Medical ultrasound: Difference between revisions
→Displaying the image: removed an obsolete method |
|||
| Line 134: | Line 134: | ||
=== Displaying the image === |
=== Displaying the image === |
||
Images from the |
Images from the ultrasound scanner are transferred and displayed using the [[DICOM]] standard. Normally, very little post processing is applied to ultrasound images. |
||
== Sound in the body == |
== Sound in the body == |
||
Revision as of 19:54, 10 October 2014
| Medical ultrasonography | |
|---|---|
 Sonographer doing pediatric echocardiography | |
| ICD-10-PCS | B?4 |
| ICD-9-CM | 88.7 |
| MeSH | D014463 |
| OPS-301 code | 3-03...3-05 |

Diagnostic sonography (ultrasonography) is an ultrasound-based diagnostic imaging technique used for visualizing internal body structures including tendons, muscles, joints, vessels and internal organs for possible pathology or lesions. The practice of examining pregnant women using ultrasound is called obstetric sonography, and is widely used.
In physics, 'ultrasound' refers to sound waves with a frequency too high for humans to hear. Ultrasound images (sonograms) are made by sending a pulse of ultrasound into tissue using an ultrasound transducer (probe). The sound reflects (echoes) from parts of the tissue; these echoes are recorded and displayed as an image to the operator.
Many different types of images can be formed using ultrasound. The most well-known type is a B-mode image, which displays a two-dimensional cross-section of the tissue being imaged. Other types of image can display blood flow, motion of tissue over time, the location of blood, the presence of specific molecules, the stiffness of tissue, or the anatomy of a three-dimensional region.
Compared to other prominent methods of medical imaging, ultrasonography has several advantages. It provides images in real-time (rather than after an acquisition or processing delay), it is portable and can be brought to a sick patient's bedside, it is substantially lower in cost, and it does not use harmful ionizing radiation. Drawbacks of ultrasonography include various limits on its field of view including difficulty imaging structures behind bone and air, and its relative dependence on a skilled operator.
Diagnostic applications

Typical diagnostic sonographic scanners operate in the frequency range of 1 to 18 megahertz, though frequencies up to 50–100 megahertz have been used experimentally in a technique known as biomicroscopy in special regions, such as the anterior chamber of the eye.[3] The choice of frequency is a trade-off between spatial resolution of the image and imaging depth: lower frequencies produce less resolution but image deeper into the body. Higher frequency sound waves have a smaller wavelength and thus are capable of reflecting or scattering from smaller structures. Higher frequency sound waves also have a larger attenuation coefficient and thus are more readily absorbed in tissue, limiting the depth of penetration of the sound wave into the body (for details, see Acoustic attenuation.)
Sonography (ultrasonography) is widely used in medicine. It is possible to perform both diagnosis and therapeutic procedures, using ultrasound to guide interventional procedures (for instance biopsies or drainage of fluid collections). Sonographers are medical professionals who perform scans which are then typically interpreted by radiologists, physicians who specialize in the application and interpretation of a wide variety of medical imaging modalities, or by cardiologists in the case of cardiac ultrasonography (echocardiography). Sonographers typically use a hand-held probe (called a transducer) that is placed directly on and moved over the patient. Increasingly, clinicians (physicians and other healthcare professionals who provide direct patient care) are using ultrasound in their office and hospital practices.
Sonography is effective for imaging soft tissues of the body. Superficial structures such as muscles, tendons, testes, breast, thyroid and parathyroid glands, and the neonatal brain are imaged at a higher frequency (7–18 MHz), which provides better axial and lateral resolution. Deeper structures such as liver and kidney are imaged at a lower frequency 1–6 MHz with lower axial and lateral resolution but greater penetration.
Medical sonography is used in the study of many different systems:
| System/Specialty | Description | See also |
|---|---|---|
| Anesthesiology | Ultrasound is commonly used by anesthesiologists (Anaesthetists) to guide injecting needles when placing local anaesthetic solutions near nerves | |
| Angiology | Duplex ultrasound (B Mode vessels imaging combined with Doppler flow measurement) is daily used in angiology to diagnose arterial and venous disease all over the body. | see angiology |
| Cardiology | Echocardiography is an essential tool in cardiology, to diagnose e.g. dilatation of parts of the heart and function of heart ventricles and valves | see echocardiography |
| Emergency Medicine | Point of care ultrasound has many applications in the Emergency Department, including the Focused Assessment with Sonography for Trauma (FAST) exam for assessing significant hemoperitoneum or pericardial tamponade after trauma. Ultrasound is routinely used in the Emergency Department to expedite the care of patients with right upper quadrant abdominal pain who may have gallstones or cholecystitis. | see FAST exam, Emergency ultrasound. |
| Gastroenterology/Colorectal surgery | In abdominal sonography, the solid organs of the abdomen such as the pancreas, aorta, inferior vena cava, liver, gall bladder, bile ducts, kidneys, and spleen are imaged. Sound waves are blocked by gas in the bowel and attenuated in different degree by fat, therefore there are limited diagnostic capabilities in this area. The appendix can sometimes be seen when inflamed (as in e.g.: appendicitis). Endoanal ultrasound is used particularly in the investigation of anorectal symptoms such as fecal incontinence or obstructed defecation. It images the immediate perianal anatomy and is able to detect occult defects such as tearing of the anal sphincter. | See abdominal ultrasonography, Endoanal ultrasound |
| Gynecology | see gynecologic ultrasonography | |
| Head and Neck Surgery/Otolaryngology | Most structures of the neck, including the thyroid and parathryoid glands, lymph nodes, and salivary glands, are well-visualized by high-frequency ultrasound with exceptional anatomic detail. Ultrasound is the preferred imaging modality for thyroid tumors and lesions, and ultrasonography is critical in the evaluation, preoperative planning, and postoperative surveillance of patients with thyroid cancer. Many other benign and malignant conditions in the head and neck can be evaluated and managed with the help of diagnostic ultrasound and ultrasound-guided procedures. | |
| Neonatology | for basic assessment of intracerebral structural abnormalities, bleeds, ventriculomegaly or hydrocephalus and anoxic insults (Periventricular leukomalacia). The ultrasound can be performed through the soft spots in the skull of a newborn infant (Fontanelle) until these completely close at about 1 year of age and form a virtually impenetrable acoustic barrier for the ultrasound. The most common site for cranial ultrasound is the anterior fontanelle. The smaller the fontanelle, the poorer the quality of the picture. | Intracerebral: see Transcranial Doppler |
| Neurology | for assessing blood flow and stenoses in the carotid arteries (Carotid ultrasonography) and the big intracerebral arteries | see Carotid ultrasonography. Intracerebral: see Transcranial Doppler |
| Obstetrics | Obstetrical sonography is commonly used during pregnancy to check on the development of the fetus. | see obstetric ultrasonography |
| Ophthalmology | Ultrasound images of the eyes, also known as ocular ultrasonography | see A-scan ultrasonography, B-scan ultrasonography |
| Pulmonology | Endobronchial Ultrasound (EBUS) probes are applied to standard flexible endoscopic probes and used by pulmonologists to allow for direct visualization of endobronchial lesions and lymph nodes prior to transbronchial needle aspiration. Among its many uses, EBUS aids in lung cancer staging by allowing for lymph node sampling without the need for major surgery.[4] | |
| Urology | to determine, for example, the amount of fluid retained in a patient's bladder. In a pelvic sonogram, organs of the pelvic region are imaged. This includes the uterus and ovaries or urinary bladder. Males are sometimes given a pelvic sonogram to check on the health of their bladder, the prostate, or their testicles (for example to distinguish epididymitis from testicular torsion). In young males, it is used to distinguish more benign testicular masses (varicocele or hydrocele) from testicular cancer, which is highly curable but which must be treated to preserve health and fertility. There are two methods of performing a pelvic sonography – externally or internally. The internal pelvic sonogram is performed either transvaginally (in a woman) or transrectally (in a man). Sonographic imaging of the pelvic floor can produce important diagnostic information regarding the precise relationship of abnormal structures with other pelvic organs and it represents a useful hint to treat patients with symptoms related to pelvic prolapse, double incontinence and obstructed defecation. It is used to diagnose and, at higher frequencies, to treat (break up) kidney stones or kidney crystals (nephrolithiasis).[5] | |
| Musculoskeletal | tendons, muscles, nerves, ligaments, soft tissue masses, and bone surfaces[6] | |
| Cardiovascular system | To assess patency and possible obstruction of arteries Arterial sonography, diagnose deep vein thrombosis (Thrombosonography) and determine extent and severity of venous insufficiency (venosonography) | Intravascular ultrasound |
Other types of uses include:
- Interventional ultrasonography; biopsy, emptying fluids, intrauterine Blood transfusion (Hemolytic disease of the newborn)
- Contrast-enhanced ultrasound
A general-purpose sonographic machine may be used for most imaging purposes. Usually specialty applications may be served only by use of a specialty transducer. Most ultrasound procedures are done using a transducer on the surface of the body, but improved diagnostic confidence is often possible if a transducer can be placed inside the body. For this purpose, specialty transducers, including endovaginal, endorectal, and transesophageal transducers are commonly employed. At the extreme of this, very small transducers can be mounted on small diameter catheters and placed into blood vessels to image the walls and disease of those vessels.
From sound to image
The creation of an image from sound is done in three steps – producing a sound wave, receiving echoes, and interpreting those echoes.
Producing a sound wave

A sound wave is typically produced by a piezoelectric transducer encased in a plastic housing. Strong, short electrical pulses from the ultrasound machine drive the transducer at the desired frequency. The frequencies can be anywhere between 1 and 18 MHz. Older technology transducers focused their beam with physical lenses. Newer technology transducers use phased array techniques to enable the sonographic machine to change the direction and depth of focus.
The sound is focused either by the shape of the transducer, a lens in front of the transducer, or a complex set of control pulses from the ultrasound scanner machine (Beamforming). This focusing produces an arc-shaped sound wave from the face of the transducer. The wave travels into the body and comes into focus at a desired depth.
Materials on the face of the transducer enable the sound to be transmitted efficiently into the body (often a rubbery coating, a form of impedance matching). In addition, a water-based gel is placed between the patient's skin and the probe.
The sound wave is partially reflected from the layers between different tissues or scattered from smaller structures. Specifically, sound is reflected anywhere there are acoustic impedance changes in the body: e.g. blood cells in blood plasma, small structures in organs, etc. Some of the reflections return to the transducer.
Receiving the echoes
The return of the sound wave to the transducer results in the same process that it took to send the sound wave, except in reverse. The return sound wave vibrates the transducer, the transducer turns the vibrations into electrical pulses that travel to the ultrasonic scanner where they are processed and transformed into a digital image.
Forming the image
The sonographic scanner must determine three things from each received echo:
- How long it took the echo to be received from when the sound was transmitted.
- From this the focal length for the phased array is deduced, enabling a sharp image of that echo at that depth (this is not possible while producing a sound wave).
- How strong the echo was. It could be noted that sound wave is not a click, but a pulse with a specific carrier frequency. Moving objects change this frequency on reflection, so that it is only a matter of electronics to have simultaneous Doppler sonography.
Once the ultrasonic scanner determines these three things, it can locate which pixel in the image to light up and to what intensity and at what hue if frequency is processed (see redshift for a natural mapping to hue).
Transforming the received signal into a digital image may be explained by using a blank spreadsheet as an analogy. First picture a long, flat transducer at the top of the sheet. Send pulses down the 'columns' of the spreadsheet (A, B, C, etc.). Listen at each column for any return echoes. When an echo is heard, note how long it took for the echo to return. The longer the wait, the deeper the row (1,2,3, etc.). The strength of the echo determines the brightness setting for that cell (white for a strong echo, black for a weak echo, and varying shades of grey for everything in between.) When all the echoes are recorded on the sheet, we have a greyscale image.
Displaying the image
Images from the ultrasound scanner are transferred and displayed using the DICOM standard. Normally, very little post processing is applied to ultrasound images.
Sound in the body
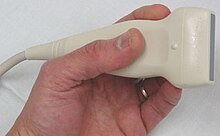
Ultrasonography (sonography) uses a probe containing multiple acoustic transducers to send pulses of sound into a material. Whenever a sound wave encounters a material with a different density (acoustical impedance), part of the sound wave is reflected back to the probe and is detected as an echo. The time it takes for the echo to travel back to the probe is measured and used to calculate the depth of the tissue interface causing the echo. The greater the difference between acoustic impedances, the larger the echo is. If the pulse hits gases or solids, the density difference is so great that most of the acoustic energy is reflected and it becomes impossible to see deeper.
The frequencies used for medical imaging are generally in the range of 1 to 18 MHz. Higher frequencies have a correspondingly smaller wavelength, and can be used to make sonograms with smaller details. However, the attenuation of the sound wave is increased at higher frequencies, so in order to have better penetration of deeper tissues, a lower frequency (3–5 MHz) is used.
Seeing deep into the body with sonography is very difficult. Some acoustic energy is lost every time an echo is formed, but most of it (approximately ) is lost from acoustic absorption. (See also Acoustic attenuation for further details on modeling of acoustic attenuation and absorption.)
The speed of sound varies as it travels through different materials, and is dependent on the acoustical impedance of the material. However, the sonographic instrument assumes that the acoustic velocity is constant at 1540 m/s. An effect of this assumption is that in a real body with non-uniform tissues, the beam becomes somewhat de-focused and image resolution is reduced.
To generate a 2D-image, the ultrasonic beam is swept. A transducer may be swept mechanically by rotating or swinging. Or a 1D phased array transducer may be used to sweep the beam electronically. The received data is processed and used to construct the image. The image is then a 2D representation of the slice into the body.
3D images can be generated by acquiring a series of adjacent 2D images. Commonly a specialised probe that mechanically scans a conventional 2D-image transducer is used. However, since the mechanical scanning is slow, it is difficult to make 3D images of moving tissues. Recently, 2D phased array transducers that can sweep the beam in 3D have been developed. These can image faster and can even be used to make live 3D images of a beating heart.
Doppler ultrasonography is used to study blood flow and muscle motion. The different detected speeds are represented in color for ease of interpretation, for example leaky heart valves: the leak shows up as a flash of unique color. Colors may alternatively be used to represent the amplitudes of the received echoes.
Modes of sonography
Several modes of ultrasound are used in medical imaging.[7][8] These are:
- A-mode: A-mode (amplitude mode) is the simplest type of ultrasound. A single transducer scans a line through the body with the echoes plotted on screen as a function of depth. Therapeutic ultrasound aimed at a specific tumor or calculus is also A-mode, to allow for pinpoint accurate focus of the destructive wave energy.
- B-mode or 2D mode: In B-mode (brightness mode) ultrasound, a linear array of transducers simultaneously scans a plane through the body that can be viewed as a two-dimensional image on screen. More commonly known as 2D mode now.
- C-mode: A C-mode image is formed in a plane normal to a B-mode image. A gate that selects data from a specific depth from an A-mode line is used; then the transducer is moved in the 2D plane to sample the entire region at this fixed depth. When the transducer traverses the area in a spiral, an area of 100 cm2 can be scanned in around 10 seconds.[8]
- M-mode: In M-mode (motion mode) ultrasound, pulses are emitted in quick succession – each time, either an A-mode or B-mode image is taken. Over time, this is analogous to recording a video in ultrasound. As the organ boundaries that produce reflections move relative to the probe, this can be used to determine the velocity of specific organ structures.
- Doppler mode: This mode makes use of the Doppler effect in measuring and visualizing blood flow
- Color Doppler: Velocity information is presented as a color-coded overlay on top of a B-mode image
- Continuous Doppler: Doppler information is sampled along a line through the body, and all velocities detected at each time point are presented (on a time line)
- Pulsed wave (PW) Doppler: Doppler information is sampled from only a small sample volume (defined in 2D image), and presented on a timeline
- Duplex: a common name for the simultaneous presentation of 2D and (usually) PW Doppler information. (Using modern ultrasound machines, color Doppler is almost always also used; hence the alternative name Triplex.)
- Pulse inversion mode: In this mode, two successive pulses with opposite sign are emitted and then subtracted from each other. This implies that any linearly responding constituent will disappear while gases with non-linear compressibility stand out. Pulse inversion may also be used in a similar manner as in Harmonic mode; see below:
- Harmonic mode: In this mode a deep penetrating fundamental frequency is emitted into the body and a harmonic overtone is detected. This way noise and artifacts due to reverberation and aberration are greatly reduced. Some also believe that penetration depth can be gained with improved lateral resolution; however, this is not well documented.
Expansions
An additional expansion or additional technique of ultrasound is biplanar ultrasound, in which the probe has two 2D planes that are perpendicular to each other, providing more efficient localization and detection.[9] Furthermore, an omniplane probe is one that can rotate 180° to obtain multiple images.[9] In 3D ultrasound, many 2D planes are digitally added together to create a 3-dimensional image of the object. In contrast-enhanced ultrasound, microbubble contrast agents enhance the ultrasound waves, resulting in increased contrast.
Doppler ultrasonography



Sonography can be enhanced with Doppler measurements, which employ the Doppler effect to assess whether structures (usually blood)[10] are moving towards or away from the probe, and its relative velocity. By calculating the frequency shift of a particular sample volume, for example flow in an artery or a jet of blood flow over a heart valve, its speed and direction can be determined and visualised. This is particularly useful in cardiovascular studies (sonography of the vascular system and heart) and essential in many areas such as determining reverse blood flow in the liver vasculature in portal hypertension. The Doppler information is displayed graphically using spectral Doppler, or as an image using color Doppler (directional Doppler) or power Doppler (non directional Doppler). This Doppler shift falls in the audible range and is often presented audibly using stereo speakers: this produces a very distinctive, although synthetic, pulsating sound.
Most modern sonographic machines use pulsed Doppler to measure velocity. Pulsed wave machines transmit and receive series of pulses. The frequency shift of each pulse is ignored, however the relative phase changes of the pulses are used to obtain the frequency shift (since frequency is the rate of change of phase). The major advantages of pulsed Doppler over continuous wave is that distance information is obtained (the time between the transmitted and received pulses can be converted into a distance with knowledge of the speed of sound) and gain correction is applied. The disadvantage of pulsed Doppler is that the measurements can suffer from aliasing. The terminology "Doppler ultrasound" or "Doppler sonography", has been accepted to apply to both pulsed and continuous Doppler systems despite the different mechanisms by which the velocity is measured.
It should be noted here that there are no standards for the display of color Doppler. Some laboratories show arteries as red and veins as blue, as medical illustrators usually show them, even though some vessels may have portions flowing towards and portions flowing away from the transducer. This results in the illogical appearance of a vessel being partly a vein and partly an artery. Other laboratories use red to indicate flow toward the transducer and blue away from the transducer. Still other laboratories prefer to display the sonographic Doppler color map more in accord with the prior published physics with the red shift representing longer waves of echoes (scattered) from blood flowing away from the transducer; and with blue representing the shorter waves of echoes reflecting from blood flowing toward the transducer. Because of this confusion and lack of standards in the various laboratories, the sonographer must understand the underlying acoustic physics of color Doppler and the physiology of normal and abnormal blood flow in the human body (see Red shift[11][12][13]).
Although Angiography and Venography which both use X-ray and contrast injection material are more accurate than Doppler Sonography, Doppler Sonography may be chosen because it is faster, less expensive, and non-invasive.[14]
Contrast ultrasonography (ultrasound contrast imaging)
A contrast medium for medical ultrasonography is a formulation of encapsulated gaseous microbubbles[15] to increase echogenicity of blood, discovered by Dr Raymond Gramiak in 1968[16] and named contrast-enhanced ultrasound. This contrast medical imaging modality is clinically used throughout the world,[17] in particular for echocardiography in the USA and for ultrasound radiology in Europe and Asia.
Microbubbles-based contrast media is administrated intravenously in patient blood stream during the medical ultrasonography examination. The microbubbles being too large in diameter, they stay confined in blood vessels and cannot extravasate towards the interstitial fluid. An ultrasound contrast media is therefore purely intravascular, making it an ideal agent to image organ microvascularization for diagnostic purposes. A typical clinical use of contrast ultrasonography is detection of a hypervascular metastatic tumor, which exhibits a contrast uptake (kinetics of microbubbles concentration in blood circulation) faster than healthy biological tissue surrounding the tumor.[18] Other clinical applications using contrast exist, such as in echocardiography to improve delineation of left ventricle for visually checking contractibility of heart after a myocardial infarction. Finally, applications in quantitative perfusion[19] (relative measurement of blood flow [20]) emerge for identifying early patient response to an anti-cancerous drug treatment (methodology and clinical study by Dr Nathalie Lassau in 2011[21]), enabling to determine the best oncological therapeutic options.[22]

In oncological practice of medical contrast ultrasonography, clinicians use the method of parametric imaging of vascular signatures[23] invented by Dr Nicolas Rognin in 2010.[24] This method is conceived as a cancer aided diagnostic tool, facilitating characterization of a suspicious tumor (malignant versus benign) in an organ. This method is based on medical computational science [25][26] to analyze a time sequence of ultrasound contrast images, a digital video recorded in real-time during patient examination. Two consecutive signal processing steps are applied to each pixel of the tumor:
- calculation of a vascular signature (contrast uptake difference with respect to healthy tissue surrounding the tumor);
- automatic classification of the vascular signature into a unique parameter, this last coded in one of the four following colors:
- green for continuous hyper-enhancement (contrast uptake higher than healthy tissue one),
- blue for continuous hypo-enhancement (contrast uptake lower than healthy tissue one),
- red for fast hyper-enhancement (contrast uptake before healthy tissue one) or
- yellow for fast hypo-enhancement (contrast uptake after healthy tissue one).
Once signal processing in each pixel completed, a color spatial map of the parameter is displayed on a computer monitor, summarizing all vascular information of the tumor in a single image called parametric image (see last figure of press article [27] as clinical examples). This parametric image is interpreted by clinicians based on predominant colorization of the tumor: red indicates a suspicion of malignancy (risk of cancer), green or yellow – a high probability of benignity. In the first case (suspicion of malignant tumor), the clinician typically prescribes a biopsy to confirm the diagnostic or a CT scan examination as a second opinion. In the second case (quasi-certain of benign tumor), only a follow-up is needed with a contrast ultrasonography examination a few months later. The main clinical benefits are to avoid a systematic biopsy (risky invasive procedure) of benign tumors or a CT scan examination exposing the patient to X-ray radiation. The parametric imaging of vascular signatures method proved to be effective in humans for characterization of tumors in the liver.[28] In a cancer screening context, this method might be potentially applicable to other organs such as breast[29] or prostate.
Molecular ultrasonography (ultrasound molecular imaging)
The future of contrast ultrasonography is in molecular imaging with potential clinical applications expected in cancer screening to detect malignant tumors at their earliest stage of appearance. Molecular ultrasonography (or ultrasound molecular imaging) uses targeted microbubbles originally designed by Dr Alexander Klibanov in 1997;[30][31] such targeted microbubbles specifically bind or adhere to tumoral microvessels by targeting biomolecular cancer expression (overexpression of certain biomolecules occurs during neo-angiogenesis[32][33] or inflammation[34] processes in malignant tumors). As a result, a few minutes after their injection in blood circulation, the targeted microbubbles accumulate in the malignant tumor; facilitating its localization in a unique ultrasound contrast image. In 2013, the very first exploratory clinical trial in humans for prostate cancer was completed at Amsterdam in the Netherlands by Dr Hessel Wijkstra.[35]
In molecular ultrasonography, the technique of acoustic radiation force (also used for shear wave elastography) is applied in order to literally push the targeted microbubbles towards microvessels wall; firstly demonstrated by Dr Paul Dayton in 1999.[36] This allows to maximize binding to the malignant tumor; the targeted microbubbles being in more direct contact with cancerous biomolecules expressed at the inner surface of tumoral microvessels. At the stage of scientific preclinical research, the technique of acoustic radiation force was implemented as a prototype in clinical ultrasound systems and validated in vivo in 2D[37] and 3D[38][39] imaging modes.
Elastography (ultrasound elasticity imaging)
Ultrasound is also used for elastography. This can be useful in medical diagnoses, as elasticity can discern healthy from unhealthy tissue for specific organs/growths. In some cases unhealthy tissue may have a lower system Q, meaning that the system acts more like a large heavy spring as compared to higher values of system Q (healthy tissue) that respond to higher forcing frequencies. Ultrasonic elastography is different from conventional ultrasound, as a transceiver (pair) and a transmitter are used instead of only a transceiver. One transducer acts as both the transmitter and receiver to image the region of interest over time. The extra transmitter is a very low frequency transmitter, and perturbs the system so the unhealthy tissue oscillates at a low frequency and the healthy tissue does not. The transceiver, which operates at a high frequency (typically MHz) then measures the displacement of the unhealthy tissue (oscillating at a much lower frequency). The movement of the slowly oscillating tissue is used to determine the elasticity of the material, which can then be used to distinguish healthy tissue from the unhealthy tissue.
Compression ultrasonography
Compression ultrasonography is a simplified technique used for quick deep vein thrombosis diagnosis. The examination is limited to common femoral vein and popliteal vein only, instead to spend time performing the full examination, lower limbs venous ultrasonography. It is performed using only one test: vein compression.[40]
Compression ultrasonography has both high sensitivity and specificity for detecting proximal deep vein thrombosis only in symptomatic patients. Results are not reliable when the patient is symptomless and must be checked, for example in high risk postoperative patients mainly in orthopedic patients.[41][42]
Attributes
As with all imaging modalities, ultrasonography has its list of positive and negative attributes.
Strengths
- It images muscle, soft tissue, and bone surfaces very well and is particularly useful for delineating the interfaces between solid and fluid-filled spaces.
- It renders "live" images, where the operator can dynamically select the most useful section for diagnosing and documenting changes, often enabling rapid diagnoses. Live images also allow for ultrasound-guided biopsies or injections, which can be cumbersome with other imaging modalities.
- It shows the structure of organs.
- It has no known long-term side effects and rarely causes any discomfort to the patient.
- Equipment is widely available and comparatively flexible.
- Small, easily carried scanners are available; examinations can be performed at the bedside.
- Relatively inexpensive compared to other modes of investigation, such as computed X-ray tomography, DEXA or magnetic resonance imaging.
- Spatial resolution is better in high frequency ultrasound transducers than it is in most other imaging modalities.
- Through the use of an Ultrasound research interface, an ultrasound device can offer a relatively inexpensive, real-time, and flexible method for capturing data required for special research purposes for tissue characterization and development of new image processing techniques
Weaknesses

- Sonographic devices have trouble penetrating bone. For example, sonography of the adult brain is very limited though improvements are being made in transcranial ultrasonography.
- Sonography performs very poorly when there is a gas between the transducer and the organ of interest, due to the extreme differences in acoustic impedance. For example, overlying gas in the gastrointestinal tract often makes ultrasound scanning of the pancreas difficult, and lung imaging is not possible (apart from demarcating pleural effusions).
- Even in the absence of bone or air, the depth penetration of ultrasound may be limited depending on the frequency of imaging. Consequently, there might be difficulties imaging structures deep in the body, especially in obese patients.
- Body habitus has a large influence on image quality. Image quality and accuracy of diagnosis is limited with obese patients, overlying subcutaneous fat attenuates the sound beam and a lower frequency transducer is required (with lower resolution)
- The method is operator-dependent. A high level of skill and experience is needed to acquire good-quality images and make accurate diagnoses.
- There is no scout image as there is with CT and MRI. Once an image has been acquired there is no exact way to tell which part of the body was imaged.
Risks and side-effects
Ultrasonography is generally considered a safe imaging modality.[43]
Diagnostic ultrasound studies of the fetus are generally considered to be safe during pregnancy. This diagnostic procedure should be performed only when there is a valid medical indication, and the lowest possible ultrasonic exposure setting should be used to gain the necessary diagnostic information under the "as low as reasonably practicable" or ALARP principle.
World Health Organizations technical report series 875 (1998).[44] supports that ultrasound is harmless: "Diagnostic ultrasound is recognized as a safe, effective, and highly flexible imaging modality capable of providing clinically relevant information about most parts of the body in a rapid and cost-effective fashion". Although there is no evidence ultrasound could be harmful for the fetus, US Food and Drug Administration views promotion, selling, or leasing of ultrasound equipment for making "keepsake fetal videos" to be an unapproved use of a medical device.
Medical ultrasonography should not be performed without a medical indication to perform it. To do otherwise would be to perform unnecessary health care to patients, which bring unwarranted costs and may lead to other testing. Overuse of ultrasonography is reported in the United States, especially as routine screening for deep vein thrombosis after orthopedic surgeries in patients who are not at heightened risk for having that condition.[45]
Studies on the safety of ultrasound
- A meta-analysis of several ultrasonography studies published in 2000 found no statistically significant harmful effects from ultrasonography, but mentioned that there was a lack of data on long-term substantive outcomes such as neurodevelopment.[46]
- A study at the Yale School of Medicine published in 2006 found a small but significant correlation between prolonged and frequent use of ultrasound and abnormal neuronal migration in mice.[47]
- A study performed in Sweden in 2001[48] has shown that subtle effects of neurological damage linked to ultrasound were implicated by an increased incidence in left-handedness in boys (a marker for brain problems when not hereditary) and speech delays.[49][50]
- The above findings, however, were not confirmed in a later follow-up study.[51]
- A later study, however, performed on a larger sample of 8865 children, has established a statistically significant, albeit weak association of ultrasonography exposure and being non-right handed later in life.[52] (See Handedness#Ultrasound).
Obstetric ultrasound
Obstetric ultrasound can be used to identify many conditions that would be harmful to the mother and the baby. Many health care professionals consider the risk of leaving these conditions undiagnosed to be much greater than the very small risk, if any, associated with undergoing an ultrasound scan.
Sonography is used routinely in obstetric appointments during pregnancy, but the FDA discourages its use for non-medical purposes such as fetal keepsake videos and photos, even though it is the same technology used in hospitals.[53]
Obstetric ultrasound is primarily used to:
- Date the pregnancy (gestational age)
- Confirm fetal viability
- Determine location of fetus, intrauterine vs ectopic
- Check the location of the placenta in relation to the cervix
- Check for the number of fetuses (multiple pregnancy)
- Check for major physical abnormalities.
- Assess fetal growth (for evidence of intrauterine growth restriction (IUGR))
- Check for fetal movement and heartbeat.
- Determine the sex of the baby
Its results are occasionally incorrect, producing a false positive (the Cochrane Collaboration is a relevant effort to improve the reliability of health care trials). False detection may result in patients being warned of birth defects when no such defect exists. Sex determination is only accurate after 12 weeks gestation. When balancing risk and reward, there are recommendations to avoid the use of routine ultrasound for low risk pregnancies. In many countries ultrasound is used routinely in the management of all pregnancies.
According to the European Committee of Medical Ultrasound Safety (ECMUS)[54]
Ultrasonic examinations should only be performed by competent personnel who are trained and updated in safety matters. Ultrasound produces heating, pressure changes and mechanical disturbances in tissue. Diagnostic levels of ultrasound can produce temperature rises that are hazardous to sensitive organs and the embryo/fetus. Biological effects of non-thermal origin have been reported in animals but, to date, no such effects have been demonstrated in humans, except when a microbubble contrast agent is present.
Nonetheless, care should be taken to use low power settings and avoid pulsed wave scanning of the fetal brain unless specifically indicated in high risk pregnancies.
Ultrasound scanners have different Doppler-techniques to visualize arteries and veins. The most common is colour doppler or power doppler, but also other techniques like b-flow are used to show bloodflow in an organ. By using pulsed wave doppler or continuous wave doppler bloodflow velocities can be calculated.
Figures released for the period 2005–2006 by the UK Government (Department of Health) show that non-obstetric ultrasound examinations constituted more than 65% of the total number of ultrasound scans conducted.
Society and Culture
Recent studies have stressed the importance of framing “reproductive health matters cross-culturally”, particularly when understanding the “new phenomenon” of “the proliferation of ultrasound imaging” in developing countries.[55] In 2007 Tine Gammeltoft interviewed 400 women in Hanoi’s “obstetrics and Gynaecology Hospital”, each “had an average of 6.6 scans during her pregnancy”, much higher than five years ago when in Vietnam, “a pregnant woman might or might not have had a single scan during her pregnancy”.[55] Gammeltoft explains that “many Asian countries” see “the foetus as an ambiguous being” unlike in Western medicine where it is common to think of the foetus as “materially stable”.[55] Therefore, although women, particularly in Asian countries, “express intense uncertainties regarding the safety and credibility of this technology”, it is overused for its “immediate reassurance”.[55]
Regulation
Diagnostic and therapeutic ultrasound equipment is regulated in the USA by the FDA, and worldwide by other national regulatory agencies. The FDA limits acoustic output using several metrics. Generally other regulatory agencies around the world accept the FDA-established guidelines.
Currently New Mexico is the only state in the USA which regulates diagnostic medical sonographers. Certification examinations for sonographers are available in the US from three organizations: the American Registry for Diagnostic Medical Sonography, Cardiovascular Credentialing International and the American Registry of Radiologic Technologists.
The primary regulated metrics are MI (Mechanical Index) a metric associated with the cavitation bio-effect, and TI (Thermal Index) a metric associated with the tissue heating bio-effect. The FDA requires that the machine not exceed limits that they have established. This requires self-regulation on the part of the manufacturer in terms of the calibration of the machine. The established limits are reasonably conservative so as to maintain diagnostic ultrasound as a safe imaging modality.[56]
Ultrasound-based pre-natal care and sex screening technologies were launched in India in the 1980s. With concerns about its misuse for sex-selective abortion, the Government of India passed the Pre-natal Diagnostic Techniques Act (PNDT) in 1994 to regulate legal and illegal uses of ultrasound equipment.[57] This law was further amended into the Pre-Conception and Pre-natal Diagnostic Techniques (Regulation and Prevention of Misuse) (PCPNDT) Act in 2004 to deter and punish prenatal sex screening and sex selective abortion.[58] It is currently illegal and a punishable crime in India to determine or disclose the sex of a fetus using an ultrasound equipment.[59]
History
France
In his book "L'investigation vasculaire par ultrasonographie Doppler" (Ed Masson, 1977) [10] Dr Claude Franceschi laid down the Doppler Ultrasound fundamentals of the hemodynamics semiotics, which are still in use in current Doppler arterial and venous Duplex Ultrasound investigations.
Scotland
Parallel developments in Glasgow, Scotland by Professor Ian Donald and colleagues at the Glasgow Royal Maternity Hospital (GRMH) led to the first diagnostic applications of the technique. Donald was an obstetrician with a self-confessed "childish interest in machines, electronic and otherwise", who, having treated the wife of one of the company's directors, was invited to visit the Research Department of boilermakers Babcock & Wilcox at Renfrew, where he used their industrial ultrasound equipment to conduct experiments on various morbid anatomical specimens and assess their ultrasonic characteristics. Together with the medical physicist Tom Brown and fellow obstetrician Dr John MacVicar, Donald refined the equipment to enable differentiation of pathology in live volunteer patients. These findings were reported in The Lancet on 7 June 1958[60] as "Investigation of Abdominal Masses by Pulsed Ultrasound" – possibly one of the most important papers ever published in the field of diagnostic medical imaging.
At GRMH, Professor Donald and Dr James Willocks then refined their techniques to obstetric applications including fetal head measurement to assess the size and growth of the fetus. With the opening of the new Queen Mother's Hospital in Yorkhill in 1964, it became possible to improve these methods even further. Dr Stuart Campbell's pioneering work on fetal cephalometry led to it acquiring long-term status as the definitive method of study of foetal growth. As the technical quality of the scans was further developed, it soon became possible to study pregnancy from start to finish and diagnose its many complications such as multiple pregnancy, fetal abnormality and placenta praevia. Diagnostic ultrasound has since been imported into practically every other area of medicine.
Sweden
Medical ultrasonography was used in 1953 at Lund University by cardiologist Inge Edler and Carl Hellmuth Hertz, the son of Gustav Ludwig Hertz, who was a graduate student at the department of nuclear physics.
Edler had asked Hertz if it was possible to use radar to look into the body, but Hertz said this was impossible. However, he said, it might be possible to use ultrasonography. Hertz was familiar with using ultrasonic reflectoscopes for nondestructive materials testing, and together they developed the idea of using this method in medicine.
The first successful measurement of heart activity was made on October 29, 1953 using a device borrowed from the ship construction company Kockums in Malmö. On December 16 the same year, the method was used to generate an echo-encephalogram (ultrasonic probe of the brain). Edler and Hertz published their findings in 1954.[61]
United States
Ultrasonic energy was first applied to the human body for medical purposes by Dr George Ludwig at the Naval Medical Research Institute, Bethesda, Maryland in the late 1940s.[62][63] English born and educated John Wild (1914–2009) first used ultrasound to assess the thickness of bowel tissue as early as 1949: for his early work he has been described as the "father of medical ultrasound".[64]
In 1962, after about two years of work, Joseph Holmes, William Wright, and Ralph Meyerdirk developed the first compound contact B-mode scanner. Their work had been supported by U.S. Public Health Services and the University of Colorado. Wright and Meyerdirk left the University to form Physionic Engineering Inc., which launched the first commercial hand-held articulated arm compound contact B-mode scanner in 1963. This was the start of the most popular design in the history of ultrasound scanners.[65]
In the late 1960s Dr Gene Strandness and the bio-engineering group at the University of Washington conducted research on Doppler ultrasound as a diagnostic tool for vascular disease. Eventually, they developed technologies to use duplex imaging, or Doppler in conjunction with B-mode scanning, to view vascular structures in real-time, while also providing hemodynamic information.[66]
The first demonstration of color Doppler was by Geoff Stevenson, who was involved in the early developments and medical use of Doppler shifted ultrasonic energy.[67]
See also
References
- ^ Dubose, T. J. (1985). "Fetal Biometry: Vertical Calvarial Diameter and Calvarial Volume". Journal of Diagnostic Medical Sonography. 1 (5): 205. doi:10.1177/875647938500100504.
- ^ "3D BPD Correction". July 2000. Retrieved 2008-09-27.[dead link]
- ^ Pavlin, Charles; Foster, F. Stuart (1994). Ultrasound Biomicroscopy of the Eye. Springer. ISBN 0-387-94206-8.
- ^ Herth, F J F; Eberhardt, R; Vilmann, P; Krasnik, M; Ernst, A (2006). "Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes". Thorax. 61 (9): 795–8. doi:10.1136/thx.2005.047829. PMC 2117082. PMID 16738038.
- ^ Piloni, Vittorio Luigi; Spazzafumo, Liana (June 2007). "Sonography of the female pelvic floor:clinical indications and techniques". Pelviperineology. 26 (2): 59–65.
- ^ Arend CF. Ultrasound of the Shoulder. Porto Alegre: Master Medical Books; 2013. (Free access at ShoulderUS.com)[page needed]
- ^ The Gale Encyclopedia of Medicine, 2nd Edition, Vol. 1 A-B. p. 4
- ^ a b Cobbold, Richard S. C. (2007). Foundations of Biomedical Ultrasound. Oxford University Press. pp. 422–423. ISBN 978-0-19-516831-0.
- ^ a b Page 161 (part II > Two-dimensional Echocardiography) in: Reves, J. G.; Estafanous, Fawzy G.; Barash, Paul G. (2001). Cardiac anesthesia: principles and clinical practice. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-2195-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Claude Franceschi (1978). L'Investigation vasculaire par ultrasonographie doppler. Masson. ISBN 2-225-63679-6.
- ^ Ellis, George FR, Williams, Ruth M. (2000). Flat and Curved Space-Times (2nd ed.). Oxford University Press. ISBN 0-19-850656-2.
{{cite book}}: CS1 maint: multiple names: authors list (link)[page needed] - ^ Dubose, T. J.; Baker, A. L. (2009). "Confusion and Direction in Diagnostic Doppler Sonography". Journal of Diagnostic Medical Sonography. 25 (3): 173. doi:10.1177/8756479309335681.
- ^ "Doppler Ultrasound History". Retrieved January 25, 2008.
- ^ "Information and Resources Doppler Ultrasound". Retrieved July 22, 2013.
- ^ Schneider, Michel (1999). "Characteristics of SonoVue™". Echocardiography. 16 (7, Pt 2): 743–746. doi:10.1111/j.1540-8175.1999.tb00144.x. PMID 11175217.
- ^ Gramiak, Raymond; Shah, Pravin M. (1968). "Echocardiography of the Aortic Root". Investigative Radiology. 3 (5): 356–66. doi:10.1097/00004424-196809000-00011. PMID 5688346.
- ^ "CEUS Around the World - The International Contrast Ultrasound Society (ICUS)" (PDF). October 2013. Retrieved 2013-10-27.
- ^ Claudon, Michel; Dietrich, Christoph F.; Choi, Byung Ihn; Cosgrove, David O.; Kudo, Masatoshi; Nolsøe, Christian P.; Piscaglia, Fabio; Wilson, Stephanie R.; Barr, Richard G.; Chammas, Maria C.; Chaubal, Nitin G.; Chen, Min-Hua; Clevert, Dirk Andre; Correas, Jean Michel; Ding, Hong; Forsberg, Flemming; Fowlkes, J. Brian; Gibson, Robert N.; Goldberg, Barry B.; Lassau, Nathalie; Leen, Edward L.S.; Mattrey, Robert F.; Moriyasu, Fuminori; Solbiati, Luigi; Weskott, Hans-Peter; Xu, Hui-Xiong; World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound (2013). "Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver – Update 2012". Ultrasound in Medicine & Biology. 39 (2): 187–210. doi:10.1016/j.ultrasmedbio.2012.09.002. PMID 23137926.
- ^ Piscaglia, F.; Nolsøe, C.; Dietrich, C.; Cosgrove, D.; Gilja, O.; Bachmann Nielsen, M.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; Claudon, M.; Clevert, D.; Correas, J.; d'Onofrio, M.; Drudi, F.; Eyding, J.; Giovannini, M.; Hocke, M.; Ignee, A.; Jung, E.; Klauser, A.; Lassau, N.; Leen, E.; Mathis, G.; Saftoiu, A.; Seidel, G.; Sidhu, P.; Ter Haar, G.; Timmerman, D.; Weskott, H. (2011). "The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications". Ultraschall in der Medizin. 33 (1): 33–59. doi:10.1055/s-0031-1281676. PMID 21874631.
- ^ Tang, M.- X.; Mulvana, H.; Gauthier, T.; Lim, A. K. P.; Cosgrove, D. O.; Eckersley, R. J.; Stride, E. (2011). "Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability". Interface Focus. 1 (4): 520–39. doi:10.1098/rsfs.2011.0026. PMC 3262271. PMID 22866229.
- ^ Lassau, N.; Koscielny, S.; Chami, L.; Chebil, M.; Benatsou, B.; Roche, A.; Ducreux, M.; Malka, D.; Boige, V. (2010). "Advanced Hepatocellular Carcinoma: Early Evaluation of Response to Bevacizumab Therapy at Dynamic Contrast-enhanced US with Quantification--Preliminary Results". Radiology. 258 (1): 291–300. doi:10.1148/radiol.10091870. PMID 20980447.
{{cite journal}}: Invalid|display-authors=9(help) - ^ Sugimoto, Katsutoshi; Moriyasu, Fuminori; Saito, Kazuhiro; Rognin, Nicolas; Kamiyama, Naohisa; Furuichi, Yoshihiro; Imai, Yasuharu (2013). "Hepatocellular carcinoma treated with sorafenib: Early detection of treatment response and major adverse events by contrast-enhanced US". Liver International. 33 (4): 605–15. doi:10.1111/liv.12098. PMID 23305331.
- ^ Rognin, N G; Arditi, M; Mercier, L; Frinking, P J A; Schneider, M; Perrenoud, G; Anaye, A; Meuwly, J; Tranquart, F (2010). "Parametric imaging for characterizing focal liver lesions in contrast-enhanced ultrasound". IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 57 (11): 2503–11. doi:10.1109/TUFFC.2010.1716. PMID 21041137.
{{cite journal}}: Invalid|display-authors=9(help) - ^ Rognin N; et al. (2010). "Parametric images based on dynamic behavior over time". International Patent. World Intellectual Property Organization (WIPO). pp. 1–44.
{{cite web}}: Explicit use of et al. in:|author=(help) - ^ Tranquart, F.; Mercier, L.; Frinking, P.; Gaud, E.; Arditi, M. (2012). "Perfusion Quantification in Contrast-Enhanced Ultrasound (CEUS) – Ready for Research Projects and Routine Clinical Use". Ultraschall in der Medizin. 33: S31–8. doi:10.1055/s-0032-1312894. PMID 22723027.
- ^ Angelelli, Paolo; Nylund, Kim; Gilja, Odd Helge; Hauser, Helwig (2011). "Interactive visual analysis of contrast-enhanced ultrasound data based on small neighborhood statistics". Computers & Graphics. 35 (2): 218. doi:10.1016/j.cag.2010.12.005.
- ^ Barnes E, Contrast US processing tool shows malignant liver lesions, AuntMinnie.com, 2010.
- ^ Anaye, A.; Perrenoud, G.; Rognin, N.; Arditi, M.; Mercier, L.; Frinking, P.; Ruffieux, C.; Peetrons, P.; Meuli, R.; Meuwly, J.-Y. (2011). "Differentiation of Focal Liver Lesions: Usefulness of Parametric Imaging with Contrast-enhanced US". Radiology. 261 (1): 300–10. doi:10.1148/radiol.11101866. PMID 21746815.
- ^ Yuan, Zhang; Quan, Jiang; Yunxiao, Zhang; Jian, Chen; Zhu, He; Liping, Gong (2013). "Diagnostic Value of Contrast-Enhanced Ultrasound Parametric Imaging in Breast Tumors". Journal of Breast Cancer. 16 (2): 208–13. doi:10.4048/jbc.2013.16.2.208. PMC 3706868. PMID 23843855.
- ^ Klibanov, A. L.; Hughes, M. S.; Marsh, J. N.; Hall, C. S.; Miller, J. G.; Wilble, J. H.; Brandenburger, G. H. (1997). "Targeting of ultrasound contrast material. An in vitro feasibility study". Acta Radiologica Supplementum. 412: 113–120. PMID 9240089.
- ^ Klibanov, A (1999). "Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging". Advanced Drug Delivery Reviews. 37 (1–3): 139–157. doi:10.1016/S0169-409X(98)00104-5. PMID 10837732.
- ^ Pochon, S; Tardy, I; Bussat, P; Bettinger, T; Brochot, J; Von Wronski, M; Passantino, L; Schneider, M (2010). "BR55: A lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis". Investigative radiology. 45 (2): 89–95. doi:10.1097/RLI.0b013e3181c5927c. PMID 20027118.
- ^ Willmann, J. K.; Kimura, R. H.; Deshpande, N.; Lutz, A. M.; Cochran, J. R.; Gambhir, S. S. (2010). "Targeted Contrast-Enhanced Ultrasound Imaging of Tumor Angiogenesis with Contrast Microbubbles Conjugated to Integrin-Binding Knottin Peptides". Journal of Nuclear Medicine. 51 (3): 433–40. doi:10.2967/jnumed.109.068007. PMID 20150258.
- ^ Lindner, JR (2004). "Molecular imaging with contrast ultrasound and targeted microbubbles". Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 11 (2): 215–21. doi:10.1016/j.nuclcard.2004.01.003. PMID 15052252.
- ^ Clinical trial number NCT01253213 for "BR55 in Prostate Cancer: an Exploratory Clinical Trial" at ClinicalTrials.gov
- ^ Dayton, Paul; Klibanov, Alexander; Brandenburger, Gary; Ferrara, Kathy (1999). "Acoustic radiation force in vivo: A mechanism to assist targeting of microbubbles". Ultrasound in Medicine & Biology. 25 (8): 1195. doi:10.1016/S0301-5629(99)00062-9.
- ^ Frinking, Peter J.A.; Tardy, Isabelle; Théraulaz, Martine; Arditi, Marcel; Powers, Jeffry; Pochon, Sibylle; Tranquart, François (2012). "Effects of Acoustic Radiation Force on the Binding Efficiency of BR55, a VEGFR2-Specific Ultrasound Contrast Agent". Ultrasound in Medicine & Biology. 38 (8): 1460–9. doi:10.1016/j.ultrasmedbio.2012.03.018. PMID 22579540.
- ^ Gessner, Ryan C.; Streeter, Jason E.; Kothadia, Roshni; Feingold, Steven; Dayton, Paul A. (2012). "An In Vivo Validation of the Application of Acoustic Radiation Force to Enhance the Diagnostic Utility of Molecular Imaging Using 3-D Ultrasound". Ultrasound in Medicine & Biology. 38 (4): 651–60. doi:10.1016/j.ultrasmedbio.2011.12.005. PMID 22341052.
- ^ Rognin N; et al. (2013). "Molecular Ultrasound Imaging Enhancement by Volumic Acoustic Radiation Force (VARF): Pre-clinical in vivo Validation in a Murine Tumor Model". World Molecular Imaging Congress, Savannah, GA, USA.
{{cite journal}}: Explicit use of et al. in:|last=(help) - ^ Cogo, A.; Lensing, A. W A; Koopman, M. M W; Piovella, F.; Siragusa, S.; Wells, P. S; Villalta, S.; Büller, H. R; Turpie, A. G G; Prandoni, P. (1998). "Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: Prospective cohort study". BMJ. 316 (7124): 17–20. doi:10.1136/bmj.316.7124.17. PMC 2665362. PMID 9451260.
- ^ Kearon, Clive; Julian, JA; Newman, TE; Ginsberg, JS (1998). "Noninvasive Diagnosis of Deep Venous Thrombosis". Annals of Internal Medicine. 128 (8): 663–77. doi:10.7326/0003-4819-128-8-199804150-00011. PMID 9537941.
- ^ Jongbloets, L.M.M.; Koopman, M.M.W.; Büller, H.R.; Ten Cate, J.W.; Lensing, A.W.A. (1994). "Limitations of compression ultrasound for the detection of symptomless postoperative deep vein thrombosis". The Lancet. 343 (8906): 1142–4. doi:10.1016/S0140-6736(94)90240-2. PMID 7910237.
- ^ Merritt, CR (1989). "Ultrasound safety: What are the issues?". Radiology. 173 (2): 304–6. doi:10.1148/radiology.173.2.2678243. PMID 2678243.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ "Training in Diagnostic Ultrasound: essentials, principles and standards" (PDF). WHO. 1998. p. 2.
- ^ American Academy of Orthopaedic Surgeons (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Academy of Orthopaedic Surgeons, retrieved 19 May 2013, which cites
- Members of 2007 and 2011 AAOS Guideline Development Work Groups on PE/VTED Prophylaxis; Mont, M; Jacobs, J; Lieberman, J; Parvizi, J; Lachiewicz, P; Johanson, N; Watters, W (Apr 18, 2012). "Preventing venous thromboembolic disease in patients undergoing elective total hip and knee arthroplasty". The Journal of bone and joint surgery. American volume. 94 (8): 673–4. doi:10.2106/JBJS.9408edit. PMC 3326687. PMID 22517384.
{{cite journal}}: CS1 maint: numeric names: authors list (link)
- Members of 2007 and 2011 AAOS Guideline Development Work Groups on PE/VTED Prophylaxis; Mont, M; Jacobs, J; Lieberman, J; Parvizi, J; Lachiewicz, P; Johanson, N; Watters, W (Apr 18, 2012). "Preventing venous thromboembolic disease in patients undergoing elective total hip and knee arthroplasty". The Journal of bone and joint surgery. American volume. 94 (8): 673–4. doi:10.2106/JBJS.9408edit. PMC 3326687. PMID 22517384.
- ^ Bricker, L; Garcia, J; Henderson, J; Mugford, M; Neilson, J; Roberts, T; Martin, MA (2000). "Ultrasound screening in pregnancy: A systematic review of the clinical effectiveness, cost-effectiveness and women's views". Health technology assessment. 4 (16): i–vi, 1–193. PMID 11070816.
- ^ Ang, E. S. B. C.; Gluncic, V.; Duque, A.; Schafer, M. E.; Rakic, P. (2006). "Prenatal exposure to ultrasound waves impacts neuronal migration in mice". Proceedings of the National Academy of Sciences. 103 (34): 12903–10. doi:10.1073/pnas.0605294103. PMC 1538990. PMID 16901978.[non-primary source needed]
- ^ Kieler, Helle; Cnattingius, Sven; Haglund, Bengt; Palmgren, Juni; Axelsson, Ove (2001). "Sinistrality—a side-effect of prenatal sonography: A comparative study of young men". Epidemiology. 12 (6): 618–23. doi:10.1097/00001648-200111000-00007. PMID 11679787.[non-primary source needed]
- ^ Salvesen, K A; Vatten, L J; Eik-Nes, S H; Hugdahl, K; Bakketeig, L S (1993). "Routine ultrasonography in utero and subsequent handedness and neurological development". BMJ. 307 (6897): 159–64. doi:10.1136/bmj.307.6897.159. PMC 1678377. PMID 7688253.[non-primary source needed]
- ^ Kieler, Helle; Axelsson, Ove; Haglund, Bengt; Nilsson, Staffan; Salvesen, Kjell Å. (1998). "Routine ultrasound screening in pregnancy and the children's subsequent handedness". Early Human Development. 50 (2): 233–45. doi:10.1016/S0378-3782(97)00097-2. PMID 9483394.[non-primary source needed]
- ^ Heikkilä, K.; Vuoksimaa, E.; Oksava, K.; Saari-Kemppainen, A.; Iivanainen, M. (2011). "Handedness in the Helsinki Ultrasound Trial". Ultrasound in Obstetrics & Gynecology. 37 (6): 638. doi:10.1002/uog.8962.[non-primary source needed]
- ^ Salvesen, K. Å. (2011). "Ultrasound in pregnancy and non-right handedness: Meta-analysis of randomized trials". Ultrasound in Obstetrics & Gynecology. 38 (3): 267. doi:10.1002/uog.9055.
- ^ Fetal Keepsake Videos. Fda.gov (2009-04-21). Retrieved on 2011-11-13.
- ^ Clinical Safety Statements. Efsumb.org. Retrieved on 2011-11-13.
- ^ a b c d Gammeltoft, Tine (2007). "Sonography and Sociality: Obstetrical Ultrasound Imaging in Urban Vietnam". Medical Anthropology Quarterly. 21 (2): 134. doi:10.1525/maq.2007.21.2.133.
- ^ Deane, Collin (2002). "Safety of diagnostic ultrasound in fetal scanning". In Kypros Nicolaides, Giuseppe Rizzo, Kurt Hecker and Renato Ximenes (ed.). Doppler in Obstetrics.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ MTP and PCPNDT Initiatives Report Government of India (2011)
- ^ IMPLEMENTATION OF THE PCPNDT ACT IN INDIA - Perspectives and Challenges Public Health Foundation of India, Supported by United Nations FPA (2010)
- ^ "THE PRE-NATAL DIAGNOSTIC TECHNIQUES (REGULATION AND PREVENTION OF MISUSE) ACT, 1994". mohfw.nic.in. 20 September 1994.
- ^ Donald, Ian; MacVicar, J; Brown, T.G (1958). "Investigation of Abdominal Masses by Pulsed Ultrasound". The Lancet. 271 (7032): 1188–95. doi:10.1016/S0140-6736(58)91905-6. PMID 13550965.
- ^ Edler, I.; Hertz, C. H. (2004). "The Use of Ultrasonic Reflectoscope for the Continuous Recording of the Movements of Heart Walls". Clinical Physiology and Functional Imaging. 24 (3): 118–36. doi:10.1111/j.1475-097X.2004.00539.x. PMID 15165281.
- ^ "History of the AIUM". Archived from the original on November 3, 2005. Retrieved November 15, 2005.
- ^ "The History of Ultrasound: A collection of recollections, articles, interviews and images". www.obgyn.net. Retrieved 2006-05-11.[dead link]
- ^ Watts, G. (2009). "John Wild". BMJ. 339: b4428. doi:10.1136/bmj.b4428.
- ^ Woo, Joseph (2002). "A short History of the development of Ultrasound in Obstetrics and Gynecology". ob-ultrasound.net. Retrieved 2007-08-26.
- ^ Zierler, R. Eugene (November 1, 2002). "D. Eugene Strandness, Jr, MD, 1928–2002". Journal of Ultrasound. 21 (11): 1323–1325.
- ^ "Medical Imaging Past Present and Future" http://books.google.fr/books?id=DForAgAAQBAJ&printsec=frontcover&dq=inauthor:%22XRayCeRT.com%22&hl=fr&sa=X&ei=Ynv0U5DlDIqd0QXV1IHYAw&ved=0CCgQ6AEwAQ#v=onepage&q&f=false
External links
- About the discovery of medical ultrasonography
- History of medical sonography (ultrasound)
- Procedures in Ultrasound (Sonography) for patients, from RadiologyInfo.org

