Endonuclease: Difference between revisions
m Fixed minor grammatical error. |
rearrange by inserting notation and further discussions heading to separate pertinent contents from the categories section |
||
| (45 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| ⚫ | '''Endonucleases''' are [[enzyme]]s that cleave the [[phosphodiester bond]] within a [[polynucleotide]] chain. There are a small amount of significant classes of endonucleases that cleave only at the specific nucleotide sequences (such as the ''restriction endonucleases'' which are so vital in biotechnology).<ref ="Cox_Nelson_Lehninger_2005">{{cite book | author = Cox M, Nelson DR, Lehninger AL | title = Lehninger principles of biochemistry | publisher = W.H. Freeman | location = San Francisco | year = 2005 | pages = 952 | isbn = 0-7167-4339-6 }}</ref>. At the extreme ends of a sequence there are [[restriction endonucleases]], usually called restriction enzymes. These are endonucleases from eubacteria and archea that recognize a specific DNA sequence<ref>{{cite book |author=Stephen T. Kilpatrick; Jocelyn E. Krebs; Lewin, Benjamin; Goldstein, Elliott |title=Lewin's genes X |publisher=Jones and Bartlett |location=Boston |year=2011 |pages= |isbn=0-7637-6632-1 |oclc= |doi= |accessdate=}}</ref>. The nucleotide sequence recognized for cleavage by a restriction enzyme is called the restriction site. Typically, a restriction site will be a [[palindromic]] sequence of about four to six nucleotides long. Most restriction endonucleases cleave the DNA strand unevenly, leaving complementary single-stranded ends. These ends can reconnect through hybridization and are termed "sticky ends." Once paired, the phosphodiester bonds of the fragments can be joined by [[DNA ligase]]. There are hundreds of restriction endonucleases known, each attacking a different restriction site. The DNA fragments cleaved by the same endonuclease can be joined together regardless of the origin of the DNA. Such DNA is called [[recombinant DNA]]; DNA formed by the joining of genes into new combinations.<ref ="Cox_Nelson_Lehninger_2005">{{cite book | author = Cox M, Nelson DR, Lehninger AL | title = Lehninger principles of biochemistry | publisher = W.H. Freeman | location = San Francisco | year = 2005 | pages = 307 | isbn = 0-7167-4339-6 }}</ref>. ''Restriction endonucleases'' ([[restriction enzyme]]s) are divided into three categories, Type I, Type II, and Type III, according to their mechanism of action. These enzymes are often used in [[genetic engineering]] to make [[recombinant DNA]] for introduction into bacterial, plant, or animal cells, as well as in [[synthetic biology]].<ref name = "Simon_2010">{{cite book | author = Simon M | title = Emergent computation: Emphasizing Bioinformatics | url = | year = 2010 |publisher = Springer | location = New York | isbn = 1441919635 | pages = 437 }}</ref>{{rp|375-390}} |
||
{{Refimprove|date=December 2009}} |
|||
| ⚫ | '''Endonucleases''' are [[enzyme]]s that cleave the [[phosphodiester bond]] within a [[polynucleotide]] chain |
||
| ⚫ | The commonly used notation for restriction endonucleases is of the form "''vwxyz''", where "''vwx''" names the life form (bacteria) where this restriction endonuclease may be found, "''y''" names the strain (and is optional), and "''z''" (in Roman numerals) indicates different restriction endonucleases in the same life form (bacteria). Thus for example, "EcoRI" means that the restriction endonuclease is found in ''Escherichia coli'' ("Eco"); strain RY13 ("R"), restriction endonuclease number "I". Another example: "HaeII" and "HaeIII" refer to bacterium ''Haemophilus aegyptius'', number II and number III, respectively.<ref> |
||
[[File:Endonuclease.jpg|thumb|Endonuclease]] |
|||
| ⚫ | Restriction endonucleases come in several types. A restriction endonuclease typically requires a recognition site and a cleavage pattern (typically of nucleotide bases: A, C, G, T). If the recognition site is outside the region of the cleavage pattern, then the restriction endonuclease is referred to as Type I. If the recognition sequence overlaps with the cleavage sequence, then the restriction endonuclease [[restriction enzyme]] is Type II. |
||
| ⚫ | |||
== Categories == |
|||
Ultimately, there are three categories of [[restriction endonucleases]] that relatively contribute to the cleavage of specific sequences. The types I and III are large multisubunit complexes that include both the [[endonucleases]] and [[methylase]] activities. Type I can cleave at random sites of about 1000 base pairs or more from the recognition sequence and it requires ATP as source of energy. The type II behaves slightly differently and was first isolated by Hamilton Smith in 1970. They are simpler versions of the endonucleases and requires no ATP in its degradation processes. Some examples of the type II restriction endonucleases include ''BamHI, EcoRI, EcoRV'', and ''Haelll''. The type III, however, cleaves the DNA at about 25 base pairs from the recognition sequence and also requires ATP in the process.<ref name="Cox_Nelson_Lehninger_2005">{{cite book | author = Cox M, Nelson DR, Lehninger AL | title = Lehninger principles of biochemistry | publisher = W.H. Freeman | location = San Francisco | year = 2005 | pages = 1100 | isbn = 0-7167-4339-6 }}</ref> |
|||
==Notations== |
|||
| ⚫ | The commonly used notation for restriction endonucleases is of the form "''vwxyz''", where "''vwx''" names the life form (bacteria) where this restriction endonuclease may be found, "''y''" names the strain (and is optional), and "''z''" (in Roman numerals) indicates different restriction endonucleases in the same life form (bacteria). Thus for example, "EcoRI" means that the restriction endonuclease is found in ''Escherichia coli'' ("Eco"); strain RY13 ("R"), restriction endonuclease number "I". Another example: "HaeII" and "HaeIII" refer to bacterium ''Haemophilus aegyptius'', number II and number III, respectively.<ref name="Cox_Nelson_Lehninger_2005"/>{{rp|64-64}} The restriction enzymes used in molecular biology usually recognize short target sequences of about 4 – 8 base pairs. For instance, the ''EcoRI'' enzyme recognizes and cleaves the sequence 5' – GAATTC – 3'.<ref name="isbn0-8053-9592-X">{{cite book | author = Losick R, Watson JD, Baker TA, Bell S, Gann S, Levine MW | title = Molecular biology of the gene | publisher = Pearson/Benjamin Cummings | location = San Francisco | year = 2008 | pages = | isbn = 0-8053-9592-X | oclc = | doi = | accessdate = }}</ref> |
||
[[File:Restriction enzyme Eco RI.JPG|thumb|Restriction enzyme Eco RI]] |
|||
| ⚫ | Restriction endonucleases come in several types. A restriction endonuclease typically requires a recognition site and a cleavage pattern (typically of nucleotide bases: A, C, G, T). If the recognition site is outside the region of the cleavage pattern, then the restriction endonuclease is referred to as Type I. If the recognition sequence overlaps with the cleavage sequence, then the restriction endonuclease [[restriction enzyme]] is Type II. |
||
==Further Discussions== |
|||
| ⚫ | |||
* Standard dsDNA |
* Standard dsDNA |
||
* Non-standard DNA |
* Non-standard DNA |
||
# Holliday junctions [[Holliday junction]] |
# Holliday junctions [[Holliday junction]] |
||
# Triple-stranded DNA [[triple-stranded DNA]], quadruple-stranded DNA ([[G-quadruplex]]), etc. |
# Triple-stranded DNA [[triple-stranded DNA]], quadruple-stranded DNA ([[G-quadruplex]]), etc. |
||
# Double-stranded hybrids of DNA and RNA (one strand is DNA, the other strand is RNA)<ref> |
# Double-stranded hybrids of DNA and RNA (one strand is DNA, the other strand is RNA)<ref name="Cox_Nelson_Lehninger_2005"/>{{rp|72-73}} |
||
# Synthetic or artificial DNA (for example, containing bases other than A, C, G, T, refer to the work of [[Eric T. Kool]]). Research with synthetic [[codons]], refer to the research by S. Benner, and enlarging the amino acid set in polypeptides, thus enlarging the proteome or [[proteomics]], see the research by P. Schultz.<ref> |
# Synthetic or artificial DNA (for example, containing bases other than A, C, G, T, refer to the work of [[Eric T. Kool]]). Research with synthetic [[codons]], refer to the research by S. Benner, and enlarging the amino acid set in polypeptides, thus enlarging the proteome or [[proteomics]], see the research by P. Schultz.<ref name="Cox_Nelson_Lehninger_2005"/>{{rp|chapter 3}} |
||
In addition, research is now underway to construct synthetic or artificial restriction endonucleases, especially with recognition sites that are unique within a genome. |
In addition, research is now underway to construct synthetic or artificial restriction endonucleases, especially with recognition sites that are unique within a genome. |
||
Restriction endonucleases or [[restriction enzymes]] typically cleave in two ways: blunt-ended or sticky-ended patterns. An example of a Type I restriction endonuclease |
Restriction endonucleases or [[restriction enzymes]] typically cleave in two ways: blunt-ended or sticky-ended patterns. An example of a Type I restriction endonuclease.<ref name="Cox_Nelson_Lehninger_2005"/>{{rp|64}} |
||
== DNA repair == |
|||
Endonucleases play a role in DNA repair. [[AP endonuclease]], specifically, catalyze the incision of DNA exclusively at AP sites, and therefore prepare DNA for subsequent excision, repair synthesis and DNA ligation. For example, when depurination occurs, this lesion leaves a deoxyribose sugar with a missing base.<ref name="isbn1-55581-319-4">{{cite book | author = Ellenberger T, Friedberg EC, Walker GS, Wolfram S, Wood RJ, Schultz R | title = DNA repair and mutagenesis | publisher = ASM Press | location = Washington, D.C | year = 2006 | pages = | isbn = 1-55581-319-4 | oclc = | doi = | accessdate = }}</ref> The AP enodnuclease recognizes this sugar and essentially cuts the DNA at this site and then allows for DNA repair to continue.<ref name="isbn0-8153-3218-1">{{cite book | author = Alberts B | title = Molecular biology of the cell | publisher = Garland Science | location = New York | year = 2002 | pages = | isbn = 0-8153-3218-1 }}</ref> E. coli cells contain two AP endonucleases: endonuclease IV (endoIV) and exonuclease III (exoIII). While in Eukaryotes, there is only one AP enodnuclease.<ref name="pmid12483517">{{cite journal | author = Nishino T, Morikawa K | title = Structure and function of nucleases in DNA repair: shape, grip and blade of the DNA scissors | journal = Oncogene | volume = 21 | issue = 58 | pages = 9022–32 | year = 2002 | month = December | pmid = 12483517 | doi = 10.1038/sj.onc.1206135 }}</ref> |
|||
[[File:APEndonucleasecartoon.gif|APEndonucleasecartoon]] |
|||
== Common endonucleases == |
|||
Below are tables of common prokaryotic and eukaryotic endonucleases <ref>{{cite book |author=Tania A. Baker; Kornberg, Arthur |title=DNA replication |publisher=University Science |location= |year=2005 |pages= |isbn=1-891389-44-0 |oclc= |doi= |accessdate=}}</ref>. |
|||
{| class="wikitable" |
|||
|- |
|||
! Prokaryotic Enzyme !! Source !! Comments |
|||
|- |
|||
| RecBCD enonuclease || E. coli || Partially ATP dependent; also an exonuclease; functions in recombination and repar |
|||
|- |
|||
| T7 endonuclease || phage T7 encoded (gene 3) || Essential for replication; preference for single stranded over double stranded DNA |
|||
|- |
|||
| T4 endonuclease IV || phage T4 encoded (denA) || Splits -TpC- sequence to yield 5'-dCMP- terminated oligonucleotides; chain length of product varies with conditions |
|||
|- |
|||
| Bal 31 endonuclease || Alteromonas espejiana || Also an exonuclease; nibbles away 3' and 5' ends of duplex DNA |
|||
|- |
|||
| EndonucleaseI (endo I) || E. coli (endA) || Periplasmic location; average chain length of product is 7; inhibited by tRNA; produces double stranded DNA break; proudces nick when complexed with tRNA; endo I mutants grow normally |
|||
|- |
|||
| Micrococcal nuclease || Staphylococcus || Produces 3'-P termini; requires Ca2+; also acts on RNA; prefers single stranded DNA and AT-rich regions |
|||
|- |
|||
| Endonuclease II (endo VI, exo III) || E. coli (xth) || Cleavage next to AP site; also a 3'-->5' exonuclease; phosphomonoesterase on 3'-P termini |
|||
|} |
|||
{| class="wikitable" |
|||
|- |
|||
! Eukaryotic Enzyme !! Source !! Comments |
|||
|- |
|||
| Neurospora endonuclease || Neurospora crassa|| Also acts on RNA |
|||
|- |
|||
| S1-nuclease || Aspergillus oryzae || Also acts on RNA |
|||
|- |
|||
| P1-nuclease || Penicillium citrinum || Also acts on RNA |
|||
|- |
|||
| Mung bean nuclease I || mung bean sprouts || Also acts on RNA |
|||
|- |
|||
| Ustilago nuclease (Dnase I) || Ustilago maydis || Also acts on RNA |
|||
|- |
|||
| Dnase I || Bovine pancreas || Average chain length of product is 4; produces double strand break in presence of Mn2+ |
|||
|- |
|||
| AP endonuclease || Nucleus, mitochondria || Involved in DNA Base Excision Repair pathway |
|||
|- |
|||
| Endo R|| HeLa cells || Specific for GC sites |
|||
|} |
|||
== |
== Mutations == |
||
Restriction endonucleases (ENases) are most commonly produced by bacteria, and can be used to map a piece of DNA. |
|||
Xeroderma pigmentosa is a rare, autosomal recessive disease caused by a defective UV-specific endonuclease. Patients with mutations are unable to repair DNA damage caused by sunlight.<ref name="isbn0-470-65451-1">{{cite book | author = | title = Medical Biochemistry at a Glance | publisher = Wiley | location = New York | year = 2012 | pages = | isbn = 0-470-65451-1 }}</ref> |
|||
ENases are widespread amongst [[prokaryotes]] (bacteria and archaea), but are produced by some eukaryotic organisms as well, although this is very rare.<ref>Madigan, Michael T., John M. Martinko, Thomas D. Brock, and David P. Clark. Biology of Microorganisms. 12th ed. San Francisco, CA: Pearson/Benjamin Cummings, 2009. Print. p. 314</ref> |
|||
Sickle Cell anemia is a disease caused by a point mutation. The sequence altered by the mutation eliminates the recognition site for the restriction endonuclease MstII that recognizes the nucleotide sequence.<ref name="isbn0-7817-6960-4">{{cite book | author = Ferrier DR, Champe PC, Harvey RP | title = Biochemistry | publisher = Wolters Kluwer/Lippincott Williams & Wilkins | location = Philadelphia | year = 2008 | pages = | isbn = 0-7817-6960-4 | oclc = | doi = | accessdate = }}</ref> |
|||
Some endonucleases have actions on RNA, such as the [[Dicer]] enzyme, which initiates the formation of RNA-induced silencing complexes. These may also be termed [[endoribonuclease]]s. |
|||
tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Pontocerebellar hypoplasias (PCH) represent a group of neurodegenerative autosomal recessive disorders that is caused by mutations in three of the four different subunits of the tRNA-splicing endonuclease complex.<ref name="pmid18711368">{{cite journal | author = Budde BS, Namavar Y, Barth PG, Poll-The BT, Nürnberg G, Becker C, van Ruissen F, Weterman MA, Fluiter K, te Beek ET, Aronica E, van der Knaap MS, Höhne W, Toliat MR, Crow YJ, Steinling M, Voit T, Roelenso F, Brussel W, Brockmann K, Kyllerman M, Boltshauser E, Hammersen G, Willemsen M, Basel-Vanagaite L, Krägeloh-Mann I, de Vries LS, Sztriha L, Muntoni F, Ferrie CD, Battini R, Hennekam RC, Grillo E, Beemer FA, Stoets LM, Wollnik B, Nürnberg P, Baas F | title = tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia | journal = Nat. Genet. | volume = 40 | issue = 9 | pages = 1113–8 | year = 2008 | month = September | pmid = 18711368 | doi = 10.1038/ng.204 }}</ref> |
|||
===Bacterial=== |
|||
# [[UvrABC endonuclease]] is a well-documented endonuclease found in ''E.coli''. |
|||
==See also== |
==See also== |
||
* [[Exonuclease]] |
* [[Exonuclease]] |
||
* [[Restriction endonuclease]] |
|||
* [[Nuclease]] |
* [[Nuclease]] |
||
* [[Ribonuclease]] |
* [[Ribonuclease]] |
||
* [[AP endonuclease]] |
|||
==References== |
==References== |
||
| ⚫ | |||
{{Ibid|date=January 2012}} |
|||
| ⚫ | |||
{{Nucleases}} |
{{Nucleases}} |
||
Revision as of 01:12, 15 December 2012
Endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain. There are a small amount of significant classes of endonucleases that cleave only at the specific nucleotide sequences (such as the restriction endonucleases which are so vital in biotechnology).[1]. At the extreme ends of a sequence there are restriction endonucleases, usually called restriction enzymes. These are endonucleases from eubacteria and archea that recognize a specific DNA sequence[2]. The nucleotide sequence recognized for cleavage by a restriction enzyme is called the restriction site. Typically, a restriction site will be a palindromic sequence of about four to six nucleotides long. Most restriction endonucleases cleave the DNA strand unevenly, leaving complementary single-stranded ends. These ends can reconnect through hybridization and are termed "sticky ends." Once paired, the phosphodiester bonds of the fragments can be joined by DNA ligase. There are hundreds of restriction endonucleases known, each attacking a different restriction site. The DNA fragments cleaved by the same endonuclease can be joined together regardless of the origin of the DNA. Such DNA is called recombinant DNA; DNA formed by the joining of genes into new combinations.[3]. Restriction endonucleases (restriction enzymes) are divided into three categories, Type I, Type II, and Type III, according to their mechanism of action. These enzymes are often used in genetic engineering to make recombinant DNA for introduction into bacterial, plant, or animal cells, as well as in synthetic biology.[4]: 375–390
Categories
Ultimately, there are three categories of restriction endonucleases that relatively contribute to the cleavage of specific sequences. The types I and III are large multisubunit complexes that include both the endonucleases and methylase activities. Type I can cleave at random sites of about 1000 base pairs or more from the recognition sequence and it requires ATP as source of energy. The type II behaves slightly differently and was first isolated by Hamilton Smith in 1970. They are simpler versions of the endonucleases and requires no ATP in its degradation processes. Some examples of the type II restriction endonucleases include BamHI, EcoRI, EcoRV, and Haelll. The type III, however, cleaves the DNA at about 25 base pairs from the recognition sequence and also requires ATP in the process.[5]
Notations
The commonly used notation for restriction endonucleases is of the form "vwxyz", where "vwx" names the life form (bacteria) where this restriction endonuclease may be found, "y" names the strain (and is optional), and "z" (in Roman numerals) indicates different restriction endonucleases in the same life form (bacteria). Thus for example, "EcoRI" means that the restriction endonuclease is found in Escherichia coli ("Eco"); strain RY13 ("R"), restriction endonuclease number "I". Another example: "HaeII" and "HaeIII" refer to bacterium Haemophilus aegyptius, number II and number III, respectively.[5]: 64–64 The restriction enzymes used in molecular biology usually recognize short target sequences of about 4 – 8 base pairs. For instance, the EcoRI enzyme recognizes and cleaves the sequence 5' – GAATTC – 3'.[6]
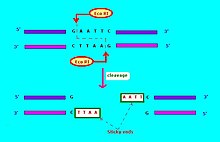
Restriction endonucleases come in several types. A restriction endonuclease typically requires a recognition site and a cleavage pattern (typically of nucleotide bases: A, C, G, T). If the recognition site is outside the region of the cleavage pattern, then the restriction endonuclease is referred to as Type I. If the recognition sequence overlaps with the cleavage sequence, then the restriction endonuclease restriction enzyme is Type II.
Further Discussions
Restriction endonucleases may be found that cleave standard dsDNA (double-stranded DNA), or ssDNA (single-stranded DNA), or even RNA. This discussion is restricted to dsDNA, however, the discussion can be extended to the following:
- Standard dsDNA
- Non-standard DNA
- Holliday junctions Holliday junction
- Triple-stranded DNA triple-stranded DNA, quadruple-stranded DNA (G-quadruplex), etc.
- Double-stranded hybrids of DNA and RNA (one strand is DNA, the other strand is RNA)[5]: 72–73
- Synthetic or artificial DNA (for example, containing bases other than A, C, G, T, refer to the work of Eric T. Kool). Research with synthetic codons, refer to the research by S. Benner, and enlarging the amino acid set in polypeptides, thus enlarging the proteome or proteomics, see the research by P. Schultz.[5]: chapter 3
In addition, research is now underway to construct synthetic or artificial restriction endonucleases, especially with recognition sites that are unique within a genome.
Restriction endonucleases or restriction enzymes typically cleave in two ways: blunt-ended or sticky-ended patterns. An example of a Type I restriction endonuclease.[5]: 64
DNA repair
Endonucleases play a role in DNA repair. AP endonuclease, specifically, catalyze the incision of DNA exclusively at AP sites, and therefore prepare DNA for subsequent excision, repair synthesis and DNA ligation. For example, when depurination occurs, this lesion leaves a deoxyribose sugar with a missing base.[7] The AP enodnuclease recognizes this sugar and essentially cuts the DNA at this site and then allows for DNA repair to continue.[8] E. coli cells contain two AP endonucleases: endonuclease IV (endoIV) and exonuclease III (exoIII). While in Eukaryotes, there is only one AP enodnuclease.[9]
Common endonucleases
Below are tables of common prokaryotic and eukaryotic endonucleases [10].
| Prokaryotic Enzyme | Source | Comments |
|---|---|---|
| RecBCD enonuclease | E. coli | Partially ATP dependent; also an exonuclease; functions in recombination and repar |
| T7 endonuclease | phage T7 encoded (gene 3) | Essential for replication; preference for single stranded over double stranded DNA |
| T4 endonuclease IV | phage T4 encoded (denA) | Splits -TpC- sequence to yield 5'-dCMP- terminated oligonucleotides; chain length of product varies with conditions |
| Bal 31 endonuclease | Alteromonas espejiana | Also an exonuclease; nibbles away 3' and 5' ends of duplex DNA |
| EndonucleaseI (endo I) | E. coli (endA) | Periplasmic location; average chain length of product is 7; inhibited by tRNA; produces double stranded DNA break; proudces nick when complexed with tRNA; endo I mutants grow normally |
| Micrococcal nuclease | Staphylococcus | Produces 3'-P termini; requires Ca2+; also acts on RNA; prefers single stranded DNA and AT-rich regions |
| Endonuclease II (endo VI, exo III) | E. coli (xth) | Cleavage next to AP site; also a 3'-->5' exonuclease; phosphomonoesterase on 3'-P termini |
| Eukaryotic Enzyme | Source | Comments |
|---|---|---|
| Neurospora endonuclease | Neurospora crassa | Also acts on RNA |
| S1-nuclease | Aspergillus oryzae | Also acts on RNA |
| P1-nuclease | Penicillium citrinum | Also acts on RNA |
| Mung bean nuclease I | mung bean sprouts | Also acts on RNA |
| Ustilago nuclease (Dnase I) | Ustilago maydis | Also acts on RNA |
| Dnase I | Bovine pancreas | Average chain length of product is 4; produces double strand break in presence of Mn2+ |
| AP endonuclease | Nucleus, mitochondria | Involved in DNA Base Excision Repair pathway |
| Endo R | HeLa cells | Specific for GC sites |
Mutations
Xeroderma pigmentosa is a rare, autosomal recessive disease caused by a defective UV-specific endonuclease. Patients with mutations are unable to repair DNA damage caused by sunlight.[11]
Sickle Cell anemia is a disease caused by a point mutation. The sequence altered by the mutation eliminates the recognition site for the restriction endonuclease MstII that recognizes the nucleotide sequence.[12]
tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Pontocerebellar hypoplasias (PCH) represent a group of neurodegenerative autosomal recessive disorders that is caused by mutations in three of the four different subunits of the tRNA-splicing endonuclease complex.[13]
See also
References
- ^ Cox M, Nelson DR, Lehninger AL (2005). Lehninger principles of biochemistry. San Francisco: W.H. Freeman. p. 952. ISBN 0-7167-4339-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Stephen T. Kilpatrick; Jocelyn E. Krebs; Lewin, Benjamin; Goldstein, Elliott (2011). Lewin's genes X. Boston: Jones and Bartlett. ISBN 0-7637-6632-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Cox M, Nelson DR, Lehninger AL (2005). Lehninger principles of biochemistry. San Francisco: W.H. Freeman. p. 307. ISBN 0-7167-4339-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Simon M (2010). Emergent computation: Emphasizing Bioinformatics. New York: Springer. p. 437. ISBN 1441919635.
- ^ a b c d e Cox M, Nelson DR, Lehninger AL (2005). Lehninger principles of biochemistry. San Francisco: W.H. Freeman. p. 1100. ISBN 0-7167-4339-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Losick R, Watson JD, Baker TA, Bell S, Gann S, Levine MW (2008). Molecular biology of the gene. San Francisco: Pearson/Benjamin Cummings. ISBN 0-8053-9592-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Ellenberger T, Friedberg EC, Walker GS, Wolfram S, Wood RJ, Schultz R (2006). DNA repair and mutagenesis. Washington, D.C: ASM Press. ISBN 1-55581-319-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Alberts B (2002). Molecular biology of the cell. New York: Garland Science. ISBN 0-8153-3218-1.
- ^ Nishino T, Morikawa K (2002). "Structure and function of nucleases in DNA repair: shape, grip and blade of the DNA scissors". Oncogene. 21 (58): 9022–32. doi:10.1038/sj.onc.1206135. PMID 12483517.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Tania A. Baker; Kornberg, Arthur (2005). DNA replication. University Science. ISBN 1-891389-44-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Medical Biochemistry at a Glance. New York: Wiley. 2012. ISBN 0-470-65451-1.
- ^ Ferrier DR, Champe PC, Harvey RP (2008). Biochemistry. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. ISBN 0-7817-6960-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Budde BS, Namavar Y, Barth PG, Poll-The BT, Nürnberg G, Becker C, van Ruissen F, Weterman MA, Fluiter K, te Beek ET, Aronica E, van der Knaap MS, Höhne W, Toliat MR, Crow YJ, Steinling M, Voit T, Roelenso F, Brussel W, Brockmann K, Kyllerman M, Boltshauser E, Hammersen G, Willemsen M, Basel-Vanagaite L, Krägeloh-Mann I, de Vries LS, Sztriha L, Muntoni F, Ferrie CD, Battini R, Hennekam RC, Grillo E, Beemer FA, Stoets LM, Wollnik B, Nürnberg P, Baas F (2008). "tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia". Nat. Genet. 40 (9): 1113–8. doi:10.1038/ng.204. PMID 18711368.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)

