Color: Difference between revisions
ClueBot NG (talk | contribs) m Reverting possible vandalism by 194.83.25.92 to version by AlexiusHoratius. False positive? Report it. Thanks, ClueBot NG. (603221) (Bot) |
|||
| Line 13: | Line 13: | ||
== Physics == |
== Physics == |
||
Yellow lorry, red lorry, yellow lorry, red lorry, yellow lolly, red lolly, lol! |
|||
[[Image:Rendered Spectrum.png|thumb|400px|Continuous optical spectrum rendered into the [[sRGB]] color space.]] |
|||
{|class=wikitable width=400 align="right" style="margin: 1em 0em 1em 1em; clear: right" |
|||
|+'''The colors of the visible light spectrum'''<ref>{{cite book | title = Fundamentals of Atmospheric Radiation: An Introduction with 400 Problems | author = Craig F. Bohren | publisher = Wiley-VCH | year = 2006 | isbn = 3527405038 | url = http://books.google.com/?id=1oDOWr_yueIC&pg=PA214&lpg=PA214&dq=indigo+spectra+blue+violet+date:1990-2007 }}</ref> |
|||
|- |
|||
!style="text-align: left"|color |
|||
! abbr="wavelength" | wavelength interval |
|||
! abbr="frequency" | frequency interval |
|||
|-align="center" bgcolor="red" style="color:white;" |
|||
!style="text-align: left"|[[red]] |
|||
|~ 700–635 nm |
|||
|~ 430–480 THz |
|||
|-align="center" bgcolor="#FF8000" style="color:white;" |
|||
!style="text-align: left"|[[orange (colour)|orange]] |
|||
|~ 635–590 nm |
|||
|~ 480–510 THz |
|||
|-align="center" bgcolor="#FFFF00" style="color:green;" |
|||
!style="text-align: left"|[[yellow]] |
|||
|~ 590–560 nm |
|||
|~ 510–540 THz |
|||
|-align="center" bgcolor="#00FF00" style="color:black;" |
|||
!style="text-align: left"|[[green]] |
|||
|~ 560–490 nm |
|||
|~ 540–610 THz |
|||
|-align="center" bgcolor="#0000FF" style="color:white;" |
|||
!style="text-align: left"|[[blue]] |
|||
|~ 490–450 nm |
|||
|~ 610–670 THz |
|||
|-align="center" bgcolor="#8000FF" style="color:white;" |
|||
!style="text-align: left"|[[violet (color)|violet]] |
|||
|~ 450–400 nm |
|||
|~ 670–750 THz |
|||
|} |
|||
{|class=wikitable width=400 align="right" style="margin: 1em 0em 1em 1em; clear:right;" |
|||
|+'''Color, wavelength, frequency and energy of light''' |
|||
|- |
|||
!style="text-align: left"|Color |
|||
!<math>\lambda \,\!</math> |
|||
(nm) |
|||
!<math>\nu \,\!</math> |
|||
(THz) |
|||
!<math>\nu_b \,\!</math> |
|||
(μm<sup>−1</sup>) |
|||
!<math>E \,\!</math> |
|||
(eV) |
|||
!<math>E \,\!</math> |
|||
(kJ mol<sup>−1</sup>) |
|||
|-align="right" |
|||
!style="text-align: left"|[[Infrared]] |
|||
|>1000 |
|||
|<300 |
|||
|<1.00 |
|||
|<1.24 |
|||
|<120 |
|||
|-align="right" |
|||
!style="text-align: left"|Red |
|||
|700 |
|||
|428 |
|||
|1.43 |
|||
|1.77 |
|||
|171 |
|||
|-align="right" |
|||
!style="text-align: left"|Orange |
|||
|620 |
|||
|484 |
|||
|1.61 |
|||
|2.00 |
|||
|193 |
|||
|-align="right" |
|||
!style="text-align: left"|Yellow |
|||
|580 |
|||
|517 |
|||
|1.72 |
|||
|2.14 |
|||
|206 |
|||
|-align="right" |
|||
!style="text-align: left"|Green |
|||
|530 |
|||
|566 |
|||
|1.89 |
|||
|2.34 |
|||
|226 |
|||
|-align="right" |
|||
!style="text-align: left"|Blue |
|||
|470 |
|||
|638 |
|||
|2.13 |
|||
|2.64 |
|||
|254 |
|||
|-align="right" |
|||
!style="text-align: left"|Violet |
|||
|420 |
|||
|714 |
|||
|2.38 |
|||
|2.95 |
|||
|285 |
|||
|-align="right" |
|||
!style="text-align: left"|Near [[ultraviolet]] |
|||
|300 |
|||
|1000 |
|||
|3.33 |
|||
|4.15 |
|||
|400 |
|||
|-align="right" |
|||
!style="text-align: left"|Far ultraviolet |
|||
|<200 |
|||
|>1500 |
|||
|>5.00 |
|||
|>6.20 |
|||
|>598 |
|||
|} |
|||
<!--no empty line here--> |
|||
[[Electromagnetic radiation]] is characterized by its [[wavelength]] (or [[frequency]]) and its [[Luminous intensity|intensity]]. When the wavelength is within the visible spectrum (the range of wavelengths humans can perceive, approximately from 390 [[nanometre|nm]] to 750 nm), it is known as "visible light". |
|||
Most light sources emit light at many different wavelengths; a source's ''spectrum'' is a distribution giving its intensity at each wavelength. Although the spectrum of light arriving at the eye from a given direction determines the color [[Wikt:sensation|sensation]] in that direction, there are many more possible spectral combinations than color sensations. In fact, one may formally define a color as a class of spectra that give rise to the same color sensation, although such classes would vary widely among different species, and to a lesser extent among individuals within the same species. In each such class the members are called ''[[Metamerism (color)|metamers]]'' of the color in question. |
|||
=== Spectral colors === |
|||
The familiar colors of the [[rainbow]] in the [[Optical spectrum|spectrum]] – named using the [[Latin]] word for ''appearance'' or ''apparition'' by [[Isaac Newton]] in 1671 – include all those colors that can be produced by [[visible light]] of a single wavelength only, the ''pure spectral'' or [[monochromatic color|''monochromatic'' colors]]. The table at right shows approximate frequencies (in [[hertz|terahertz]]) and wavelengths (in [[nanometre|nanometers]]) for various pure spectral colors. The wavelengths are measured in air or [[vacuum]] (see [[refraction]]). |
|||
The color table should not be interpreted as a definitive list – the pure spectral colors form a continuous [[spectrum]], and how it is divided into distinct colors [[language|linguistically]] is a matter of culture and historical contingency (although people everywhere have been shown to ''perceive'' colors in the same way<ref>[[Brent Berlin|Berlin, B.]] and [[Paul Kay|Kay, P.]], ''[[Basic Color Terms: Their Universality and Evolution]]'', Berkeley: [[University of California Press]], 1969.</ref>). A common list identifies six main bands: red, orange, yellow, green, blue, and violet. Newton's conception included a seventh color, [[indigo]], between blue and violet. Optical scientists Hardy and Perrin list indigo as between 446 and 464 nm wavelength.<ref>Arthur C. Hardy and Fred H. Perrin. ''[http://apps.isiknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=22&SID=2EdCK2KejLbni4FJpgB&page=1&doc=1&colname=BIOSIS The Principles of Optics.]'' McGraw-Hill Book Co., Inc., New York. 1932.</ref> |
|||
The ''intensity'' of a spectral color, relative to the context in which it is viewed, may alter its perception considerably; for example, a low-intensity orange-yellow is [[brown]], and a low-intensity yellow-green is olive-green. |
|||
For discussion of non-spectral colors, see [[#Spectral colors and color reproduction|below]]. |
|||
=== Color of objects === |
|||
The color of an object depends on both the physics of the object in its environment and the characteristics of the perceiving eye and brain. Physically, objects can be said to have the color of the light leaving their surfaces, which normally depends on the spectrum of the incident illumination and the reflectance properties of the surface, as well as potentially on the angles of illumination and viewing. Some objects not only reflect light, but also transmit light or emit light themselves (see below), which contribute to the color also. And a viewer's perception of the object's color depends not only on the spectrum of the light leaving its surface, but also on a host of contextual cues, so that the color tends to be perceived as relatively constant: that is, relatively independent of the lighting spectrum, viewing angle, etc. This effect is known as [[color constancy]]. |
|||
[[File:Optical grey squares orange brown.svg|right|thumb|250px|The upper disk and the lower disk have exactly the same objective color, and are in identical gray surroundings; based on context differences, humans perceive the squares as having different reflectances, and may interpret the colors as different color categories; see [[same color illusion]].]] |
|||
Some generalizations of the physics can be drawn, neglecting perceptual effects for now: |
|||
* Light arriving at an [[Opacity (optics)|opaque]] surface is either [[Reflection (physics)|reflect]]ed "[[Specular reflection|specularly]]" (that is, in the manner of a mirror), [[scattering|scatter]]ed (that is, reflected with diffuse scattering), or [[absorption (electromagnetic radiation)|absorbed]] – or some combination of these. |
|||
* Opaque objects that do not reflect specularly (which tend to have rough surfaces) have their color determined by which wavelengths of light they scatter more and which they scatter less (with the light that is not scattered being absorbed). If objects scatter all wavelengths, they appear white. If they absorb all wavelengths, they appear black. |
|||
* Opaque objects that specularly reflect light of different wavelengths with different efficiencies look like mirrors tinted with colors determined by those differences. An object that reflects some fraction of impinging light and absorbs the rest may look black but also be faintly reflective; examples are black objects coated with layers of enamel or lacquer. |
|||
* Objects that [[Transparency and translucency|transmit light]] are either ''translucent'' (scattering the transmitted light) or ''transparent'' (not scattering the transmitted light). If they also absorb (or reflect) light of various wavelengths differentially, they appear tinted with a color determined by the nature of that absorption (or that reflectance). |
|||
* Objects may emit light that they generate themselves, rather than merely reflecting or transmitting light. They may do so because of their elevated temperature (they are then said to be ''[[incandescence|incandescent]]''), as a result of certain chemical reactions (a phenomenon called ''[[chemoluminescence]]''), or for other reasons (see the articles [[Phosphorescence]] and [[List of light sources]]). |
|||
* Objects may absorb light and then as a consequence emit light that has different properties. They are then called ''[[fluorescence|fluorescent]]'' (if light is emitted only while light is absorbed) or ''phosphorescent'' (if light is emitted even after light ceases to be absorbed; this term is also sometimes loosely applied to light emitted because of chemical reactions). |
|||
For further treatment of the color of objects, see [[#Structural color|structural color]], below. |
|||
To summarize, the color of an object is a complex result of its surface properties, its transmission properties, and its emission properties, all of which factors contribute to the mix of wavelengths in the light leaving the surface of the object. The perceived color is then further conditioned by the nature of the ambient illumination, and by the color properties of other objects nearby, via the effect known as [[color constancy]] and via other characteristics of the perceiving eye and brain. |
|||
== Perception == |
== Perception == |
||
Revision as of 12:41, 21 September 2011

Color or colour (see spelling differences) is the visual perceptual property corresponding in humans to the categories called red, green, blue and others. Color derives from the spectrum of light (distribution of light energy versus wavelength) interacting in the eye with the spectral sensitivities of the light receptors. Color categories and physical specifications of color are also associated with objects, materials, light sources, etc., based on their physical properties such as light absorption, reflection, or emission spectra. By defining a color space, colors can be identified numerically by their coordinates.
Because perception of color stems from the varying spectral sensitivity of different types of cone cells in the retina to different parts of the spectrum, colors may be defined and quantified by the degree to which they stimulate these cells. These physical or physiological quantifications of color, however, do not fully explain the psychophysical perception of color appearance.
The science of color is sometimes called chromatics, colorimetry, or simply color science. It includes the perception of color by the human eye and brain, the origin of color in materials, color theory in art, and the physics of electromagnetic radiation in the visible range (that is, what we commonly refer to simply as light).
Physics
Yellow lorry, red lorry, yellow lorry, red lorry, yellow lolly, red lolly, lol!
Perception
Development of theories of color vision
Although Aristotle and other ancient scientists had already written on the nature of light and color vision, it was not until Newton that light was identified as the source of the color sensation. In 1810, Goethe published his comprehensive Theory of Colors. In 1801 Thomas Young proposed his trichromatic theory, based on the observation that any color could be matched with a combination of three lights. This theory was later refined by James Clerk Maxwell and Hermann von Helmholtz. As Helmholtz puts it, "the principles of Newton's law of mixture were experimentally confirmed by Maxwell in 1856. Young's theory of color sensations, like so much else that this marvellous investigator achieved in advance of his time, remained unnoticed until Maxwell directed attention to it."[1]
At the same time as Helmholtz, Ewald Hering developed the opponent process theory of color, noting that color blindness and afterimages typically come in opponent pairs (red-green, blue-orange, yellow-purple, and black-white). Ultimately these two theories were synthesized in 1957 by Hurvich and Jameson, who showed that retinal processing corresponds to the trichromatic theory, while processing at the level of the lateral geniculate nucleus corresponds to the opponent theory.[2]
In 1931, an international group of experts known as the Commission internationale de l'éclairage (CIE) developed a mathematical color model, which mapped out the space of observable colors and assigned a set of three numbers to each.
Color in the eye

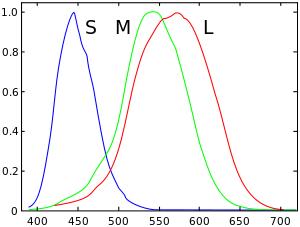
The ability of the human eye to distinguish colors is based upon the varying sensitivity of different cells in the retina to light of different wavelengths. Humans being trichromatic, the retina contains three types of color receptor cells, or cones. One type, relatively distinct from the other two, is most responsive to light that we perceive as violet, with wavelengths around 420 nm; cones of this type are sometimes called short-wavelength cones, S cones, or blue cones. The other two types are closely related genetically and chemically. One of them, sometimes called long-wavelength cones, L cones, or red cones, is most sensitive to light we perceive as greenish yellow, with wavelengths around 564 nm; the other type, known as middle-wavelength cones, M cones, or green cones is most sensitive to light perceived as green, with wavelengths around 534 nm.
Light, no matter how complex its composition of wavelengths, is reduced to three color components by the eye. For each location in the visual field, the three types of cones yield three signals based on the extent to which each is stimulated. These amounts of stimulation are sometimes called tristimulus values.
The response curve as a function of wavelength for each type of cone is illustrated above. Because the curves overlap, some tristimulus values do not occur for any incoming light combination. For example, it is not possible to stimulate only the mid-wavelength (so-called "green") cones; the other cones will inevitably be stimulated to some degree at the same time. The set of all possible tristimulus values determines the human color space. It has been estimated that humans can distinguish roughly 10 million different colors.[3]
The other type of light-sensitive cell in the eye, the rod, has a different response curve. In normal situations, when light is bright enough to strongly stimulate the cones, rods play virtually no role in vision at all.[4] On the other hand, in dim light, the cones are understimulated leaving only the signal from the rods, resulting in a colorless response. (Furthermore, the rods are barely sensitive to light in the "red" range.) In certain conditions of intermediate illumination, the rod response and a weak cone response can together result in color discriminations not accounted for by cone responses alone. These effects, combined, are summarized also in the Kruithof curve, that describes the change of color perception and pleasingness of light as function of temperature and intensity.
Color in the brain

While the mechanisms of color vision at the level of the retina are well-described in terms of tristimulus values (see above), color processing after that point is organized differently. A dominant theory of color vision proposes that color information is transmitted out of the eye by three opponent processes, or opponent channels, each constructed from the raw output of the cones: a red-green channel, a blue-yellow channel and a black-white "luminance" channel. This theory has been supported by neurobiology, and accounts for the structure of our subjective color experience. Specifically, it explains why we cannot perceive a "reddish green" or "yellowish blue," and it predicts the color wheel: it is the collection of colors for which at least one of the two color channels measures a value at one of its extremes.
The exact nature of color perception beyond the processing already described, and indeed the status of color as a feature of the perceived world or rather as a feature of our perception of the world, is a matter of complex and continuing philosophical dispute (see qualia).
Nonstandard color perception
Color deficiency
If one or more types of a person's color-sensing cones are missing or less responsive than normal to incoming light, that person can distinguish fewer colors and is said to be color deficient or color blind (though this latter term can be misleading; almost all color deficient individuals can distinguish at least some colors). Some kinds of color deficiency are caused by anomalies in the number or nature of cones in the retina. Others (like central or cortical achromatopsia) are caused by neural anomalies in those parts of the brain where visual processing takes place.
Tetrachromacy
While most humans are trichromatic (having three types of color receptors), many animals, known as tetrachromats, have four types. These include some species of spiders, most marsupials, birds, reptiles, and many species of fish. Other species are sensitive to only two axes of color or do not perceive color at all; these are called dichromats and monochromats respectively. A distinction is made between retinal tetrachromacy (having four pigments in cone cells in the retina, compared to three in trichromats) and functional tetrachromacy (having the ability to make enhanced color discriminations based on that retinal difference). As many as half of all women are retinal tetrachromats.[5]: p.256 The phenomenon arises when an individual receives two slightly different copies of the gene for either the medium- or long-wavelength cones, which are carried on the x-chromosome. To have two different genes, a person must have two x-chromosomes, which is why the phenomenon only occurs in women.[5] For some of these retinal tetrachromats, color discriminations are enhanced, making them functional tetrachromats.[5]
Synesthesia
In certain forms of synesthesia, perceiving letters and numbers (grapheme–color synesthesia) or hearing musical sounds (music–color synesthesia) will lead to the unusual additional experiences of seeing colors. Behavioral and functional neuroimaging experiments have demonstrated that these color experiences lead to changes in behavioral tasks and lead to increased activation of brain regions involved in color perception, thus demonstrating their reality, and similarity to real color percepts, albeit evoked through a non-standard route.
Afterimages

After exposure to strong light in their sensitivity range, photoreceptors of a given type become desensitized. For a few seconds after the light ceases, they will continue to signal less strongly than they otherwise would. Colors observed during that period will appear to lack the color component detected by the desensitized photoreceptors. This effect is responsible for the phenomenon of afterimages, in which the eye may continue to see a bright figure after looking away from it, but in a complementary color.
Afterimage effects have also been utilized by artists, including Vincent van Gogh.
Color constancy
There is an interesting phenomenon which occurs when an artist uses a limited color palette: the eye tends to compensate by seeing any grey or neutral color as the color which is missing from the color wheel. For example, in a limited palette consisting of red, yellow, black and white, a mixture of yellow and black will appear as a variety of green, a mixture of red and black will appear as a variety of purple, and pure grey will appear bluish.[6]
The trichromatic theory discussed above is strictly true when the visual system is in a fixed state of adaptation. In reality, the visual system is constantly adapting to changes in the environment and compares the various colors in a scene to reduce the effects of the illumination. If a scene is illuminated with one light, and then with another, as long as the difference between the light sources stays within a reasonable range, the colors in the scene appear relatively constant to us. This was studied by Edwin Land in the 1970s and led to his retinex theory of color constancy.
It should be noted, that both phenomena described above are readily explained and mathematical modeled with modern theories of chromatic adaptation and color appearance (e.g., CIECAM02, iCAM).[7] There is no need to dismiss the trichromatic theory of vision, but rather it must be enhanced with an understanding of how the visual system adapts (adjusts) to changes in the viewing environment.
Color naming
Colors vary in several different ways, including hue (shades of red, orange, yellow, green, blue, and violet), saturation, brightness, and gloss. Some color words are derived from the name of an object of that color, such as "orange" or "salmon", while others are abstract, like "red".
Different cultures have different terms for colors, and may also assign some color names to slightly different parts of the spectrum: for instance, the Chinese character 青 (rendered as qīng in Mandarin and ao in Japanese) has a meaning that covers both blue and green; blue and green are traditionally considered shades of "青." South Korea, on the other hand, differentiates between blue and green by using "綠 (녹)" for green and "靑 (청)" for blue.
In the 1969 study Basic Color Terms: Their Universality and Evolution, Brent Berlin and Paul Kay describe a pattern in naming "basic" colors (like "red" but not "red-orange" or "dark red" or "blood red", which are "shades" of red). All languages that have two "basic" color names distinguish dark/cool colors from bright/warm colors. The next colors to be distinguished are usually red and then yellow or green. All languages with six "basic" colors include black, white, red, green, blue and yellow. The pattern holds up to a set of twelve: black, gray, white, pink, red, orange, yellow, green, blue, purple, brown, and azure (distinct from blue in Russian and Italian but not English).
Associations
Individual colors have a variety of cultural associations such as national colors (in general described in individual color articles and color symbolism). The field of color psychology attempts to identify the effects of color on human emotion and activity. Chromotherapy is a form of alternative medicine attributed to various Eastern traditions. Colors have different associations in different countries and cultures.[8]
Different colors have been demonstrated to have affects on cognition. For example, researchers at the University of Linz in Austria demonstrated that the color red significantly decreases cognitive functioning in men.[9]
Spectral colors and color reproduction

Most light sources are mixtures of various wavelengths of light. However, many such sources can still have a spectral color insofar as the eye cannot distinguish them from monochromatic sources. For example, most computer displays reproduce the spectral color orange as a combination of red and green light; it appears orange because the red and green are mixed in the right proportions to allow the eye's red and green cones to respond the way they do to orange.
A useful concept in understanding the perceived color of a non-monochromatic light source is the dominant wavelength, which identifies the single wavelength of light that produces a sensation most similar to the light source. Dominant wavelength is roughly akin to hue.
There are many color perceptions that by definition cannot be pure spectral colors due to desaturation or because they are purples (mixtures of red and violet light, from opposite ends of the spectrum). Some examples of necessarily non-spectral colors are the achromatic colors (black, gray and white) and colors such as pink, tan, and magenta.
Two different light spectra that have the same effect on the three color receptors in the human eye will be perceived as the same color. This is exemplified by the white light emitted by fluorescent lamps, which typically has a spectrum of a few narrow bands, while daylight has a continuous spectrum. The human eye cannot tell the difference between such light spectra just by looking into the light source, although reflected colors from objects can look different. (This is often exploited e.g. to make fruit or tomatoes look more intensely red.)
Similarly, most human color perceptions can be generated by a mixture of three colors called primaries. This is used to reproduce color scenes in photography, printing, television and other media. There are a number of methods or color spaces for specifying a color in terms of three particular primary colors. Each method has its advantages and disadvantages depending on the particular application.
No mixture of colors, though, can produce a fully pure color perceived as completely identical to a spectral color, although one can get very close for the longer wavelengths, where the chromaticity diagram above has a nearly straight edge. For example, mixing green light (530 nm) and blue light (460 nm) produces cyan light that is slightly desaturated, because response of the red color receptor would be greater to the green and blue light in the mixture than it would be to a pure cyan light at 485 nm that has the same intensity as the mixture of blue and green.
Because of this, and because the primaries in color printing systems generally are not pure themselves, the colors reproduced are never perfectly saturated colors, and so spectral colors cannot be matched exactly. However, natural scenes rarely contain fully saturated colors, thus such scenes can usually be approximated well by these systems. The range of colors that can be reproduced with a given color reproduction system is called the gamut. The CIE chromaticity diagram can be used to describe the gamut.
Another problem with color reproduction systems is connected with the acquisition devices, like cameras or scanners. The characteristics of the color sensors in the devices are often very far from the characteristics of the receptors in the human eye. In effect, acquisition of colors that have some special, often very "jagged," spectra caused for example by unusual lighting of the photographed scene can be relatively poor.
Species that have color receptors different from humans, e.g. birds that may have four receptors, can differentiate some colors that look the same to a human. In such cases, a color reproduction system 'tuned' to a human with normal color vision may give very inaccurate results for the other observers.
The different color response of different devices can be problematic if not properly managed. For color information stored and transferred in digital form, color management techniques, such as those based on ICC profiles, can help to avoid distortions of the reproduced colors. Color management does not circumvent the gamut limitations of particular output devices, but can assist in finding good mapping of input colors into the gamut that can be reproduced.
Pigments and reflective media
Pigments are chemicals that selectively absorb and reflect different spectra of light. When a surface is painted with a pigment, light hitting the surface is reflected, minus some wavelengths. This subtraction of wavelengths produces the appearance of different colors. Most paints are a blend of several chemical pigments, intended to produce a reflection of a given color.
Pigment manufacturers assume the source light will be white, or of roughly equal intensity across the spectrum. If the light is not a pure white source (as in the case of nearly all forms of artificial lighting), the resulting spectrum will appear a slightly different color. Red paint, viewed under blue light, may appear black. Red paint is red because it reflects only the red components of the spectrum. Blue light, containing none of these, will create no reflection from red paint, creating the appearance of black.
Structural color
Structural colors are colors caused by interference effects rather than by pigments. Color effects are produced when a material is scored with fine parallel lines, formed of one or more parallel thin layers, or otherwise composed of microstructures on the scale of the color's wavelength. If the microstructures are spaced randomly, light of shorter wavelengths will be scattered preferentially to produce Tyndall effect colors: the blue of the sky (Rayleigh scattering, caused by structures much smaller than the wavelength of light, in this case air molecules), the luster of opals, and the blue of human irises. If the microstructures are aligned in arrays, for example the array of pits in a CD, they behave as a diffraction grating: the grating reflects different wavelengths in different directions due to interference phenomena, separating mixed "white" light into light of different wavelengths. If the structure is one or more thin layers then it will reflect some wavelengths and transmit others, depending on the layers' thickness.
Structural color is studied in the field of thin-film optics. A layman's term that describes particularly the most ordered or the most changeable structural colors is iridescence. Structural color is responsible for the blues and greens of the feathers of many birds (the blue jay, for example), as well as certain butterfly wings and beetle shells. Variations in the pattern's spacing often give rise to an iridescent effect, as seen in peacock feathers, soap bubbles, films of oil, and mother of pearl, because the reflected color depends upon the viewing angle. Numerous scientists have carried out research in butterfly wings and beetle shells, including Isaac Newton and Robert Hooke. Since 1942, electron micrography has been used, advancing the development of products that exploit structural color, such as "photonic" cosmetics.[10]
Additional terms
- Colorfulness, chroma, purity, or saturation: how "intense" or "concentrated" a color is. Technical definitions distinguish between colorfulness, chroma, and saturation as distinct perceptual attributes and include purity as a physical quantity. These terms, and others related to light and color are internationally agreed upon and published in the CIE Lighting Vocabulary.[11] More readily available texts on colorimetry also define and explain these terms.[12][13]
- Dichromatism: a phenomenon where the hue is dependent on concentration and/or thickness of the absorbing substance.
- Hue: the color's direction from white, for example in a color wheel or chromaticity diagram.
- Shade: a color made darker by adding black.
- Tint: a color made lighter by adding white.
- Value, brightness, lightness, or luminosity: how light or dark a color is.
See also
- CIECAM02
- Color mapping
- Complementary color
- Impossible color
- International Color Consortium
- International Commission on Illumination
- List of colors
- List of colors (compact)
- Neutral color
- Pearlescent coating including Metal effect pigments
- Primary color
- Rainbow
- Secondary color
- Tertiary color
References
- ^ Hermann von Helmholtz, Physiological Optics – The Sensations of Vision, 1866, as translated in Sources of Color Science, David L. MacAdam, ed., Cambridge: MIT Press, 1970.
- ^ Palmer, S.E. (1999). Vision Science: Photons to Phenomenology, Cambridge, MA: MIT Press. ISBN 0-262-16183-4.
- ^ a b Judd, Deane B. (1975). Color in Business, Science and Industry. Wiley Series in Pure and Applied Optics (third ed.). New York: Wiley-Interscience. p. 388. ISBN 0471452122.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Under well-lit viewing conditions (photopic vision), cones ...are highly active and rods are inactive." Hirakawa, K. (2005). Chromatic Adaptation and White-Balance Problem (PDF). IEEE ICIP. doi:10.1109/ICIP.2005.1530559.
{{cite conference}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Jameson, K. A., Highnote, S. M., & Wasserman, L. M. (2001). "Richer color experience in observers with multiple photopigment opsin genes" (PDF). Psychonomic Bulletin and Review. 8 (2): 244–261. doi:10.1038/351652a0. PMID 1904993.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Depauw, Robert C. "United States Patent". Retrieved 20 March 2011.
- ^ M.D. Fairchild, Color Appearance Models, 2nd Ed., Wiley, Chichester (2005).
- ^ "Chart: Color Meanings by Culture". Retrieved 2010-06-29.
- ^ Gnambs, Timo; Appel, Markus; Batinic, Bernad. (2010). Color red in web-based knowledge testing. Computers in Human Behavior, 26, p1625-1631.
- ^ "Economic and Social Research Council - Science in the Dock, Art in the Stocks". Retrieved 2007-10-07.
- ^ CIE Pub. 17-4, International Lighting Vocabulary, 1987. http://www.cie.co.at/publ/abst/17-4-89.html
- ^ R.S. Berns, Principles of Color Technology, 3rd Ed., Wiley, New York (2001).
- ^ M.D. Fairchild, Color Appearance Models, 2nd Ed., Wiley, Chichester (2005).
External links and sources
- Bibliography Database on Color Theory, Buenos Aires University
- Barry Maund. "Color". In Zalta, Edward N. (ed.). Stanford Encyclopedia of Philosophy.
- Why Should Engineers and Scientists Be Worried About Color?
- Robert Ridgway's A Nomenclature of Colors (1886) and Color Standards and Color Nomenclature (1912) - text-searchable digital facsimiles at Linda Hall Library
- Albert Henry Munsell's A Color Notation, (1907) at Project Gutenberg