Metformin: Difference between revisions
m Reverted edit(s) by 122.150.101.152 identified as test/vandalism using STiki |
Bluerasberry (talk | contribs) →Type 2 diabetes: wikilink... |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 71: | Line 71: | ||
===Type 2 diabetes=== |
===Type 2 diabetes=== |
||
The main use for metformin is as the [[first-line therapy]] in the treatment of [[diabetes mellitus type 2]].<ref name="BBDdiabetesM2">{{Citation |author1 = Consumer Reports Health |
|||
| ⚫ | |||
Best Buy Drugs |author1-link = Consumer Reports |date = |title = The Oral Diabetes Drugs: Treating Type 2 Diabetes |publisher = [[Consumer Reports]] |work = Best Buy Drugs |page =2 |url = http://www.consumerreports.org/health/resources/pdf/best-buy-drugs/DiabetesUpdate-FINAL-Feb09.pdf |accessdate = September 18 2012}}</ref> [[Specialty (medicine)|Specialty]] [[professional organizations]] recommend metformin because it is an older drug, its effects are well-understood, it is safer than alternatives for most people, it is highly effective in treating type 2 diabetes, and because it is the least expensive option.<ref name="BBDdiabetesM2"/> It also usually can manage the condition by itself, even though some people may also take a secondary diabetes drug.<ref name="BBDdiabetesM2"/> |
|||
| ⚫ | In overweight populations, over 10 years of treatment, metformin reduced diabetes complications and overall mortality by about 30% when compared with [[insulin]] and [[sulfonylurea]]s ([[glibenclamide]] and [[chlorpropamide]]) and by about 40% when compared with the group only given dietary advice.<ref name = UKPDS/> This difference held in people who were followed up for five to 10 years after the study.<ref name="pmid18784090">{{vcite journal |author=Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA |title=10-year follow-up of intensive glucose control in type 2 diabetes |journal=N Engl J Med |volume=359 |issue=15 |pages=1577–89 |year=2008 |month=October |pmid=18784090 |doi=10.1056/NEJMoa0806470 |url=http://content.nejm.org/cgi/content/full/359/15/1577}}</ref> Since intensive glucose control with metformin appears to decrease the risk of diabetes-related endpoints in overweight people with diabetes, and is associated with less weight gain and fewer hypoglycaemic attacks than are insulin and sulphonylureas, it may be the first-line pharmacological therapy of choice in this group. In addition, metformin had no effect on body weight: Over the 10-year treatment period, the metformin group gained about 1 kg, the same as the dietary advice group, while the sulfonylureas group gained 3 kg, and the insulin group, 6 kg.<ref name = UKPDS>{{vcite journal |title=Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group |journal=Lancet |volume=352 |issue=9131 |pages=854–65 |year=1998 |pmid=9742977|doi=10.1016/S0140-6736(98)07037-8}}</ref><ref>{{vcite journal |author=Selvin E, Bolen S, Yeh HC, ''et al.'' |author.= |title=Cardiovascular outcomes in trials of oral diabetes medications: a systematic review |journal=Arch Intern Med |volume=168 |issue=19 |pages=2070–80 |year=2008 |month=October |pmid=18955635 |doi=10.1001/archinte.168.19.2070 |url=http://archinte.ama-assn.org/cgi/content/full/168/19/2070}}</ref> As metformin affords a similar level of [[blood sugar]] control to insulin and sulfonylureas, it appears to decrease mortality primarily through decreasing heart attacks, strokes and other cardiovascular complications. |
||
Metformin has a lower risk of hypoglycemia than the sulfonylureas,<ref name=Umesh/><ref name=Bolen/> although it has uncommonly occurred during intense exercise, calorie deficit, or when used with other agents to lower blood glucose.<ref name="isbn0071416137 ">{{vcite book |author=DiPiro, Joseph T.; Talbert, Robert L.; Yee, Gary C.; Matzke, Gary R.; Wells, Barbara G.; Posey, L. Michael|title=Pharmacotherapy: a pathophysiologic approach |publisher=McGraw-Hill |location=New York |year=2005 |isbn=0-07-141613-7 }}</ref><ref>[http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18054 "Glucophage package insert"]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.</ref> Metformin is also not associated with weight gain, and modestly reduces [[low density lipoprotein|LDL]] and [[triglyceride]] levels.<ref name=Umesh/><ref name=Bolen/> |
Metformin has a lower risk of hypoglycemia than the sulfonylureas,<ref name=Umesh/><ref name=Bolen/> although it has uncommonly occurred during intense exercise, calorie deficit, or when used with other agents to lower blood glucose.<ref name="isbn0071416137 ">{{vcite book |author=DiPiro, Joseph T.; Talbert, Robert L.; Yee, Gary C.; Matzke, Gary R.; Wells, Barbara G.; Posey, L. Michael|title=Pharmacotherapy: a pathophysiologic approach |publisher=McGraw-Hill |location=New York |year=2005 |isbn=0-07-141613-7 }}</ref><ref>[http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18054 "Glucophage package insert"]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.</ref> Metformin is also not associated with weight gain, and modestly reduces [[low density lipoprotein|LDL]] and [[triglyceride]] levels.<ref name=Umesh/><ref name=Bolen/> |
||
Revision as of 13:38, 19 September 2012

| |

| |
| Names | |
|---|---|
| Other names
N,N-Dimethylimidodicarbonimidic diamide[citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.010.472 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H11N5 | |
| Molar mass | 129.167 g·mol−1 |
| log P | 1.254 |
| Pharmacology | |
| License data | |
| Oral | |
| Pharmacokinetics: | |
| 50–60% | |
| 6.2 hours | |
| Renal | |
| Legal status | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Metformin (BP, pronounced /mɛtˈfɔːrm[invalid input: 'ɨ']n/, met-FAWR-min; originally sold as Glucophage) is an oral antidiabetic drug in the biguanide class. It is the first-line drug of choice for the treatment of type 2 diabetes, in particular, in overweight and obese people and those with normal kidney function.[1][2][3] Its use in gestational diabetes has been limited by safety concerns. It is also used in the treatment of polycystic ovary syndrome, and has been investigated for other diseases where insulin resistance may be an important factor. Metformin works by suppressing glucose production by the liver.
Metformin is the only antidiabetic drug that has been conclusively shown to prevent the cardiovascular complications of diabetes. It helps reduce LDL cholesterol and triglyceride levels, and is not associated with weight gain. As of 2010[update], metformin is one of only two oral antidiabetics in the World Health Organization Model List of Essential Medicines (the other being glibenclamide).[4]
When prescribed appropriately, metformin causes few adverse effects (the most common is gastrointestinal upset) and is associated with a low risk of hypoglycemia. Lactic acidosis (a buildup of lactate in the blood) can be a serious concern in overdose and when it is prescribed to people with contraindications, but otherwise, there is no significant risk.
First synthesized and found to reduce blood sugar in the 1920s, metformin was forgotten for the next two decades as research shifted to insulin and other antidiabetic drugs. Interest in metformin was rekindled in the late 1940s after several reports that it could reduce blood sugar levels in people, and in 1957, French physician Jean Sterne published the first clinical trial of metformin as a treatment for diabetes. It was introduced to the United Kingdom in 1958, Canada in 1972, and the United States in 1995. Metformin is now believed to be the most widely prescribed antidiabetic drug in the world; in the United States alone, more than 48 million prescriptions were filled in 2010 for its generic formulations.[5][6]
Medical uses
Metformin is primarily used for type 2 diabetes, but is increasingly being used in polycystic ovary syndrome (PCOS),[7] non-alcoholic fatty liver disease (NAFLD)[8] and premature puberty,[9] three other diseases that feature insulin resistance; these indications are still[update] considered experimental. The benefit of metformin in NAFLD has not been extensively studied and may be only temporary;[10] although some randomized controlled trials have found significant improvement with its use, the evidence is still insufficient.[11][12]
Type 2 diabetes
The main use for metformin is as the first-line therapy in the treatment of diabetes mellitus type 2.[13] Specialty professional organizations recommend metformin because it is an older drug, its effects are well-understood, it is safer than alternatives for most people, it is highly effective in treating type 2 diabetes, and because it is the least expensive option.[13] It also usually can manage the condition by itself, even though some people may also take a secondary diabetes drug.[13]
In overweight populations, over 10 years of treatment, metformin reduced diabetes complications and overall mortality by about 30% when compared with insulin and sulfonylureas (glibenclamide and chlorpropamide) and by about 40% when compared with the group only given dietary advice.[14] This difference held in people who were followed up for five to 10 years after the study.[15] Since intensive glucose control with metformin appears to decrease the risk of diabetes-related endpoints in overweight people with diabetes, and is associated with less weight gain and fewer hypoglycaemic attacks than are insulin and sulphonylureas, it may be the first-line pharmacological therapy of choice in this group. In addition, metformin had no effect on body weight: Over the 10-year treatment period, the metformin group gained about 1 kg, the same as the dietary advice group, while the sulfonylureas group gained 3 kg, and the insulin group, 6 kg.[14][16] As metformin affords a similar level of blood sugar control to insulin and sulfonylureas, it appears to decrease mortality primarily through decreasing heart attacks, strokes and other cardiovascular complications.
Metformin has a lower risk of hypoglycemia than the sulfonylureas,[17][18] although it has uncommonly occurred during intense exercise, calorie deficit, or when used with other agents to lower blood glucose.[19][20] Metformin is also not associated with weight gain, and modestly reduces LDL and triglyceride levels.[17][18]
Prediabetes
Metformin treatment of people at risk for type 2 diabetes may decrease their chances of developing the disease, although intensive physical exercise and dieting work significantly better for this purpose. In a large U.S. study known as the Diabetes Prevention Program, participants were divided into groups and given either placebo, metformin, or lifestyle intervention, and followed for an average of three years. The intensive program of lifestyle modifications included a 16-lesson training on dieting and exercise followed by monthly individualized sessions with the goals to decrease the body weight by 7% and engage in a physical activity for at least 150 minutes per week. The incidence of diabetes was 58% lower in the lifestyle group and 31% lower in those given metformin. Among younger people with a higher body mass index, lifestyle modification was no more effective than metformin, and for older individuals with a lower body mass index, metformin was no better than placebo in preventing diabetes.[21] After ten years, the incidence of diabetes was 34% lower in the group of participants given diet and exercise and 18% lower in those given metformin.[22] It is unclear whether metformin slowed down the progression of prediabetes to diabetes (true preventive effect), or the decrease of diabetes in the treated population was simply due to its glucose-lowering action (treatment effect).[23]
Polycystic ovary syndrome
Antidiabetic therapy has been proposed as a treatment for polycystic ovary syndrome (PCOS), a condition frequently associated with insulin resistance, since the late 1980s.[24] The use of metformin in PCOS was first reported in 1994, in a small study conducted at the University of the Andes, Venezuela.[25][26] The United Kingdom's National Institute for Health and Clinical Excellence recommended in 2004 that women with PCOS and a body mass index above 25 be given metformin for anovulation and infertility when other therapies have failed to produce results.[27] However, two large clinical studies completed in 2006–2007 returned mostly negative results, with metformin being no better than placebo, and a metformin-clomifene combination no better than clomifene alone.[28][29] Reflecting this, subsequent reviews noted large randomized controlled trials have, in general, not shown the promise suggested by the early small studies. U.K. and international clinical practice guidelines do not recommend metformin as a first-line treatment[30] or do not recommend it at all, except for women with glucose intolerance.[31] The guidelines suggest clomiphene as the first medication option and emphasize lifestyle modification independently from the drug treatment.
In a dissenting opinion, a systematic review of four head-to-head comparative trials of metformin and clomifene found them equally effective for infertility.[32] A BMJ editorial noted four positive studies of metformin were in people not responding to clomifene, while the population in the negative studies was drug-naive or uncontrolled for the previous treatment. The editorial suggested metformin should be used as a second-line drug if clomifene treatment fails.[33] Another review recommended metformin unreservedly as a first-line treatment option because it has positive effects not only on anovulation, but also on insulin resistance, hirsutism, and obesity often associated with PCOS.[34] A large Cochrane Collaboration review of 27 randomized clinical trials found metformin improves ovulation and pregnancy rates, particularly when combined with clomifene, but is not associated with any increase in the number of live births.[35]
The design of the negative trials may be one of the explanations for the contradictory results. For example, using live birth rate instead of pregnancy as the endpoint may have biased some trials against metformin, which works slower than clomifene.[36] Another explanation may be different efficacy of metformin in different populations. The negative trials contained a large percentage of obese and previously untreated people whose response to metformin may be weaker.[36]
Gestational diabetes
Several observational studies and randomized, controlled trials have found metformin is as effective and safe as insulin for the management of gestational diabetes,[37][38][39] and a small case-control study has suggested the children of women given metformin instead of insulin may be healthier in the neonatal period.[40] Nonetheless, several concerns have been raised regarding studies published thus far, and evidence on the long-term safety of metformin for both mother and child is still lacking.[41]
Investigational findings
Prevention of weight gain
A single randomized, controlled trial suggested metformin may reduce weight gain in people taking atypical antipsychotics, in particular, when combined with lifestyle interventions (education, dieting, and exercise).[42]
Cancer prevention
A large case-control study conducted at M.D. Anderson Cancer Center has suggested metformin may protect against pancreatic cancer. The risk of pancreatic cancer in study participants having taken metformin was found to be 62% lower than in participants never having taken it, whereas participants who had used insulin or secretagogues (such as the sulfonylureas) were found to have a 5-fold and 2.5-fold higher risk of pancreatic cancer, respectively, compared to participants that had been treated with neither.[43] The study had several limitations, however, and the reason for this risk reduction is still unclear.[43] Observational studies conducted by the University of Dundee have shown a decrease of 25–37% in cancer cases in diabetics taking metformin.[44][45] Several epidemiological and case-controlled studies found diabetics using metformin may have lower cancer risk in comparison to those using other antidiabetic medications. The causes of this phenomenon are unclear, and the results require confirmation in controlled studies.[46] A direct action of metformin on cancer cells is suspected. Indeed, metformin exhibits a strong and consistent antiproliferative action on several cancer cell lines, including breast, colon, ovarian, pancreatic, lung and prostate cancer cells. These cellular studies were generally completed by preclinical studies showing a reliable antitumoral effect in various mouse models. In addition, the first clinical trials demonstrated a beneficial effect in breast and colon cancer.[47]
Contraindications
Metformin is contraindicated in people with any condition that could increase the risk of lactic acidosis, including kidney disorders (creatinine levels over 150 μmol/l (1.7 mg/dL),[48] although this is an arbitrary limit), lung disease and liver disease. According to the prescribing information, heart failure, in particular, unstable or acute congestive heart failure, increases risk of lactic acidosis with metformin.[49] A 2007 systematic review of controlled trials, however, suggested metformin is the only antidiabetic drug not associated with any measurable harm in people with heart failure, and that it may reduce mortality in comparison with other antidiabetic agents.[50]
Metformin is recommended to be temporarily discontinued before any radiographic study involving iodinated contrast agents, (such as a contrast-enhanced CT scan or angiogram), as the contrast dye may temporarily impair kidney function, indirectly leading to lactic acidosis by causing retention of metformin in the body.[51][52] Metformin can be resumed after two days, assuming kidney function is normal.[51][52]
Adverse effects
The most common adverse effect of metformin is gastrointestinal upset, including diarrhea, cramps, nausea, vomiting and increased flatulence; metformin is more commonly associated with gastrointestinal side effects than most other antidiabetic drugs.[18] The most serious potential side effect of metformin use is lactic acidosis; this complication is very rare, and the vast majority of these cases seem to be related to comorbid conditions, such as impaired liver or kidney function, rather than to the metformin itself.[53]
Metformin has also been reported to decrease the blood levels of thyroid-stimulating hormone in people with hypothyroidism,[54] and, in men, testosterone.[55][56] The clinical significance of these changes is still unknown.
Gastrointestinal
In a clinical trial of 286 subjects, 53.2% of the 141 given immediate-release metformin (as opposed to placebo) reported diarrhea, versus 11.7% for placebo, and 25.5% reported nausea/vomiting, versus 8.3% for those on placebo.[57]
Gastrointestinal upset can cause severe discomfort; it is most common when metformin is first administered, or when the dose is increased. The discomfort can often be avoided by beginning at a low dose (1 to 1.7 grams per day) and increasing the dose gradually. Gastrointestinal upset after prolonged, steady use is less common.[citation needed]
Long-term use of metformin has been associated with increased homocysteine levels[58] and malabsorption of vitamin B12.[59][60] Higher doses and prolonged use are associated with increased incidence of vitamin B12 deficiency,[61] and some researchers recommend screening or prevention strategies.[62]
Lactic acidosis
The most serious potential adverse effect of biguanide use is lactic acidosis, the incidence for which is 9 per 100,000 person-years.[63] Phenformin, another biguanide, was withdrawn from the market because of an increased risk of lactic acidosis (rate of 40-64 per 100,000 patient-years).[63] However, metformin is safer than phenformin, and the risk of developing lactic acidosis is not increased by the medication as long as it is not prescribed to known high-risk groups.[64]
Lactate uptake by the liver is diminished with metformin administration because lactate is a substrate for hepatic gluconeogenesis, a process that metformin inhibits. In healthy individuals, this slight excess is simply cleared by other mechanisms (including uptake by the kidneys, when their function is unimpaired), and no significant elevation in blood levels of lactate occurs.[17] When there is impaired renal function, however, clearance of metformin and lactate is reduced, leading to increased levels of both, and possibly causing lactic acidosis due to a buildup of lactic acid. Because metformin decreases liver uptake of lactate, any condition that may precipitate lactic acidosis is a contraindication to its use. Common causes of increased lactic acid production include alcoholism (due to depletion of NAD+ stores), heart failure, and respiratory disease (due to inadequate oxygenation of tissues); the most common cause of impaired lactic acid excretion is kidney disease.[65]
Metformin has also been suggested to increase production of lactate in the small intestine; this could potentially contribute to lactic acidosis in those with risk factors.[66] However, the clinical significance of this is unknown, and the risk of metformin-associated lactic acidosis is most commonly attributed to decreased hepatic uptake rather than increased intestinal production.[17][65][67]
Overdose
A review of intentional and accidental metformin overdoses reported to poison control centers over a five-year period found serious adverse events were rare, though the elderly appeared to be at greater risk.[68] A similar study where cases were reported to Texas poison control centers between the years 2000 and 2006 found ingested doses of more than 5,000 mg were more likely to involve serious medical outcomes in adults.[69] Survival following intentional overdoses with up to 63,000 mg (63 g) of metformin have been reported in the medical literature.[70] Fatalities following overdose are rare, but do occur.[71][72][73] In healthy children, unintentional doses of less than 1,700 mg are unlikely to cause any significant toxic effects.[74]
The most common symptoms following overdose appear to include vomiting, diarrhea, abdominal pain, tachycardia, drowsiness, and, rarely, hypoglycemia or hyperglycemia.[69][72] The major potentially life-threatening complication of metformin overdose is lactic acidosis, which is due to lactate accumulation.[75][76] Treatment of metformin overdose is generally supportive, as there is no specific antidote. Lactic acidosis is initially treated with sodium bicarbonate, although high doses are not recommended, as this may increase intracellular acidosis.[73] Acidosis that does not respond to administration of sodium bicarbonate may require further management with standard hemodialysis or continuous veno-venous hemofiltration. In addition, due to metformin’s low molecular weight and lack of plasma protein binding, these techniques also have the benefit of efficiently removing metformin from blood plasma, preventing further lactate overproduction.[77][78][79]
Metformin may be quantitated in blood, plasma, or serum to monitor therapy, confirm a diagnosis of poisoning, or assist in a medicolegal death investigation. Blood or plasma metformin concentrations are usually in a range of 1–4 mg/L in persons receiving the drug therapeutically, 40–120 mg/L in victims of acute overdosage, and 80–200 mg/L in fatalities. Chromatographic techniques are commonly employed.[80][81]
Interactions
The H2-receptor antagonist cimetidine causes an increase in the plasma concentration of metformin, by reducing clearance of metformin by the kidneys;[82] both metformin and cimetidine are cleared from the body by tubular secretion, and both, particularly the cationic (positively charged) form of cimetidine, may compete for the same transport mechanism.[83] A small double-blind, randomized study found the antibiotic cephalexin to also increase metformin concentrations by a similar mechanism;[84] theoretically, other cationic medications may produce the same effect.[83]
Mechanism of action
Metformin improves hyperglycemia primarily by suppressing glucose production by the liver (hepatic gluconeogenesis).[66] The "average" person with type 2 diabetes has three times the normal rate of gluconeogenesis; metformin treatment reduces this by over one third.[85] Metformin activates AMP-activated protein kinase (AMPK), an enzyme that plays an important role in insulin signaling, whole body energy balance, and the metabolism of glucose and fats;[86] activation of AMPK is required for metformin's inhibitory effect on the production of glucose by liver cells.[87] Research published in 2008 further elucidated metformin's mechanism of action, showing activation of AMPK is required for an increase in the expression of SHP, which in turn inhibits the expression of the hepatic gluconeogenic genes PEPCK and Glc-6-Pase.[88] Metformin is frequently used in research along with AICAR as an AMPK agonist. The mechanism by which biguanides increase the activity of AMPK remains uncertain; however, research suggests that metformin increases the amount of cytosolic AMP (as opposed to a change in total AMP or total AMP/ATP).[89]
In addition to suppressing hepatic glucose production, metformin increases insulin sensitivity, enhances peripheral glucose uptake (by phosphorylating GLUT-4 enhancer factor), increases fatty acid oxidation,[90] and decreases absorption of glucose from the gastrointestinal tract. Increased peripheral utilization of glucose may be due to improved insulin binding to insulin receptors.[91] AMPK probably also plays a role, as metformin administration increases AMPK activity in skeletal muscle.[92] AMPK is known to cause GLUT4 deployment to the plasma membrane, resulting in insulin-independent glucose uptake. Some metabolic actions of metformin do appear to occur by AMPK-independent mechanisms; a 2008 study found "the metabolic actions of metformin in the heart muscle can occur independent of changes in AMPK activity and may be mediated by p38 MAPK- and PKC-dependent mechanisms."[93]
Chemistry
The usual synthesis of metformin, originally described in 1922 and reproduced in multiple later patents and publications, involves the reaction of dimethylamine hydrochloride and 2-cyanoguanidine (dicyandiamide) with heating.[94][95]
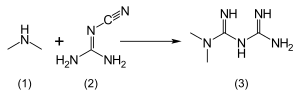
According to the procedure described in the 1975 Aron patent,[96] and the Pharmaceutical Manufacturing Encyclopedia,[97] equimolar amounts of dimethylamine and 2-cyanoguanidine are dissolved in toluene with cooling to make a concentrated solution, and an equimolar amount of hydrogen chloride is slowly added. The mixture begins to boil on its own, and after cooling, metformin hydrochloride precipitates with a 96% yield.
Structure
The structure of metformin was generally represented in a wrong tautomeric form for a number of years. This was corrected in 2005.[98] The energy difference between the correct tautomer and the generally represented tautomer is about 37 kJ/mol (9 kcal/mol). The drug is administered as metformin hydrochloride. The structure of the metformin hydrochloride was also corrected recently.[99] According to these studies, the metformin has different electronic structure compared to its protonated form. The neutral species is a simple conjugated system. Upon protonation, (i) the conjugation breaks down, (ii) the intramolecular hydrogen bond breaks down, (iii) the molecule becomes non-planar (iv) two lone pairs get accumulated at the central nitrogen (v) dynamism increases in the system via C=N rotational process and via N-inversion process. Thus, electronically, metformin hydrochloride should be treated as a simple extension of metformin. The nucleophilicity of metformin hydrochloride is moderate and that is a desired property. Metformin is shown to belong to a new class of compounds called nitreones.
Pharmacokinetics
Metformin has an oral bioavailability of 50–60% under fasting conditions, and is absorbed slowly.[83][100] Peak plasma concentrations (Cmax) are reached within one to three hours of taking immediate-release metformin and four to eight hours with extended-release formulations.[83][100] The plasma protein binding of metformin is negligible, as reflected by its very high apparent volume of distribution (300–1000 L after a single dose). Steady state is usually reached in one or two days.[83]
Metformin has acid dissociation constant values (pKa) of 2.8 and 11.5 and, therefore, exists very largely as the hydrophilic cationic species at physiological pH values. The metformin acid dissociation constant values (pKa) make metformin a stronger base than most other basic drugs with less than 0.01% unionized in blood. Furthermore, the lipid solubility of the unionized species is slight as shown by its low logP value [log(10) of the distribution coefficient of the unionized form between octanol and water] of -1.43. These chemical parameters indicate low lipophilicity and, consequently, rapid passive diffusion of metformin through cell membranes is unlikely. The logP of metformin is less than that of phenformin (-0.84) because two methyl substituents on metformin impart lesser lipophilicity than the larger phenylethyl side chain in phenformin. More lipophilic derivatives of metformin are presently being investigated with the aim of producing prodrugs with better oral absorption than metformin itself.[101]
Metformin is not metabolized. It is cleared from the body by tubular secretion and excreted unchanged in the urine; metformin is undetectable in blood plasma within 24 hours of a single oral dose.[83][102] The average elimination half-life in plasma is 6.2 hours.[83] Metformin is distributed to (and appears to accumulate in) red blood cells, with a much longer elimination half-life: 17.6 hours[83] (reported as ranging from 18.5 to 31.5 hours in a single-dose study of non-diabetic people).[102]
History
The biguanide class of antidiabetic drugs, which also includes the withdrawn agents phenformin and buformin, originates from the French lilac or goat's rue (Galega officinalis), a plant used in folk medicine for several centuries.[103]

Metformin was first described in the scientific literature in 1922, by Emil Werner and James Bell, as a product in the synthesis of N,N-dimethylguanidine.[94] In 1929, Slotta and Tschesche discovered its sugar-lowering action in rabbits, noting it was the most potent of the biguanide analogs they studied.[104] This result was completely forgotten, as other guanidine analogs, such as the synthalins, took over, and were themselves soon overshadowed by insulin.[105]
Interest in metformin, however, picked up at the end of the 1940s. In 1950, metformin, unlike some other similar compounds, was found not to decrease blood pressure and heart rate in animals.[106] That same year, a prominent Philippine physician, Eusebio Y. Garcia,[107] used metformin (he named it Fluamine) to treat influenza; he noted the drug "lowered the blood sugar to minimum physiological limit" and was nontoxic. Garcia also believed metformin to have bacteriostatic, antiviral, antimalarial, antipyretic and analgesic actions.[108] In a series of articles in 1954, Polish pharmacologist Janusz Supniewski[109] was unable to confirm most of these effects, including lowered blood sugar; he did, however, observe some antiviral effects in humans.[110][111]
While training at the Hôpital de la Pitié, French diabetologist Jean Sterne studied the antihyperglycemic properties of galegine, an alkaloid isolated from Galega officinalis, which is related in structure to metformin and had seen brief use as an antidiabetic before the synthalins were developed.[5] Later, working at Laboratoires Aron in Paris, he was prompted by Garcia's report to reinvestigate the blood sugar lowering activity of metformin and several biguanide analogs. Sterne was the first to try metformin on humans for the treatment of diabetes; he coined the name "Glucophage" (glucose eater) for the drug and published his results in 1957.[5][105]
Metformin became available in the British National Formulary in 1958. It was sold in the UK by a small Aron subsidiary called Rona. [112]
Broad interest in metformin was not rekindled until the withdrawal of the other biguanides in the 1970s. Metformin was approved in Canada in 1972,[113] but did not receive approval by the U.S. Food and Drug Administration (FDA) for type 2 diabetes until 1994.[114] Produced under license by Bristol-Myers Squibb, Glucophage was the first branded formulation of metformin to be marketed in the United States, beginning on March 3, 1995.[115] Generic formulations are now available in several countries, and metformin is believed to have become the most widely prescribed antidiabetic drug in the world.[5]
Formulations

Metformin is sold under several trade names, including Glucophage XR, Riomet, Fortamet, Glumetza, Obimet, Gluformin, Dianben, Diabex, and Diaformin.
Metformin IR (immediate release) is available in 500 mg, 850 mg, and 1000 mg tablets; all are now generic in the US.
Metformin SR (slow release) or XR (extended release) was introduced in 2004, in 500 mg and 750 mg strengths, mainly to counteract the most common gastrointestinal side effects, as well as to increase compliance by reducing pill burden. No difference in effectiveness exists between the two preparations.
Combinations with other drugs
When used for type 2 diabetes, metformin is often prescribed in combination with other drugs. Several are available as fixed-dose combinations, also with the purpose of reducing pill burden and making administration simpler and more convenient.[116]
As of 2009, the most popular brand-name combination was metformin with rosiglitazone, sold as Avandamet by GlaxoSmithKline since 2002.[117][118] Rosiglitazone actively makes cells more sensitive to insulin, complementing the action of the metformin. In 2005, all current stock of Avandamet was seized by the FDA and removed from the market, after inspections showed the factory where it was produced was violating good manufacturing practices.[119] The drug pair continued to be prescribed separately in the absence of Avandamet, which was available again by the end of that year.
In the United States, metformin is also available in combination with pioglitazone (trade name Actoplus Met), the sulfonylureas glipizide (trade name Metaglip) and glibenclamide (known as glyburide in the United States, trade name Glucovance), the dipeptidyl peptidase-4 inhibitor sitagliptin (with the combination sold under the trade name Janumet), the dipeptidyl peptidase-4 inhibitor saxagliptin (with the combination sold under the trade name Kombiglyze XR), and the meglitinide repaglinide (PrandiMet). Generic formulations of metformin/glipizide and metformin/glibenclamide are available (the latter being more popular).[6] A generic formulation of metformin/rosiglitazone from Teva has received tentative approval from the FDA, and is expected to reach the market in early 2012.[120]
In Europe, the combination of metformin and the dipeptidyl peptidase-4 inhibitor linagliptin is approved the trade name Jentadueto.[121]
References
- ^ Clinical Guidelines Task Force, International Diabetes Federation (2005). Template:PDFlink. In: Global Guideline for Type 2 Diabetes. Brussels: International Diabetes Federation, 35–8. Retrieved on November 6, 2007.
- ^ National Collaborating Centre for Chronic Conditions. Type 2 diabetes: national clinical guideline for management in primary and secondary care (update) [pdf]. London: Royal College of Physicians; 2008. ISBN 978-1-86016-333-3. p. 86.
- ^ American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32 Suppl 1:S13–61. doi:10.2337/dc09-S013. PMID 19118286.
- ^ (March 2010) Template:PDFlink, 16th edition, World Health Organization, p. 24. Retrieved on 22 December 2010.
- ^ a b c d Bailey CJ, Day C. Metformin: its botanical background. Practical Diabetes International. 2004;21(3):115–7. doi:10.1002/pdi.606.
- ^ a b Template:PDFlink. IMS Institute for Healthcare Informatics (April 2011). Retrieved on April 28, 2011.
- ^ Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327(7421):951–3. doi:10.1136/bmj.327.7421.951. PMID 14576245. PMC 259161.
- ^ Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358(9285):893–4. doi:10.1016/S0140-6736(01)06042-1. PMID 11567710.
- ^ Ibáñez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab. 2006;91(8):2888–91. doi:10.1210/jc.2006-0336. PMID 16684823.
- ^ Nair S, Diehl AM, Wiseman M, Farr GH Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20(1):23–28. doi:10.1111/j.1365-2036.2004.02025.x. PMID 15225167.
- ^ Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;24(1):CD005166. doi:10.1002/14651858.CD005166.pub2. PMID 17253544.
- ^ Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48(5):587–96. PMID 19412008.
- ^ a b c Consumer Reports Health
Best Buy Drugs, "The Oral Diabetes Drugs: Treating Type 2 Diabetes" (PDF), Best Buy Drugs, Consumer Reports, p. 2, retrieved September 18 2012
{{citation}}: Check date values in:|accessdate=(help); line feed character in|author1=at position 24 (help) - ^ a b Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. doi:10.1016/S0140-6736(98)07037-8. PMID 9742977.
- ^ Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi:10.1056/NEJMoa0806470. PMID 18784090.
- ^ Selvin E, Bolen S, Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168(19):2070–80. doi:10.1001/archinte.168.19.2070. PMID 18955635.
- ^ a b c d Maharani U. Chapter 27: Diabetes Mellitus & Hypoglycemia. In: Papadakis MA, McPhee SJ. CURRENT Medical Diagnosis and Treatment 2010. 49th ed. McGraw-Hill Medical; 2009. ISBN 0-07-162444-9. p. 1092–93.
- ^ a b c Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–99. PMID 17638715.
- ^ DiPiro, Joseph T.; Talbert, Robert L.; Yee, Gary C.; Matzke, Gary R.; Wells, Barbara G.; Posey, L. Michael. Pharmacotherapy: a pathophysiologic approach. New York: McGraw-Hill; 2005. ISBN 0-07-141613-7.
- ^ "Glucophage package insert". Princeton, NJ: Bristol-Myers Squibb Company; 2009.
- ^ Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi:10.1056/NEJMoa012512. PMID 11832527.
- ^ Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi:10.1016/S0140-6736(09)61457-4. PMID 19878986.
- ^ Lilly M, Godwin M. Treating prediabetes with metformin: systematic review and meta-analysis. Can Fam Physician. 2009;55(4):363–9. PMID 19366942.
- ^ Kidson W. Polycystic ovary syndrome: a new direction in treatment. Med J Aust. 1998;169(10):537–40. PMID 9861912.
- ^ Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metab Clin Exp. 1994;43(5):647–54. PMID 8177055.
- ^ Teede H. Insulin sensitizers in polycystic ovary syndrome. In: Kovács GT, Norman RW. Polycystic ovary syndrome. Cambridge, UK: Cambridge University Press; 2007. ISBN 0-521-84849-0. p. 65–81.
- ^ National Collaborating Centre for Women’s and Children's Health. Fertility: assessment and treatment for people with fertility problems [pdf]. London: Royal College of Obstetricians and Gynaecologists; 2004. ISBN 1-900364-97-2. p. 58–9.
- ^ Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–66. doi:10.1056/NEJMoa063971. PMID 17287476.
- ^ Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ. 2006;332(7556):1485. doi:10.1136/bmj.38867.631551.55. PMID 16769748.
- ^ Balen A. Royal College of Obstetricians and Gynaecologists. Metformin therapy for the management of infertility in women with polycystic ovary syndrome [PDF]; December 2008 [Retrieved 2009-12-13].
- ^ The Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462–77. doi:10.1093/humrep/dem426. PMID 18308833.
- ^ Palomba S, Pasquali R, Orio F, Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin. Endocrinol. (Oxf). 2009;70(2):311–21. doi:10.1111/j.1365-2265.2008.03369.x. PMID 18691273.
- ^ Al-Inany H, Johnson N. Drugs for anovulatory infertility in polycystic ovary syndrome. BMJ. 2006;332(7556):1461–2. doi:10.1136/bmj.332.7556.1461. PMID 16793784.
- ^ Radosh L. Drug treatments for polycystic ovary syndrome. Am Fam Physician. 2009;79(8):671–6. PMID 19405411.
- ^ Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2009;(4):CD003053. doi:10.1002/14651858.CD003053.pub3. PMID 19821299.
- ^ a b Palomba S, Orio F, Falbo A, Russo T, Tolino A, Zullo F. Clomiphene citrate versus metformin as first-line approach for the treatment of anovulation in infertile patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(9):3498–503. doi:10.1210/jc.2007-1009. PMID 17595241.
- ^ Tertti K, Ekblad U, Vahlberg T, Rönnemaa T. Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study. Rev Diabet Stud. 2008;5(2):95–101. doi:10.1900/RDS.2008.5.95. PMID 18795211. PMC 2556447.
- ^ Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;258(19):2003–15. doi:10.1056/NEJMoa0707193. PMID 18463376.
- ^ Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193–205. PMID 19104375.
- ^ Balani J, Hyer SL, Rodin DA, Shehata H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: a case-control study. Diabet Med. 2009;26(8):798–802. PMID 19709150.
- ^ Cheung NW. The management of gestational diabetes [pdf]. Vasc Health Risk Manag. 2009;5(1):153–64. PMID 19436673. PMC 2672462.
- ^ Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185–93. doi:10.1001/jama.2007.56-b. PMID 18182600.
- ^ a b Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–8. doi:10.1053/j.gastro.2009.04.013. PMID 19375425.
- ^ Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer of 25–37% in diabetic patients. BMJ. 2005;330:1304–5. doi:10.1136/bmj.38415.708634.F7. PMID 15849206. PMC 558205.
- ^ Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi:10.2337/dc08-2175. PMID 19564453. PMC 2732153.
- ^ Chong CR, Chabner BA. Mysterious metformin. Oncologist. 2009;14(12):1178–81. doi:10.1634/theoncologist.2009-0286. PMID 20007645.
- ^ Ben Sahra I, Le Marchand Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug?. Mol Cancer Therapeutics. 2010;9(5):1092–99. doi:10.1158/1535-7163.MCT-09-1186. PMID 20442309.
- ^ Jones G, Macklin J, Alexander W. Contraindications to the use of metformin. BMJ. 2003;326(7379):4–5. doi:10.1136/bmj.326.7379.4. PMID 12511434. PMC 1124930.
- ^ US FDA. Glucophage Prescribing Information for the U.S. [PDF] [Retrieved 2009-12-24].
- ^ Eurich DT, McAlister FA, Blackburn DF, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007;335(7618):497. doi:10.1136/bmj.39314.620174.80. PMID 17761999. PMC 1971204.
- ^ a b Weir J (March 19, 1999). Guidelines with Regard to Metformin-Induced Lactic Acidosis and X-ray Contrast Medium Agents. Royal College of Radiologists. Retrieved on 26 October 2007 through the Internet Archive.
- ^ a b Thomsen HS, Morcos SK. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003;76(908):513–8. doi:10.1259/bjr/26964464. PMID 12893691.
- ^ Khurana R, Malik IS. Metformin: safety in cardiac patients. Heart. 2010;96(2):99–102. doi:10.1136/hrt.2009.173773. PMID 19564648.
- ^ Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metab. 2006;91(1):225–7. doi:10.1210/jc.2005-1210. PMID 16219720.
- ^ Shegem NS, Nasir AM, Jbour AK, Batieha AM, El-Khateeb MS, Ajlouni KM. Effects of short term metformin administration on androgens in normal men. Saudi Med J. 2002;23(8):934–7. PMID 12235466.
- ^ Ozata M, Oktenli C, Bingol N, Ozdemir IC. The effects of metformin and diet on plasma testosterone and leptin levels in obese men. Obes Res. 2001;9(11):662–7. doi:10.1038/oby.2001.90. PMID 11707532.
- ^ Drug Facts and Comparisons 2005. St. Louis, Mo: Facts and Comparisons; 2004. ISBN 1-57439-193-3.
- ^ Wulffele MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, Donker AJ, Stehouwer CD. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2003;254(5):455–63. doi:10.1046/j.1365-2796.2003.01213.x. PMID 14535967.
- ^ Andrès E, Noel E, Goichot B. Metformin-associated vitamin B12 deficiency. Arch Intern Med. 2002;162(19):2251–2. doi:10.1001/archinte.162.19.2251-a. PMID 12390080.
- ^ Gilligan M. Metformin and vitamin B12 deficiency. Arch Intern Med. 2002;162(4):484–5. doi:10.1001/archinte.162.4.484. PMID 11863489.
- ^ de Jager J, Kooy A, Lehert P; et al. (2010). "Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial". BMJ. 340: c2181.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Ting R, Szeto C, Chan M, Ma K, Chow K. Risk factors of vitamin B(12) deficiency in patients receiving metformin. Arch Intern Med. 2006;166(18):1975–9. doi:10.1001/archinte.166.18.1975. PMID 17030830.
- ^ a b Stang M, Wysowski D K, Butler-Jones D (1999). "Incidence of lactic acidosis in metformin users". Diabetes Care. 22 (6): 925–927. doi:10.2337/diacare.22.6.925. PMID 10372243.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2003;163(21):2594–602. doi:10.1001/archinte.163.21.2594. PMID 14638559.
- ^ a b Shu AD, Myers Jr MG, Shoelson SE. Chapter 29: Pharmacology of the Endocrine Pancreas. In: Golan ED et al. (eds.). Principles of pharmacology: the pathophysiologic basis of drug therapy. Philadelphia: Lippincott, Williams & Wilkins; 2005. ISBN 0-7817-4678-7. p. 540–41.
- ^ a b Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update [PDF]. Ann Intern Med. 2002;137(1):25–33. PMID 12093242.
- ^ Davis SN. Chapter 60: Insulin, Oral Hypoglycemic Agents, and the Pharmacology of the Endocrine Pancreas. In: Brunton L, Lazo J, Parker K. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. ISBN 978-0-07-142280-2.
- ^ Spiller HA, Quadrani DA. Toxic effects from metformin exposure. Ann Pharmacother. 2004;38(5):776–80. doi:10.1345/aph.1D468. PMID 15031415.
- ^ a b Forrester MB. Adult metformin ingestions reported to Texas poison control centers, 2000–2006. Hum Exp Toxicol. 2008;27(7):575–83. doi:10.1177/0960327108090589. PMID 18829734.
- ^ Gjedde S, Christiansen A, Pedersen SB, Rungby J. Survival following a metformin overdose of 63 g: a case report. Pharmacol Toxicol. 2003;93(2):98–9. doi:10.1034/j.1600-0773.2003.930207.x. PMID 12899672.
- ^ Nisse P, Mathieu-Nolf M, Deveaux M, Forceville X, Combes A. A fatal case of metformin poisoning. J Toxicol Clin Toxicol. 2003;41(7):1035–6. doi:10.1081/CLT-120026533. PMID 14705855.
- ^ a b Suchard JR, Grotsky TA. Fatal metformin overdose presenting with progressive hyperglycemia. West J Emerg Med. 2008;9(3):160–4. PMID 19561734.
- ^ a b Teale KF, Devine A, Stewart H, Harper NJ. The management of metformin overdose. Anaesthesia. 1998;53(7):698–701. doi:10.1046/j.1365-2044.1998.436-az0549.x. PMID 9771180.
- ^ Spiller HA, Weber JA, Winter ML, Klein-Schwartz W, Hofman M, Gorman SE, Stork CM, Krenzelok EP. Multicenter case series of pediatric metformin ingestion. Ann Pharmacother. 2000;34(12):1385–8. doi:10.1345/aph.10116. PMID 11144693.
- ^ Dell'Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54(6):818–23. doi:10.1016/j.annemergmed.2009.04.023. PMID 19556031.
- ^ Lacher M, Hermanns-Clausen M, Haeffner K, Brandis M, Pohl M. Severe metformin intoxication with lactic acidosis in an adolescent. Eur J Pediatr. 2005;164(6):362–5. doi:10.1007/s00431-005-1634-y. PMID 15729560.
- ^ Harvey B, Hickman C, Hinson G, Ralph T, Mayer A. Severe lactic acidosis complicating metformin overdose successfully treated with high-volume venovenous hemofiltration and aggressive alkalinization. Pediatr Crit Care Med. 2005;6(5):598–601. doi:10.1097/01.PCC.0000162451.47034.4F. PMID 16148825.
- ^ Guo PY, Storsley LJ, Finkle SN. Severe lactic acidosis treated with prolonged hemodialysis: recovery after massive overdoses of metformin. Semin Dial. 2006;19(1):80–3. doi:10.1111/j.1525-139X.2006.00123.x. PMID 16423187.
- ^ Barrueto F, Meggs WJ, Barchman MJ. Clearance of metformin by hemofiltration in overdose. J Toxicol Clin Toxicol. 2002;40(2):177–80. doi:10.1081/CLT-120004407. PMID 12126190.
- ^ Liu A, Coleman SP. Determination of metformin in human plasma using hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chrom. B 877: 3695-3700, 2009.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 939–940.
- ^ Somogyi A, Stockley C, Keal J, Rolan P, Bochner F. Reduction of metformin renal tubular secretion by cimetidine in man. Br J Clin Pharmacol. 1987;23(5):545–51. PMID 3593625.
- ^ a b c d e f g h Bristol-Myers Squibb. U.S. Food and Drug Administration. Glucophage (metformin hydrochloride tablets) Label Information; August 27, 2008 [Retrieved 2009-12-08].
- ^ Jayasagar G, Krishna Kumar M, Chandrasekhar K, Madhusudan Rao C, Madhusudan Rao Y. Effect of cephalexin on the pharmacokinetics of metformin in healthy human volunteers. Drug Metabol Drug Interact. 2002;19(1):41–8. PMID 12222753.
- ^ Hundal R, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi S, Schumann W, Petersen K, Landau B, Shulman G. Mechanism by which metformin reduces glucose production in type 2 diabetes [PDF]. Diabetes. 2000;49(12):2063–9. doi:10.2337/diabetes.49.12.2063. PMID 11118008. PMC 2995498.
- ^ Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–41. doi:10.1161/01.RES.0000256090.42690.05. PMID 17307971.
- ^ Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman M, Goodyear L, Moller D. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi:10.1172/JCI13505. PMID 11602624. PMC 209533.
- ^ Kim YD, Park KG, Lee YS, et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 2008;57(2):306–14. doi:10.2337/db07-0381. PMID 17909097.
- ^ Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol. 2007;293(1):H457–66. doi:10.1152/ajpheart.00002.2007. PMID 17369473.
- ^ Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291(1):E182–E189. doi:10.1152/ajpendo.00272.2005. PMID 16478780.
- ^ Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–9. doi:10.1056/NEJM199602293340906. PMID 8569826.
- ^ Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–81. doi:10.2337/diabetes.51.7.2074. PMID 12086935.
- ^ Saeedi R, Parsons HL, Wambolt RB, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294(6):H2497–506. doi:10.1152/ajpheart.00873.2007. PMID 18375721.
- ^ a b Werner E, Bell J. The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyanodiamide, and methylammonium and dimethylammonium chlorides respectively. J Chem Soc, Transactions. 1921;121:1790–5. doi:10.1039/CT9222101790.
- ^ Shapiro SL, Parrino VA, Freedman L. Hypoglycemic Agents. I Chemical Properties of β-Phenethylbiguanide. A New Hypoglycemic Agent. J Am Chem Soc. 1959;81(9):2220–5. doi:10.1021/ja01518a052.
- ^ Procédé de préparation de chlorhydrate de diméthylbiguanide. Patent FR 2322860. 1975. French.
- ^ Pharmaceutical Manufacturing Encyclopedia (Sittig's Pharmaceutical Manufacturing Encyclopedia). 3rd ed. Vol. 3. Norwich, NY: William Andrew; 2007. ISBN 0-8155-1526-X. p. 2208.
- ^ Bharatam PV, Patel DS, Iqbal P,. Pharmacophoric Features of Biguanide Derivatives: An Electronic and Structural Analysis. J Med Chem. 2005;48(24):7615–7622. doi:10.1021/jm050602z.
- ^ Patel DS, Bharatam PV. Novel N(L)2 species with two lone pairs on nitrogen: systems isoelectronic to carbodicarbenes. Chem Commun. 2009;(9):1064–6. doi:10.1039/b816595e.
- ^ a b Heller JB. Metformin overdose in dogs and cats. Veterinary Medicine. 2007;(April):231–233.
- ^ Garry G. Graham, Jeroen Punt, Manit Arora, Richard O. Day, Matthew P. Doogue, Janna K. Duong, Timothy J. Furlong, Jerry R. Greenfield, Louise C. Greenup, Carl M. Kirkpatrick, John E. Ray, Peter Timmins and Kenneth M. Williams. Clinical Pharmacokinetics of Metformin. Clin Pharmacokinet 2011; 50 (2): 81-98
- ^ a b Robert F, Fendri S, Hary L, Lacroix C, Andréjak M, Lalau JD. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003;29(3):279–83. PMID 12909816.
- ^ Witters L. The blooming of the French lilac. J Clin Invest. 2001;108(8):1105–7. doi:10.1172/JCI14178. PMID 11602616. PMC 209536.
- ^ See Chemical Abstracts, v.23, 42772 (1929) Werner E, Bell J. Uber Biguanide. II. Die Blutzuckersenkende Wirkung der Biguanides. Berichte der Deutschen Chemischen Gesellschaft B: Abhandlungen. 1929;62:1398–1405.
- ^ a b Campbell IW. Metformin—life begins at 50: A symposium held on the occasion of the 43rd Annual Meeting of the European Association for the Study of Diabetes, Amsterdam, The Netherlands, September 2007. The British Journal of Diabetes & Vascular Disease. 2007;7:247–252. doi:10.1177/14746514070070051001.
- ^ Dawes GS, Mott JC. Circulatory and respiratory reflexes caused by aromatic guanidines. Br J Pharmacol Chemother. 1950;5(1):65–76. PMID 15405470.
- ^ About Eusebio Y. Garcia, see: Carteciano J. Philippines Department of Science and Technology. Search for DOST-NRCP Dr. Eusebio Y. Garcia Award; 2005 [Retrieved 2009-12-05].
- ^ Quoted from Chemical Abstracts, v.45, 24828 (1951) Garcia EY. Fluamine, a new synthetic analgesic and antiflu drug. J Philippine Med Assoc. 1950;26:287–93.
- ^ About Janusz Supniewski, see: Wołkow PP, Korbut R. Pharmacology at the Jagiellonian University in Kracow, short review of contribution to global science and cardiovascular research through 400 years of history [pdf]. J Physiol Pharmacol. 2006 [Retrieved 2009-12-05];57 Suppl 1:119–36. PMID 16766803.
- ^ See Chemical Abstracts, v.52, 22272 (1958) SUPNIEWSKI J, CHRUSCIEL T. [N-dimethyl-di-guanide and its biological properties.]. Arch Immunol Ther Exp (Warsz). 1954;2:1–15. Polish. PMID 13269290.
- ^ Quoted from Chemical Abstracts, v.49, 74699 (1955) Supniewski J, Krupinska, J. [Effect of biguanide derivatives on experimental cowpox in rabbits.]. Bulletin de l'Academie Polonaise des Sciences, Classe 3: Mathematique, Astronomie, Physique, Chimie, Geologie et Geographie. 1954;2(Classe II):161–5. French.
- ^ Hadden DR. Goat's rue – French lilac – Italian fitch – Spanish sainfoin: gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35(3):258–60. PMID 16402501.
- ^ Lucis OJ. The status of metformin in Canada. Can Med Assoc J. 1983;128(1):24–6. PMID 6847752.
- ^ Susan M. Cruzan (December 30, 1994). "FDA Approves New Diabetes Drug" (Press release). U.S. Food and Drug Administration. Archived from the original on 29 September 2007. Retrieved 2007-01-06.
- ^ GLUCOPHAGE Label and Approval History. U.S. Food and Drug Administration. Retrieved on 8 January 2007. Data available for download on FDA website.
- ^ Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab. 2009;11(6):527–33. doi:10.1111/j.1463-1326.2008.00993.x. PMID 19175373.
- ^ "FDA Approves GlaxoSmithKline's Avandamet (rosiglitazone maleate and metformin HCl), The Latest Advancement in the Treatment of Type 2 Diabetes" (Press release). GlaxoSmithKline. October 12, 2002. Retrieved 2006-12-27.
- ^ Template:PDFlink. Drug Topics (June 17, 2010). Retrieved on September 2, 2010.
- ^ "Questions and Answers about the Seizure of Paxil CR and Avandamet" (Press release). U.S. Food and Drug Administration. March 4, 2005. Archived from the original on 14 October 2007. Retrieved 2006-12-27.
- ^ "Teva Pharm announces settlement of generic Avandia, Avandamet, and Avandaryl litigation with GlaxoSmithKline" (Press release). Reuters. September 27, 2007. Retrieved 2009-02-17.
- ^ Jentadueto at the European Medicines Agency. First published 25/05/2012
External links
- Template:Dmoz
- Metformin drug information from Lexi-Comp. Includes dosage information and a comprehensive list of international brand names
- U.S. National Library of Medicine: Drug Information Portal – Metformin
