Sodium hydroxide: Difference between revisions
m Reverted edits by 167.128.111.110 to last revision by Alansohn (HG) |
No edit summary |
||
| Line 172: | Line 172: | ||
===Use in chemical analysis=== |
===Use in chemical analysis=== |
||
In [[analytical chemistry]], sodium hydroxide solutions are often used to measure the [[concentration]] of acids by [[titration]]. Since NaOH is not a [[primary standard]], solutions must first be standardised by titration against a standard such as [[Potassium hydrogen phthalate|KHP]]. [[Burette]]s exposed to NaOH should be rinsed out immediately after use to prevent "freezing" of the stopcock. Sodium hydroxide was traditionally used to test for [[cation]]s in [[Qualitative inorganic analysis|Qualitative Inorganic Analysis]], as well as to provide alkaline media for some reactions that need it, such as the [[Biuret |
In [[analytical chemistry]], sodium hydroxide solutions are often used to measure the [[concentration]] of acids by [[titration]]. Since NaOH is not a [[primary standard]], solutions must first be standardised by titration against a standard such as [[Potassium hydrogen phthalate|KHP]]. [[Burette]]s exposed to NaOH should be rinsed out immediately after use to prevent "freezing" of the stopcock. Sodium hydroxide was traditionally used to test for [[cation]]s in [[Qualitative inorganic analysis|Qualitative Inorganic Analysis]], as well as to provide alkaline media for some reactions that need it, such as the [[Biuret test]]. |
||
===Gold pennies experiment=== |
===Gold pennies experiment=== |
||
Revision as of 06:12, 11 June 2009
It has been suggested that Manufacture of Sodium hydroxide by Nelson's process be merged into this article. (Discuss) Proposed since January 2008. |

| |
| |
| Names | |
|---|---|
| IUPAC name
Sodium hydroxide
| |
| Other names
Caustic soda
Lye | |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.013.805 |
| EC Number |
|
| E number | E524 (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UN number | 1823 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| NaOH | |
| Molar mass | 39.9971 g/mol |
| Appearance | white solid, hygroscopic |
| Density | 2.13 g/cm3 |
| Melting point | 323 °C (613 °F; 596 K) |
| Boiling point | 1,388 °C (2,530 °F; 1,661 K) |
| 111 g/100 ml (20 °C) | |
| Solubility in ethanol | 13.9 g/100 mL |
| Solubility in methanol | 23.8 g/100 mL |
| Solubility in glycerol | soluble |
Refractive index (nD)
|
1.412 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Sodium hydrosulfide Sodium amide |
Other cations
|
Lithium hydroxide Potassium hydroxide Rubidium hydroxide Caesium hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
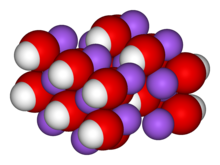
Sodium hydroxide (NaOH), also known as lye and caustic soda, is a caustic metallic base. Sodium hydroxide forms a strong alkaline solution when dissolved in a solvent such as water, however, only the hydroxide ion is basic. It is used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner. Worldwide production in 1998 was around 45 million tonnes. Sodium hydroxide is a common base in chemical laboratories.
Pure sodium hydroxide is a white solid; available in pellets, flakes, granules and as a 50% saturated solution. It is hygroscopic and readily absorbs carbon dioxide from the air, so it should be stored in an airtight container. It is very soluble in water with liberation of heat. It also dissolves in ethanol and methanol, though it exhibits lower solubility in these solvents than potassium hydroxide. It is insoluble in ether and other non-polar solvents. A sodium hydroxide solution will leave a yellow stain on fabric and paper.
Physical properties
Δ H° dissolution for diluted aqueous -44.45 kJ / mol;
From aqueous solutions at 12.3-61.8°C, it crystallizes in monohydrate, with a melting point 65.1 °C and density of 1.829 g/cm 3;
Δ H° form -734.96 kJ / mol;
Monohydrate from -28 to -24°C;
Heptahydrate from -24 to -17.7°C;
Pentahydrate from -17.7 to -5.4°C;
Tetrahydrate (α- changed), at -5 , 4 - 12.3°C Also know metastable β- NaOH 4* H2O. Which above 61.8°C are crystallized.
Chemical properties
Sodium hydroxide is completely ionic, containing sodium cations and hydroxide anions. The hydroxide anion makes sodium hydroxide a strong base which reacts with acids to form water and the corresponding salts, e.g., with hydrochloric acid, sodium chloride is formed:
In general such neutralization reactions are represented by one simple net ionic equation:
This type of reaction with a strong acid, releases heat, and hence is referred to as exothermic. Such acid-base reactions can also be used for titrations, which is a common method to determine the concentration of acids. Another type of reaction that sodium hydroxide is involved in is with acidic oxides. The reaction of carbon dioxide has already been mentioned, but other acidic oxides such as sulfur dioxide (SO2) also react completely. Such reactions are often used to "scrub" harmful acidic gases (like SO2 and H2S) and prevent their release into the atmosphere.
Sodium hydroxide slowly reacts with glass to form sodium silicate, so glass joints and stopcocks exposed to NaOH have a tendency to "freeze". Flasks and glass-lined chemical reactors are damaged by long exposure to hot sodium hydroxide, and the glass becomes frosted. Sodium hydroxide does not attack iron since iron does not have amphoteric properties. A few transition metals, however, may react with sodium hydroxide in a vigorous way.
In 1986 an aluminium road tanker in the UK was mistakenly used to transport 25% sodium hydroxide solution, causing pressurization of the contents and damage to the tanker. The pressurization was due to the hydrogen gas which is produced in the reaction between sodium hydroxide and aluminium:
2Al(s) + 6NaOH(aq) → 3H2(g) + 2Na3AlO3(aq)
Unlike NaOH, the hydroxides of most metals are insoluble, and therefore sodium hydroxide can be used to precipitate metal hydroxides. One such hydroxide is aluminium hydroxide, used as a gelatinous floc to filter out particulate matter in water treatment. Aluminium hydroxide is prepared at the treatment plant from aluminium sulfate by reacting with NaOH. This reaction is highly profitable, and is hence an important synthesis reaction.
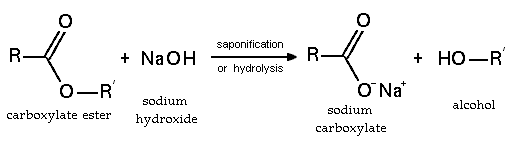
Sodium hydroxide reacts readily with carboxylic acids to form their salts and is even a strong enough base to form salts with phenols. NaOH can be used for the base-driven hydrolysis of esters (as in saponification), amides and alkyl halides. However, the limited solubility of NaOH in organic solvents means that the more soluble KOH is often preferred.
Manufacture
In 1998, total world production was around 45 million tonnes. North America and Asia collectively contributed around 14 million tonnes, while Europe produced around 10 million tonnes.
Methods of production

Sodium hydroxide is produced (along with chlorine and hydrogen) via the chloralkali process. This involves the electrolysis of an aqueous solution of sodium chloride. The sodium hydroxide builds up at the cathode, where water is reduced to hydrogen gas and hydroxide ion:
- 2Na+ + 2H2O + 2e− → H2 + 2NaOH
To produce NaOH it is necessary to prevent reaction of the NaOH with the chlorine. This is typically done in one of three ways, of which the membrane cell process is economically the most viable.
- Mercury cell process (also called the Castner-Kellner process) – Sodium ions are reduced to sodium metal, which forms an amalgam with a mercury cathode; this sodium is then reacted with water to produce NaOH. There have been concerns about mercury releases, although modern plants claim to be safe in this regard.[1]
- Diaphragm cell process – uses a steel cathode, and the reaction of NaOH with Cl2 is prevented using a porous diaphragm, often made of asbestos fibers. In the diaphragm cell process the anode area is separated from the cathode area by a permeable diaphragm. The brine is introduced into the anode compartment and flows through the diaphragm into the cathode compartment. A diluted caustic brine leaves the cell. The sodium hydroxide must usually be concentrated to 50% and the salt removed. This is done using an evaporative process with about three tonnes of steam per tonne of sodium hydroxide. The salt separated from the caustic brine can be used to saturate diluted brine. The chlorine contains oxygen and is purified by liquefaction and evaporation.[2][3]
- Membrane cell process – similar to the diaphragm cell process, with a Nafion membrane to separate the cathode and anode reactions. Only sodium ions and a little water pass through the membrane. It produces a higher quality of NaOH. Of the three processes, the membrane cell process requires the lowest consumption of electric energy and the amount of steam needed for concentration of the caustic is relatively small (less than one tonne per tonne of sodium hydroxide).[4][5]
An older method for sodium hydroxide production was the Leblanc process, which produced sodium carbonate, followed by roasting to create carbon dioxide and sodium oxide. This method is still occasionally used. It helped establish sodium hydroxide as an important commodity chemical.
The LeBlanc process was superseded by the Solvay process in the late 19th century.
Major producers
In the United States, the major producer of sodium hydroxide is the Dow Chemical Company, which has annual production around 3.7 million tonnes from sites at Freeport, Texas, and Plaquemine, Louisiana. Other major US producers include Oxychem, PPG, Olin, Pioneer Companies (which was purchased by Olin), Inc. (PIONA), and Formosa. All of these companies use the chloralkali process[6].
Uses
General applications
Sodium hydroxide is the principal strong base used in the chemical industry. In bulk it is most often handled as an aqueous solution, since solutions are cheaper and easier to handle. It is used to drive chemical reactions and also for the neutralization of acidic materials. It can be used also as a neutralizing agent in petroleum refining. It is sometimes used as a cleaner.
Alumina production - Bayer process
Sodium hydroxide is used in the refining of alumina containing ore (bauxite) to produce alumina (aluminium oxide) which is the raw material used to produce aluminium metal via the smelting process.
Soap production
Sodium hydroxide was traditionally used in soap making (cold process soap, saponification).
Oil Drilling
Sodium hydroxide is a component of drilling mud, used to increase viscosity which prevents the weighted material from settling.
Paper making
Sodium hydroxide was also widely used in making paper. Along with sodium sulfide, NaOH is a key component of the white liquor solution used to separate lignin from cellulose fibers in the Kraft process. It also plays a key role in several later stages of the process of bleaching the brown pulp resulting from the pulping process. These stages include oxygen delignification, oxidative extraction, and simple extraction, all of which require a strong alkaline environment with a pH > 10.5 at the end of the stages.
Biodiesel
For the manufacture of biodiesel, sodium hydroxide is used as a catalyst for the transesterification of methanol and triglycerides. This only works with anhydrous sodium hydroxide, because combined with water the fat would turn into soap, which would be tainted with methanol. It is used more often than potassium hydroxide because it is cheaper and a smaller quantity is needed.
Illegal drugs
Sodium hydroxide is used in the process of making methamphetamine, dimethyltryptamine, and other illegal drugs. Contrary to popular media reports, it is not actually an "ingredient" in these drugs, but simply a strong base used to manipulate the pH at various points in a chemical synthesis.
Atmospheric CO2 "scrubber"
Within contained atmospheres containing animal life (such as the International Space Station with its astronauts or within a submerged submarine with its crew), normal respiration by the occupants generates carbon dioxide.[7][8] This can rise to high enough concentration to create a toxic atmosphere and so must be restrained to non-toxic levels.[9] The property of sodium hydroxide to absorb and convert carbon dioxide has been noted above as an inconvenience, but is in this case applied as a solution to this problem. The contained atmosphere is circulated through a contacting medium containing either dry hydroxide or which is washed with an aqueous solution of the substance. One example is treating calcium hydroxide with sodium hydroxide and potassium hydroxide to form Soda lime.
Aluminium etching
Strong bases attack aluminium. Sodium hydroxide mixes with aluminium and water to create hydrogen gas. The aluminium takes the oxygen atom from sodium hydroxide(NaOH), which in turn takes the oxygen atom from the water, and releases the two hydrogen atoms. In this reaction, sodium hydroxide acts as an active catalyst. This reaction can be useful in etching through a resist, removing anodizing, or converting a polished surface to a satin-like finish, but without further passivation such as anodizing or alodining the surface may become degraded, either under normal use or in severe atmospheric conditions.
Cleansing agent
Sodium hydroxide is frequently used as a cleaner in breweries and wineries, where it is simply called "caustic". It is added to water, heated, and then used to clean the large stainless steel tanks where the beer is brewed or wine fermented, and where the resulting product is stored before bottling. It can dissolve oils and protein-based deposits. A sodium hydroxide soak solution is used as a powerful degreaser on stainless and glass bakeware. It is also the most common ingredient in oven cleaners.
Paint stripper
A solution of sodium hydroxide in water was traditionally used as the most common paint stripper on wooden objects. Due to its caustic nature and the fact that it can damage the wood surface raising the grain and staining the color, its use has become less common.
Tissue digestion
This is a process that was used with farm animals at one time. This process involves the placing of a carcass into a sealed chamber, which then puts the carcass in a mixture of sodium hydroxide and water, which breaks chemical bonds keeping the body intact. This eventually turns the body into a coffee-like liquid, and the only solid that remains are bone hulls, which could be crushed between one's fingertips. It is also of note that sodium hydroxide is frequently used in the process of decomposing roadkill dumped in landfills by animal disposal contractors[citation needed].
Sodium hydroxide has also been used by serial killers and other criminals to dispose of their victims' bodies.[10][11]
Food preparation
Food uses of sodium hydroxide include washing or chemical peeling of fruits and vegetables, chocolate and cocoa processing, caramel coloring production, poultry scalding, soft drink processing, and thickening ice cream. Olives are often soaked in sodium hydroxide to soften them, while pretzels and German lye rolls are glazed with a sodium hydroxide solution before baking to make them crisp. Due to the difficulty in obtaining food grade sodium hydroxide in small quantities for home use, Sodium carbonate is often used in place of sodium hydroxide[12].
Specific foods processed with sodium hydroxide include:
- The Scandinavian delicacy known as lutefisk (from lutfisk, "lye fish").
- Hominy is dried maize (corn) kernels reconstituted by soaking in lye-water. These expand considerably in size and may be further processed by frying to make corn nuts or by drying and grinding to make grits. Nixtamal is similar, but uses calcium hydroxide instead of sodium hydroxide.
- Sodium hydroxide is also the chemical that causes gelling of egg whites in the production of Century eggs.
- German pretzels are poached in a boiling sodium carbonate solution or cold sodium hydroxide solution before baking, which contributes to their unique crust.
- Most yellow coloured Chinese noodles are made with lye-water but are commonly mistaken for containing egg.
Domestic uses
Sodium hydroxide is used in the home as a drain cleaning agent for clearing clogged drains. It is distributed as a dry crystal or as a thick liquid gel. The chemical mechanism employed is the conversion of grease to a form of soap. Soap is water-soluble, and can be dissolved by flushing with water. Sodium hydroxide also decomposes complex molecules such as the protein that composes hair. Such drain cleaners (and their acidic versions) are highly caustic and should be handled with care.
Sodium hydroxide has been used as a relaxer to straighten hair. However, because of the high incidence and intensity of chemical burns, chemical relaxer manufacturers have now switched to other alkaline chemicals, although sodium hydroxide relaxers are still available, used mostly by professionals.
Use in chemical analysis
In analytical chemistry, sodium hydroxide solutions are often used to measure the concentration of acids by titration. Since NaOH is not a primary standard, solutions must first be standardised by titration against a standard such as KHP. Burettes exposed to NaOH should be rinsed out immediately after use to prevent "freezing" of the stopcock. Sodium hydroxide was traditionally used to test for cations in Qualitative Inorganic Analysis, as well as to provide alkaline media for some reactions that need it, such as the Biuret test.
Gold pennies experiment
Sodium hydroxide has also been used in conjunction with zinc for creation of the famous "Gold pennies" experiment. When a penny is boiled in a solution of NaOH together with some granular zinc metal (galvanized nails are one source), the color of the penny will turn silver in about 45 seconds. The penny is then held in the flame of a burner for a few seconds and it turns golden brown. The reason this happens is that granular zinc dissolves in NaOH to form Zn(OH)42- (tetrahydroxozincate). This zincate ion becomes reduced to metallic zinc on the surface of a copper penny. Zinc and copper when heated in a flame form brass.
Safety
Refer to a relevant Material Safety Data Sheet (MSDS) before handling this material

Solid sodium hydroxide or solutions of sodium hydroxide will cause chemical burns, permanent injury or scarring, and blindness if it contacts unprotected human or animal tissue. Protective equipment such as rubber gloves, safety clothing and eye protection should always be used when handling the material or its solutions.
Dissolution of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables.
Sodium hydroxide is extremely caustic, and can react with fats and oils on skin, in a reaction which creates salts. For this reason, sodium hydroxide is very dangerous, and skin should immediately be rinsed with mild acid, such as vinegar or low molar citric acid, following exposure. Do NOT wash with water following contact with this substance as this will merely exacerbate the corrosive affect.
A solution of 0.5 M or more of sodium hydroxide should be labelled corrosive, while a solution between 0.5-0.05 M should be labelled irritant.[13]
See also
References
- ^ "Chlorine Online Diagram of mercury cell process". Euro Chlor. Retrieved 2006-09-15.
- ^ "Euro Chlor - How is chlorine made?". Euro Chlor. Retrieved 2006-09-15.
- ^ "Chlorine Online Diagram of diaphragm cell process". Euro Chlor. Retrieved 2006-09-15.
- ^ "Chlorine Online Diagram of membrane cell process". Euro Chlor. Retrieved 2006-09-15.
- ^ "Euro Chlor - How is chlorine made?". Euro Chlor. Retrieved 2006-09-15.
- ^ See Kirk-Othmer in general references
- ^ DallBauman LA, Finn JE (1999). "Adsorption processes in spacecraft environmental control and life support systems". Stud Surf Sci Catal. 120: 455–71. PMID 12741388.
- ^ Brubakk, Alf O. (2003). Bennett and Elliott's physiology and medicine of diving, 5th Rev ed. United States: Saunders Ltd. p. 800. ISBN 0702025712.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lambertsen, C. J. (1971). "Carbon Dioxide Tolerance and Toxicity". Environmental Biomedical Stress Data Center, Institute for Environmental Medicine, University of Pennsylvania Medical Center. IFEM Report No. 2-71. Philadelphia, PA. Retrieved 2009-03-18.
- ^ Belle Gunness
- ^ Mexico man 'dissolved 300 bodies' BBC News 2009-01-24
- ^ "Hominy without Lye". National Center for Home Food Preservation.
- ^ Commission Directive 93/72/EEC of 1 September 1993 adapting to technical progress for the nineteenth time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. OJCE L258, 16.10.1993, pp. 29–30.
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Heaton, A. (1996) An Introduction to Industrial Chemistry, 3rd edition, New York:Blackie. ISBN 0-7514-0272-9.
- Kirk-Othmer Encyclopedia of Chemical Technology 5th edition (online, account needed), John Wiley & Sons. Accessed November 21, 2005.
- Euro Chlor - How is chlorine made? Chlorine Online
External links
- International Chemical Safety Card 0360
- NIOSH Pocket Guide to Chemical Hazards
- European Chemicals Bureau
- European Union Risk Assessment Report
- Production by brine electrolysis
- Sodium Hydroxide Site exclusively for sodium hydroxide
- Titration of acids with sodium hydroxide – freeware for data analysis, simulation of curves and pH calculation
Related to chlorine
- Chlorine Online - Facts about chlorine; sodium hydroxide is an important co-product of chlorine.
- The Chlorine Institute, Inc. website

