Metformin: Difference between revisions
→History: + |
No edit summary |
||
| Line 77: | Line 77: | ||
===Lactic acidosis=== |
===Lactic acidosis=== |
||
The most serious potential adverse effect of biguanide use is [[lactic acidosis]]. [[Phenformin]], another biguanide, was actually withdrawn from the market because of an increased risk of lactic acidosis (up to 60 cases per [[million]] patient-years). However, metformin is safer than phenformin, and the risk of developing lactic acidosis is not increased by the medication so long as it is not prescribed to known high-risk groups.<ref name = Salpeter>{{cite journal |author=Salpeter S, Greyber E, Pasternak G, Salpeter E |title=Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis |journal=[[Archives of Internal Medicine|Arch Intern Med]] |volume=163 |issue=21 |pages=2594–602 |year=2003 |pmid=14638559 |doi=10.1001/archinte.163.21.2594}}</ref> |
The most serious potential adverse effect of biguanide use is [[lactic acidosis]]. [[Phenformin]], another biguanide, was actually withdrawn from the market because of an increased risk of lactic acidosis (up to 60 cases per [[million]] patient-years). However, metformin is safer than phenformin, and the risk of developing lactic acidosis is not increased by the medication so long as it is not prescribed to known high-risk groups.<ref name = Salpeter>{{cite journal |author=Salpeter S, Greyber E, Pasternak G, Salpeter E |title=Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis |journal=[[Archives of Internal Medicine|Arch Intern Med]] |volume=163 |issue=21 |pages=2594–602 |year=2003 |pmid=14638559 |doi=10.1001/archinte.163.21.2594}}</ref> |
||
===Hormonal=== |
|||
Metformin has been reported to reduce the blood levels of [[thyroid-stimulating hormone]] in patients with [[hypothyroidism]],<ref>{{cite journal|url=http://jcem.endojournals.org/cgi/content/full/91/1/225|title=Thyrotropin suppression by metformin |author=Vigersky RA, Filmore-Nassar A, Glass AR |journal=Journal of Clinical Endocrinology & Metabolism|doi=10.1210/jc.2005-1210}}</ref> and, in men, [[lutenizing hormone]] and [[testosterone]].<ref>{{cite journal|url=http://www.ncbi.nlm.nih.gov/pubmed/12235466|title=Effects of short term metformin administration on androgens in normal men.|author=Shegem NS, Nasir AM, Jbour AK, Batieha AM, El-Khateeb MS, Ajlouni KM.|journal=Saudi Med J. |pmid=12235466}}</ref><ref>{{cite journal|title=The effects of metformin and diet on plasma testosterone and leptin levels in obese men.|author=Ozata M, Oktenli C, Bingol N, Ozdemir IC.|journal=Obes Res|pmid=11707532|year=2001|volume=9|issue=11}}</ref>. Even if testosterone deficiency ([[hypogonadism]]) is highly related with diabetes mellitus and insulin-resistance, the clinical significance of these changes is still unknown. |
|||
====Mechanism==== |
====Mechanism==== |
||
Revision as of 15:26, 4 December 2009
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 1,1-dimethylbiguanide |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 50 to 60% under fasting conditions |
| Metabolism | None |
| Elimination half-life | 6.2 hours |
| Excretion | Active renal tubular excretion by OCT2 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.472 |
| Chemical and physical data | |
| Formula | C4H11N5 |
| Molar mass | 129.164 g/mol 165.63 g/mol (hydrochloride) g·mol−1 |
| (verify) | |
Metformin (INN) (Template:Pron-en; originally sold as Glucophage) is an oral anti-diabetic drug. It is the first-line drug for the treatment of type 2 diabetes, particularly in overweight and obese people and those with normal kidney function,[2][3][4] and evidence suggests it may be the best choice for people with heart failure.[5] It is also used in the treatment of polycystic ovary syndrome.
Metformin is the most popular anti-diabetic drug in the United States and one of the most prescribed drugs in the country overall, with more than 40 million prescriptions filled in 2008 for generic metformin alone.[6] When prescribed appropriately, metformin causes few adverse effects—the most common is gastrointestinal upset—and, unlike many other anti-diabetic drugs, does not cause hypoglycemia if used alone. It also helps reduce LDL cholesterol and triglyceride levels, and may aid weight loss. As of 2009[update], metformin is one of only two oral anti-diabetics in the World Health Organization Model List of Essential Medicines (the other being glibenclamide).[7]
History
The biguanide class of anti-diabetic drugs, which also includes the withdrawn agents phenformin and buformin, originates from the French lilac (Galega officinalis), a plant known for several centuries to reduce the symptoms of diabetes mellitus.[8]
Metformin was first described in the scientific literature in 1922 by Emil Werner and James Bell as a side product in the synthesis of N,N-dimethylguanidine.[9] In 1929, Slotta and Tschesche discovered its sugar-lowering action in rabbits, noting that it was the most potent of the analogs they studied.[10] This result was completely forgotten as other guanidine analogs, such as the synthalins, took over, and were soon overshadowed by insulin.[11]
French diabetologist Jean Sterne studied the antihyperglycemic properties of galegine, a toxic extract of Galega that had also been tested in the 1920s, while training at the Hôpital de la Pitié.[12] From 1956 onwards, at Laboratoires Aron in Paris, he investigated several biguanide analogs for the treatment of diabetes; his interest in biguanides was at least partly prompted by anecdotal reports from the previous decade, when metformin (under the name flumamine) was used as a treatment for influenza and serendipitously found to have a hypoglycemic effect in humans. Sterne was first to try metformin on humans for the treatment of diabetes. He coined the name "Glucophage" for the drug and published his results in 1957.[11][12] However, his report was soon followed by trials of phenformin and buformin, which were more potent and thus adopted for clinical use.[12]
Broad interest in metformin was not rekindled until the withdrawal of the other biguanides in the 1970s. Metformin was first marketed in France in 1979, but did not receive approval by the U.S. Food and Drug Administration (FDA) for Type 2 diabetes until 1994.[13] Produced under license by Bristol-Myers Squibb, Glucophage was the first branded formulation of metformin to be marketed in the United States, beginning on March 3, 1995.[14] Generic formulations are now available in several countries.
Therapeutic uses
The main use for metformin is in the treatment of diabetes mellitus type 2, especially when this accompanies obesity and insulin resistance. Metformin is the only anti-diabetic drug that has been proven to protect against the cardiovascular complications of diabetes.[15] This was first shown in the United Kingdom Prospective Diabetes Study, a large study of overweight patients with diabetes.[16]
Unlike the other most-commonly prescribed class of oral diabetes drugs, the sulfonylureas, metformin (taken alone) does not induce hypoglycemia.[17] Hypoglycemia during intense exercise has been documented, but is extremely rare.[18] It also does not cause weight gain, and may indeed produce minor weight loss.[19] Metformin also modestly reduces LDL and triglyceride levels.[20]
Metformin is being used increasingly in polycystic ovary syndrome (PCOS),[21] non-alcoholic fatty liver disease (NAFLD)[22] and premature puberty,[23] three other diseases that feature insulin resistance; these indications are still[update] considered experimental. Although metformin is not licensed for use in PCOS, the United Kingdom's National Institute for Health and Clinical Excellence recommends that women with PCOS and a body mass index above 25 be given metformin when other therapy has failed to produce results.[24] The benefit of metformin in NAFLD has not been extensively studied and may be only temporary;[25] although some randomized controlled trials have found significant improvement with its use, the evidence is still insufficient.[26][27]
Gestational diabetes
Several observational studies and randomized controlled trials have found that metformin is as effective and safe as insulin for the management of gestational diabetes,[28][29][30] and a small case-control study has suggested that the children of women given metformin instead of insulin may be healthier in the neonatal period.[31] Nonetheless, several concerns have been raised regarding studies published thus far, and evidence on the long-term safety of metformin for both mother and child is still lacking.[32]
Investigational findings
A large case-control study conducted at M.D. Anderson Cancer Center has suggested that metformin may protect against pancreatic cancer. The risk of pancreatic cancer in study participants who took metformin was found to be 62% lower than in participants who had never taken it, whereas participants who had used insulin or secretagogues (such as the sulfonylureas) were found to have a 5-fold and 2.5-fold higher risk of pancreatic cancer, respectively, compared to participants that had been treated with neither.[33] The study had several limitations, however, and the reason for this risk reduction is still unclear.[33] Observational studies conducted by the University of Dundee have shown a decrease of 25–37% in cancer cases in diabetics taking metformin.[34][35]
A single randomized controlled trial suggested that metformin may reduce weight gain in patients taking atypical antipsychotics, particularly when combined with lifestyle interventions (education, dieting, and exercise).[36]
Contraindications
Metformin is contraindicated in people with any condition that could increase the risk of lactic acidosis, including kidney disorders (creatinine levels over 150 μmol/l,[37] although this is an arbitrary limit), lung disease and liver disease. Heart failure has long been considered a contraindication for metformin use, although a 2007 systematic review showed metformin to be the only anti-diabetic drug not associated with harm in people with heart failure.[5]
It is recommended that metformin be temporarily discontinued before any radiographic study involving iodinated contrast (such as a contrast-enhanced CT scan or angiogram), as contrast dye may temporarily impair kidney function, indirectly leading to lactic acidosis by causing retention of metformin in the body.[38][39] It is recommended that metformin be resumed after two days, assuming kidney function is normal.[38][39]
Adverse effects
The most common adverse effect of metformin is gastrointestinal upset, including diarrhea, cramps, nausea, vomiting and increased flatulence; metformin is more commonly associated with gastrointestinal side effects than most other anti-diabetic drugs.[20] The most serious potential side effect of metformin use is lactic acidosis; this complication is very rare, and seems limited to people with impaired liver or kidney function.
Metformin has also been reported to reduce the blood levels of thyroid-stimulating hormone in patients with hypothyroidism,[40] and, in men, lutenizing hormone and testosterone.[41][42] The clinical significance of these changes is still unknown.
Gastrointestinal
In a clinical trial of 286 subjects, 53.2% of the 141 who were given immediate-release metformin (as opposed to placebo) reported diarrhea, versus 11.7% for placebo, and 25.5% reported nausea/vomiting, versus 8.3% for those on placebo.[43]
Gastrointestinal upset can cause severe discomfort for patients; it is most common when metformin is first administered, or when the dose is increased. The discomfort can often be avoided by beginning at a low dose (1 to 1.7 grams per day) and increasing the dose gradually. Gastrointestinal upset after prolonged, steady use is less common.
Long-term use of metformin has been associated with increased homocysteine levels[44] and malabsorption of vitamin B12.[45][46] Higher doses and prolonged use are associated with increased incidence of B12 deficiency, and some researchers recommend screening or prevention strategies.[47]
Lactic acidosis
The most serious potential adverse effect of biguanide use is lactic acidosis. Phenformin, another biguanide, was actually withdrawn from the market because of an increased risk of lactic acidosis (up to 60 cases per million patient-years). However, metformin is safer than phenformin, and the risk of developing lactic acidosis is not increased by the medication so long as it is not prescribed to known high-risk groups.[48]
Hormonal
Metformin has been reported to reduce the blood levels of thyroid-stimulating hormone in patients with hypothyroidism,[49] and, in men, lutenizing hormone and testosterone.[50][51]. Even if testosterone deficiency (hypogonadism) is highly related with diabetes mellitus and insulin-resistance, the clinical significance of these changes is still unknown.
Mechanism
Lactate uptake by the liver is diminished with metformin administration because lactate is a substrate for hepatic gluconeogenesis, a process which metformin inhibits. In healthy individuals, this slight excess is simply cleared by other mechanisms (including uptake by the kidneys, when their function is unimpaired), and no significant elevation in blood levels of lactate occurs.[52] When there is impaired renal function, however, clearance of metformin (and lactate) is reduced and the drug may accumulate, leading to lactic acidosis. Because metformin decreases liver uptake of lactate, any condition which may precipitate lactic acidosis is a contraindication to its use. Common causes of increased lactic acid production include alcoholism (due to depletion of NAD+ stores), heart failure, and respiratory disease (due to inadequate oxygenation of tissues); the most common cause of impaired lactic acid excretion is kidney disease.[53]
It has also been suggested that metformin increases production of lactate in the small intestine; this could potentially contribute to lactic acidosis in patients with risk factors.[54] However, the clinical significance of this is unknown, and the risk of metformin-associated lactic acidosis is most commonly attributed to decreased hepatic uptake rather than increased intestinal production.[52][53][55]
Overdosage
A review of intentional and accidental metformin overdoses reported to poison control centers over a five-year period found that serious adverse events were rare, though elderly patients appeared to be at greater risk.[56] Intentional overdoses with up to 63 g of metformin have been reported in the medical literature.[57] The major potentially life-threatening complication of metformin overdose is lactic acidosis. Treatment of metformin overdose is generally supportive, but may include sodium bicarbonate to address acidosis and standard hemodialysis or continuous veno-venous hemofiltration to rapidly remove metformin and correct acidosis.[58][59]
Pharmacokinetics
Metformin has an oral bioavailability of 50–60% under fasting conditions, and is absorbed slowly.[60][61] Peak plasma concentrations (Cmax) are reached within one to three hours of taking immediate-release metformin and four to eight hours with extended-release formulations.[60][61] The plasma protein binding of metformin is negligible, as reflected by its very high apparent volume of distribution (300–1000 L after a single dose). Steady state is usually reached in one or two days.[60]
Metformin is not metabolized. It is cleared from the body by tubular secretion and excreted unchanged in the urine; metformin is undetectable in blood plasma within 24 hours of a single oral dose.[60][62] The average elimination half-life in plasma is 6.2 hours.[60] Metformin is distributed to (and appears to accumulate in) red blood cells, with a much longer elimination half-life: 17.6 hours[60] (reported as ranging from 18.5 to 31.5 hours in a single-dose study of non-diabetic people).[62]
Mechanism of action
Metformin improves hyperglycemia primarily through its suppression of hepatic glucose production (hepatic gluconeogenesis).[54] The "average" person with type 2 diabetes has three times the normal rate of gluconeogenesis; metformin treatment reduces this by over one third.[63] Metformin activates AMP-activated protein kinase (AMPK), a liver enzyme that plays an important role in insulin signaling, whole body energy balance, and the metabolism of glucose and fats;[64] activation of AMPK is required for metformin's inhibitory effect on the production of glucose by liver cells.[65] Research published in 2008 further elucidated metformin's mechanism of action, showing that activation of AMPK is required for an increase in the expression of SHP, which in turn inhibits the expression of the hepatic gluconeogenic genes PEPCK and Glc-6-Pase.[66] Metformin is frequently used in research along with AICAR as an AMPK agonist. The mechanism by which biguanides increase the activity of AMPK remains uncertain; however, research suggests that metformin increases the amount of cytosolic AMP (as opposed to a change in total AMP or total AMP/ATP).[67]
In addition to suppressing hepatic glucose production, metformin increases insulin sensitivity, enhances peripheral glucose uptake, increases fatty acid oxidation,[68] and decreases absorption of glucose from the gastrointestinal tract. Increased peripheral utilization of glucose may be due to improved insulin binding to insulin receptors.[69] AMPK probably also plays a role, as metformin administration increases AMPK activity in skeletal muscle.[70] AMPK is known to cause GLUT4 translocation, resulting in insulin-independent glucose uptake. Some metabolic actions of metformin do appear to occur by AMPK-independent mechanisms; a 2008 study found that "the metabolic actions of metformin in the heart muscle can occur independent of changes in AMPK activity and may be mediated by p38 MAPK- and PKC-dependent mechanisms."[71]
Interactions
The H2-receptor antagonist cimetidine causes an increase in the plasma concentration of metformin, by reducing clearance of metformin by the kidneys;[72] both metformin and cimetidine are cleared from the body by tubular secretion, and both, particularly the cationic (positively charged) form of cimetidine, may compete for the same transport mechanism.[73] A small double-blind, randomized study found the antibiotic cefalexin to also increase metformin concentrations by a similar mechanism;[74] theoretically, other cationic medications may produce the same effect.[73]
Formulations
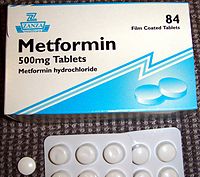
Metformin IR (immediate release) is available in 500 mg, 850 mg, and 1000 mg tablets, all now generic in the US.
Metformin SR (slow release) or XR (extended release) was introduced in 2004, in 500 mg and 750 mg strengths, mainly to counteract the most common gastrointestinal side effects, as well as to increase patient compliance by reducing pill burden. No difference in effectiveness exists between the two preparations.
Combinations with other drugs
Metformin is sometimes prescribed to type 2 diabetes patients in combination with rosiglitazone. This drug actively reduces insulin resistance, complementing the action of the metformin. In 2002, the two drugs were combined into a single product, Avandamet, marketed by GlaxoSmithKline.[75] In 2005, all current stock of Avandamet was seized by the FDA and removed from the market, after inspections showed the factory where it was produced was violating Good Manufacturing Practices.[76] The drug pair continued to be prescribed separately in the absence of Avandamet, which was available again by the end of that year.
In the United States, metformin is also available in combination with pioglitazone (trade name Actoplus Met), the sulfonylureas glipizide (trade name Metaglip) and glibenclamide (known as glyburide in the United States, trade name Glucovance), the dipeptidyl peptidase-4 inhibitor sitagliptin (trade name Janumet), and the meglitinide repaglinide (PrandiMet). Generic formulations of metformin/glipizide and metformin/glibenclamide are available. A generic formulation of metformin/rosiglitazone from Teva has received tentative approval from the FDA, and is expected to reach the market in early 2012.[77]
=Trade names
Metformin is sold under the trade names , Glucophage XR, Riomet, Fortamet, Glumetza, Obimet, Dianben, Diabex, and Diaformin.
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Clinical Guidelines Task Force, International Diabetes Federation (2005). Template:PDFlink. In: Global Guideline for Type 2 Diabetes. Brussels: International Diabetes Federation, 35–8. Retrieved on November 6, 2007.
- ^ National Institute for Health and Clinical Excellence. Clinical guideline 66: Diabetes - type 2 (update). London, 2008.
- ^ American Diabetes Association (2007). "Standards of medical care in diabetes—2007". Diabetes Care. 30 Suppl 1: S4–S41. doi:10.2337/dc07-S004. PMID 17192377.
- ^ a b Eurich DT, McAlister FA, Blackburn DF; et al. (2007). "Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review". BMJ. 335 (7618): 497. doi:10.1136/bmj.39314.620174.80. PMID 17761999.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Template:PDFlink. Drug Topics (May 26, 2009). Retrieved on July 24, 2009.
- ^ (March 2007) Template:PDFlink, 15th edition, World Health Organization, p. 21. Retrieved on 19 November 2007.
- ^ Witters L (2001). "The blooming of the French lilac". J Clin Invest. 108 (8): 1105–7. doi:10.1172/JCI14178. PMID 11602616. PMC 209536
- ^ Werner E, Bell J. (1921). "Preparation of methylguanidine and of β,β'-dimethylguanidine by the interaction of dicyanodiamide and methylammonium and dimethylammonium chlorides, respectively". J. Chem. Soc., Transactions. 121: 1790–5.
- ^ Werner E, Bell J. (1929). "Uber Biguanide. II. Die Blutzuckersenkende Wirkung der Biguanides". Berichte der Deutschen Chemischen Gesellschaft B: Abhandlungen. 62: 1398–1405.
- ^ a b Campbell IW, ed. (2007). "Metformin - life begins at 50: A symposium held on the occasion of the 43rd Annual Meeting of the European Association for the Study of Diabetes, Amsterdam, The Netherlands, September 2007". The British Journal of Diabetes & Vascular Disease. 7: 247–252.
- ^ a b c Bailey CJ (2004). "Metformin: its botanical background". Practical Diabetes International. 21 (3): 115–7. doi:10.1002/pdi.606.
- ^ Susan M. Cruzan (December 30, 1994). "FDA Approves New Diabetes Drug" (Press release). U.S. Food and Drug Administration. Archived from [www.fda.gov/bbs/topics/ANSWERS/ANS00627.html the original] on 29 September 2007. Retrieved 2007-01-06.
{{cite press release}}: Check|url=value (help) - ^ GLUCOPHAGE Label and Approval History. U.S. Food and Drug Administration. Retrieved on 8 January 2007. Data available for download on FDA website.
- ^ Selvin E, Bolen S, Yeh HC; et al. (2008). "Cardiovascular outcomes in trials of oral diabetes medications: a systematic review". Arch Intern Med. 168 (19): 2070–80. doi:10.1001/archinte.168.19.2070. PMID 18955635.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group". Lancet. 352 (9131): 854–65. 1998. doi:10.1016/S0140-6736(98)07037-8. PMID 9742977.
- ^ Kilo C, Mezitis N, Jain R, Mersey J, McGill J, Raskin P (2003). "Starting patients with type 2 diabetes on insulin therapy using once-daily injections of biphasic insulin aspart 70/30, biphasic human insulin 70/30, or NPH insulin in combination with metformin". J Diabetes Complications. 17 (6): 307–13. doi:10.1016/S1056-8727(03)00076-X. PMID 14583174.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ DiPiro, Joseph T.; Talbert, Robert L.; Yee, Gary C.; Matzke, Gary R.; Wells, Barbara G.; Posey, L. Michael (2005). Pharmacotherapy: a pathophysiologic approach. New York: McGraw-Hill. ISBN 0071416137.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE (1995). "Metabolic effects of metformin in non-insulin-dependent diabetes mellitus". N Engl J Med. 333 (9): 550–4. doi:10.1056/NEJM199508313330903. PMID 7623903.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bolen S, Feldman L, Vassy J; et al. (2007). "Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus". Ann Intern Med. 147 (6): 386–99. PMID 17638715.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Lord JM, Flight IHK, Norman RJ (2003). "Metformin in polycystic ovary syndrome: systematic review and meta-analysis". BMJ. 327 (7421): 951–3. doi:10.1136/bmj.327.7421.951. PMID 14576245.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N (2001). "Metformin in non-alcoholic steatohepatitis". Lancet. 358 (9285): 893–4. doi:10.1016/S0140-6736(01)06042-1. PMID 11567710.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ibáñez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F (2006). "Metformin treatment to prevent early puberty in girls with precocious pubarche". J. Clin. Endocrinol. Metab. 91 (8): 2888–91. doi:10.1210/jc.2006-0336. PMID 16684823.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ National Institute for Health and Clinical Excellence. 11 Clinical guideline 11 : Fertility: assessment and treatment for people with fertility problems . London, 2004.
- ^ Nair S, Diehl AM, Wiseman M, Farr GH Jr, Perrillo RP (2004). "Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial". Aliment Pharmacol Ther. 20 (1): 23–28. doi:10.1111/j.1365-2036.2004.02025.x. PMID 15225167.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F (2007). "Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis". Cochrane Database Syst Rev. 24 (1): CD005166. PMID 17253544.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H (2009). "Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review". J Pediatr Gastroenterol Nutr. 48 (5): 587–96. PMID 19412008.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tertti K, Ekblad U, Vahlberg T, Rönnemaa T (2008). "Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study". Rev Diabet Stud. 5 (2): 95–101. PMC 2556447. PMID 18795211.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: multiple names: authors list (link) - ^ Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators (2008). "Metformin versus insulin for the treatment of gestational diabetes". N Engl J Med. 258 (19): 2003–15. PMID 18463376.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E (2009). "Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review". Obstet Gynecol. 113 (1): 193–205. PMID 19104375.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Balani J, Hyer SL, Rodin DA, Shehata H (2009). "Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: a case-control study". Diabet Med. 26 (8): 798–802. PMID 19709150.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cheung NW (2009). "The management of gestational diabetes". Vasc Health Risk Manag. 5 (1): 153–64. PMC 2672462. PMID 19436673.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL (2009). "Antidiabetic therapies affect risk of pancreatic cancer". Gastroenterology. 137 (2): 482–8. doi:10.1053/j.gastro.2009.04.013. PMID 19375425.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005). "Metformin and reduced risk of cancer of 25–37% in diabetic patients". BMJ. 330: 1304–5. doi:10.1136/bmj.38415.708634.F7. PMC 558205. PMID 15849206.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM (2009). "New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes". Diabetes Care. 32: 1620–5. doi:10.2337/dc08-2175. PMC 2732153. PMID 19564453.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wu RR, Zhao JP, Jin H; et al. (2008). "Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial". JAMA. 299 (2): 185–93. doi:10.1001/jama.2007.56-b. PMID 18182600.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Jones G, Macklin J, Alexander W (2003). "Contraindications to the use of metformin". BMJ. 326 (7379): 4–5. doi:10.1136/bmj.326.7379.4. PMID 12511434.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Weir J (March 19, 1999). Guidelines with Regard to Metformin-Induced Lactic Acidosis and X-ray Contrast Medium Agents. Royal College of Radiologists. Retrieved on 26 October 2007 through the Internet Archive.
- ^ a b Thomsen HS, Morcos SK (2003). "Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines". Br J Radiol. 76 (908): 513–8. doi:10.1259/bjr/26964464. PMID 12893691.
- ^ Vigersky RA, Filmore-Nassar A, Glass AR. "Thyrotropin suppression by metformin". Journal of Clinical Endocrinology & Metabolism. doi:10.1210/jc.2005-1210.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shegem NS, Nasir AM, Jbour AK, Batieha AM, El-Khateeb MS, Ajlouni KM. "Effects of short term metformin administration on androgens in normal men". Saudi Med J. PMID 12235466.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ozata M, Oktenli C, Bingol N, Ozdemir IC. (2001). "The effects of metformin and diet on plasma testosterone and leptin levels in obese men". Obes Res. 9 (11). PMID 11707532.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Drug Facts and Comparisons 2005 (59th ed.). Lippincott Williams & Wilkins. 2004. ISBN 1574391933.
{{cite book}}: Unknown parameter|month=ignored (help) - ^ Wulffele MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, Donker AJ, Stehouwer CD. (2003). "Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial". J Intern Med. 254 (5): 455–63. doi:10.1046/j.1365-2796.2003.01213.x. PMID 14535967.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Andrès E, Noel E, Goichot B (2002). "Metformin-associated vitamin B12 deficiency". Arch Intern Med. 162 (19): 2251–2. doi:10.1001/archinte.162.19.2251-a. PMID 12390080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gilligan M (2002). "Metformin and vitamin B12 deficiency". Arch Intern Med. 162 (4): 484–5. doi:10.1001/archinte.162.4.484. PMID 11863489.
- ^ Ting R, Szeto C, Chan M, Ma K, Chow K (2006). "Risk factors of vitamin B(12) deficiency in patients receiving metformin". Arch Intern Med. 166 (18): 1975–9. doi:10.1001/archinte.166.18.1975. PMID 17030830.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Salpeter S, Greyber E, Pasternak G, Salpeter E (2003). "Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis". Arch Intern Med. 163 (21): 2594–602. doi:10.1001/archinte.163.21.2594. PMID 14638559.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vigersky RA, Filmore-Nassar A, Glass AR. "Thyrotropin suppression by metformin". Journal of Clinical Endocrinology & Metabolism. doi:10.1210/jc.2005-1210.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shegem NS, Nasir AM, Jbour AK, Batieha AM, El-Khateeb MS, Ajlouni KM. "Effects of short term metformin administration on androgens in normal men". Saudi Med J. PMID 12235466.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ozata M, Oktenli C, Bingol N, Ozdemir IC. (2001). "The effects of metformin and diet on plasma testosterone and leptin levels in obese men". Obes Res. 9 (11). PMID 11707532.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Maharani U (2009). "Chapter 27: Diabetes Mellitus & Hypoglycemia". In Papadakis MA, McPhee SJ (ed.). CURRENT Medical Diagnosis and Treatment 2010 (49 ed.). McGraw-Hill Medical. ISBN 0-07-162444-9.
- ^ a b Golan ED; et al. (2005). "Chapter 29: Pharmacology of the Endocrine Pancreas". Principles of pharmacology: the pathophysiologic basis of drug therapy. Philadelphia: Lippincott, Williams & Wilkins. ISBN 0-7817-4678-7.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ a b Kirpichnikov D, McFarlane SI, Sowers JR (2002). "Metformin: an update" (PDF). Ann Intern Med. 137 (1): 25–33. PMID 12093242. Retrieved 2008-12-30.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Davis SN (2006). "Chapter 60: Insulin, Oral Hypoglycemic Agents, and the Pharmacology of the Endocrine Pancreas". In Brunton L, Lazo J, Parker K (ed.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 978-0071422802.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Spiller HA, Quadrani DA (2004). "Toxic effects from metformin exposure". The Annals of pharmacotherapy. 38 (5): 776–80. doi:10.1345/aph.1D468. PMID 15031415.
- ^ Gjedde S, Christiansen A, Pedersen SB, Rungby J (2003). "Survival following a metformin overdose of 63 g: a case report". Pharmacol. Toxicol. 93 (2): 98–9. doi:10.1034/j.1600-0773.2003.930207.x. PMID 12899672.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Harvey B, Hickman C, Hinson G, Ralph T, Mayer A (2005). "Severe lactic acidosis complicating metformin overdose successfully treated with high-volume venovenous hemofiltration and aggressive alkalinization". Pediatr Crit Care Med. 6 (5): 598–601. doi:10.1097/01.PCC.0000162451.47034.4F. PMID 16148825.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Guo PY, Storsley LJ, Finkle SN (2006). "Severe lactic acidosis treated with prolonged hemodialysis: recovery after massive overdoses of metformin". Semin Dial. 19 (1): 80–3. doi:10.1111/j.1525-139X.2006.00123.x. PMID 16423187.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f "Glucophage Clinical Pharmacology". RxList.com. 2008. Retrieved 2009-05-16.
- ^ a b Heller, Jacqueline B. (2007). "Metformin overdose in dogs and cats" (PDF). Veterinary Medicine (April): 231–233.
- ^ a b Robert F, Fendri S, Hary L, Lacroix C, Andréjak M, Lalau JD (2003). "Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects". Diabetes Metab. 29 (3): 279–83. PMID 12909816.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hundal R, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi S, Schumann W, Petersen K, Landau B, Shulman G (2000). "Mechanism by which metformin reduces glucose production in type 2 diabetes" (PDF). Diabetes. 49 (12): 2063–9. doi:10.2337/diabetes.49.12.2063. PMID 11118008.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Towler MC, Hardie DG (2007). "AMP-activated protein kinase in metabolic control and insulin signaling". Circ Res. 100 (3): 328–41. doi:10.1161/01.RES.0000256090.42690.05. PMID 17307971.
- ^ Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman M, Goodyear L, Moller D (2001). "Role of AMP-activated protein kinase in mechanism of metformin action". J Clin Invest. 108 (8): 1167–74. doi:10.1172/JCI13505. PMID 11602624.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kim YD, Park KG, Lee YS; et al. (2008). "Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP". Diabetes. 57 (2): 306–14. doi:10.2337/db07-0381. PMID 17909097.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Zhang L, He H, Balschi JA (2007). "Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration". Am J Physiol Heart Circ Physiol. 293 (1): H457–66. doi:10.1152/ajpheart.00002.2007. PMID 17369473.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ (2006). "Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle". Am J Physiol Endocrinol Metab. 291 (1): E182–E189. doi:10.1152/ajpendo.00272.2005. PMID 16478780.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bailey CJ, Turner RC (1996). "Metformin". N Engl J Med. 334 (9): 574–9. doi:10.1056/NEJM199602293340906. PMID 8569826.
- ^ Musi N, Hirshman MF, Nygren J; et al. (2002). "Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes". Diabetes. 51 (7): 2074–81. doi:10.2337/diabetes.51.7.2074. PMID 12086935.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Saeedi R, Parsons HL, Wambolt RB; et al. (2008). "Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms". Am J Physiol Heart Circ Physiol. 294 (6): H2497–506. doi:10.1152/ajpheart.00873.2007. PMID 18375721.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Somogyi A, Stockley C, Keal J, Rolan P, Bochner F (1987). "Reduction of metformin renal tubular secretion by cimetidine in man". Br J Clin Pharmacol. 23 (5): 545–51. PMID 3593625.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Glucophage Side Effects & Drug Interactions". RxList.com. 2007. Retrieved 2007-11-19.
- ^ Jayasagar G, Krishna Kumar M, Chandrasekhar K, Madhusudan Rao C, Madhusudan Rao Y (2002). "Effect of cephalexin on the pharmacokinetics of metformin in healthy human volunteers". Drug Metabol Drug Interact. 19 (1): 41–8. PMID 12222753.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "FDA Approves GlaxoSmithKline's Avandamet (rosiglitazone maleate and metformin HCl), The Latest Advancement in the Treatment of Type 2 Diabetes" (Press release). GlaxoSmithKline. October 12, 2002. Retrieved 2006-12-27.
- ^ "Questions and Answers about the Seizure of Paxil CR and Avandamet" (Press release). U.S. Food and Drug Administration. March 4, 2005. Archived from the original on 14 October 2007. Retrieved 2006-12-27.
- ^ "Teva Pharm announces settlement of generic Avandia, Avandamet, and Avandaryl litigation with GlaxoSmithKline" (Press release). Reuters. September 27, 2007. Retrieved 2009-02-17.
External links
- Metformin at Curlie
- Metformin drug information from Lexi-Comp. Includes dosage information and a comprehensive list of international brand names
