annonc/mdh216instead. - ^ Ramage.
- ^ a b Everolimus Approved for Pancreatic Neuroendocrine Tumors. The ASCO Post. May 15, 2011, Volume 2, Issue 8 http://ascopost.com/articles/may-15-2011/everolimus-approved-for-pancreatic-neuroendocrine-tumors/
- ^ a b http://www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf
- ^ National Cancer Institute. Cancer Drug Information. FDA Approval for Sunitinib Malate. Pancreatic Neuroendocrine Tumors http://www.cancer.gov/cancertopics/druginfo/fda-sunitinib-malate
- ^ http://labeling.pfizer.com/ShowLabeling.aspx?id=607
- ^ "Pfizer Scores New Approval for Sutent in Europe". 2 Dec 2010.
- ^ Welsh JS, Kennedy AS, Thomadsen B (2006). "Selective Internal Radiation Therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma". Int J Radiat Oncol Biol Phys. 66 (2 Suppl): S62–73. doi:10.1016/j.ijrobp.2005.09.011. PMID 16979443.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Fong, T and Schoenfield LJ. Arterial Chemotherapy Infusion of the Liver (and) Chemoembolization of the Liver (TACE)[3]).
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19521311, please use {{cite journal}} with
|pmid=19521311instead. - ^ Salem 2002, [4])
- ^ Welsh, Kennedy, and Thomadsen 2006, [5]).
- ^ a b Öberg K. Neuroendocrine Gastroenteropancreatic Tumours: Current Views on Diagnosis and Treatment. Business Briefing. European Oncology Review 2005; pages 1-6. http://www.touchbriefings.com/pdf/1432/ACF237.pdf
- ^ Fortune
